Abstract
Following acute brain injury, albumin may gain access to the brain parenchyma. Clinical studies indicate a protective role for albumin in stroke but an increase in mortality associated with albumin administration following traumatic brain injury. We investigated the effects of albumin on astrocyte and microglial activation, and the role of mitogen activated protein kinases (MAPK) in these responses. Albumin activated ERK1/2, p38 MAPK and JNK signaling pathways in astrocytes, and induced the production of interleukin (IL)-1β, inducible nitric oxide (NO) synthase, the NO metabolite nitrite, and the chemokine CX3CL1 while reducing the level of S100B. The release of inflammatory markers by astrocytes was partially dependent on p38 MAPK and ERK1/2 pathways, but not JNK. In microglia, albumin exposure activated all three MAPK pathways and produced an increase in IL-1 β and nitrite. Inhibition of p38 MAPK in microglia lead to an increased level of IL1β, while inhibition of all three MAPKs suppressed the release of nitrite. These results suggest that albumin activates astrocytes and microglia, inducing inflammatory responses involved both in the mechanisms of cellular injury and repair via activation of MAPK pathways, and thereby implicate glial activation in the clinical responses to administration of albumin.
Keywords: Albumin, astrocyte, microglia, cytokines, mitogen activated protein kinase
1. Introduction
Brain injury results in the activation of glial cells which may then contribute to the mechanisms of repair, neurologic injury, or susceptibility to subsequent neurologic injury. Precedent from studies in Alzheimer’s disease and epilepsy has identified glial activation as a potential mechanism contributing to neurologic injury (Mrak and Griffin, 2005; Somera-Molina et al., 2007). There is increasing evidence to show that this activation of astrocytes and microglial cells may also result in both protective and reparative roles after brain injuries (Chen and Swanson, 2003; Laird et al., 2008; Lalancette-Hebert et al., 2007; Myer et al., 2006). Both pre-clinical (Browne et al., 2006) and clinical studies (Edwards et al., 2005) show that, under certain conditions, anti-inflammatory therapy may exacerbate neurologic injury after acute brain injury.
The integrity of the blood brain barrier may be compromised following brain injury including traumatic brain injury (TBI) (Unterberg et al., 2004) and stroke (Sandoval and Witt, 2008), resulting in the exposure of the central nervous system to blood-derived factors from which it is normally isolated. Among these factors, albumin acts as a signaling molecule in different types of cells including glia (Nadal et al., 2001). In microglia, albumin causes activation, proliferation, increased intracellular calcium and cytokine production (Hooper et al., 2005; Hooper et al., 2009). In astrocytes, albumin induces calcium signaling (Nadal et al., 2001) and increased production of the inflammatory chemokine MCP-1 (Calvo et al., 2005).
Albumin has also been implicated in the mechanisms of epileptogenesis and evolution of neurologic injury. The uptake by astrocytes and the epileptogenic effects of albumin on astrocytes are partially mediated by the transforming growth factor (TGF)-β receptor (Cacheaux et al., 2009; Ivens et al., 2007). However, the contribution of albumin to astrocyte activation, and the mechanisms which mediate this response are not well understood.
The role of albumin as a therapeutic modality in the treatment of TBI or stroke is controversial (Grande, 2008). The Saline versus Albumin Fluid Evaluation (SAFE) study showed no significant difference in the risk of death among patients treated with albumin or crystalloid (SAFE Study Investigators, 2004). A post hoc analysis of critically ill patients with TBI found a significant increase in mortality associated with resuscitation with albumin (SAFE Study Investigators, 2007). This finding is in striking contrast to the preclinical (Belayev et al., 2001) and clinical (Ginsberg et al., 2006) evidence supporting a neuroprotective benefit for albumin treatment in ischemic stroke. The mechanisms causing these discrepant responses remain to be determined.
Here, we tested the hypothesis that albumin produces activation of astrocytes and that this activation is mediated by mitogen activated protein kinases (MAPKs) including p38 MAPK, extracellular signal regulated protein kinase (ERK 1/2) and c-Jun N-terminal kinase (JNK). Albumin induces markers of glial activation including interleukin (IL)-1β, and inducible nitric oxide synthase (iNOS) but suppresses the release of S100B while producing an increase in the levels of neuroprotective chemokine, CX3CL1 (Re and Przedborski, 2006). We also investigated microglial responses to albumin under the same conditions. In microglial cultures, exposure to albumin induces an increase in IL-1β and nitrite. These responses partially involve MAPK-dependent pathways. These data implicate albumin in the mechanisms of glial activation after brain injury and identify a role for MAPK signaling in the mechanisms which mediate these responses.
2. Results
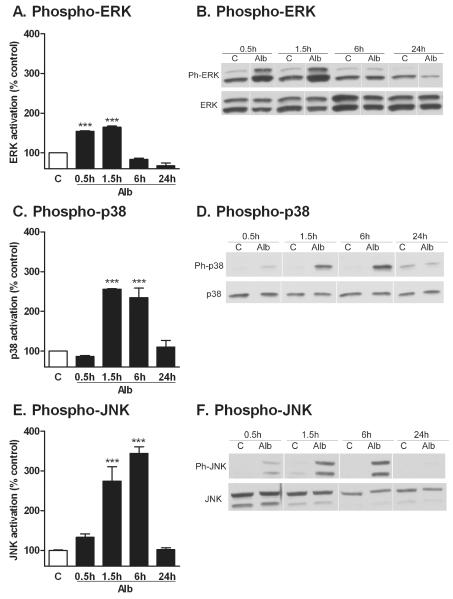
2.1. Albumin produces activation of MAPKs in astrocytes
Activation of the MAPKs, p38 MAPK, ERK1/2 and JNK was measured by quantifying the level of phosphorylation of each kinase by Western Blot (Fig. 1) up to 24 hr after exposure to albumin. Treatment of astrocytes with albumin resulted in an increase in the level of phosphorylation of the kinases, as early as 30 min for ERK1/2 (Fig. 1A and B) and 90 min for p38 and JNK (Fig. 1C-E). This increase in activated MAPKs was sustained for up to 6 hours. After 24 hr recovery, there was no significant difference between albumin-treated cells and controls in the level of phosphorylated p38 MAPK and JNK. In contrast, the level of phosphorylated ERK was significantly reduced in comparison to control cells at 24 hours. There were no differences in the levels of total ERK1/2, total p38 MAPK, and total JNK between the control and albumin group at each timepoint.
Figure 1. Albumin activates MAPK pathways in astrocytes.
Representative blots and quantification of the levels of phosphorylated and total ERK1/2 (A, B), p38 MAPK (C, D), and JNK (E, F) in astrocytes treated with either PBS control (C) or albumin (Alb) for 0.5, 1.5, 6 or 24 hours are shown. The data are representative of mean ± SEM of two independent experiments. ***P<0.001 compared to level in the control group.
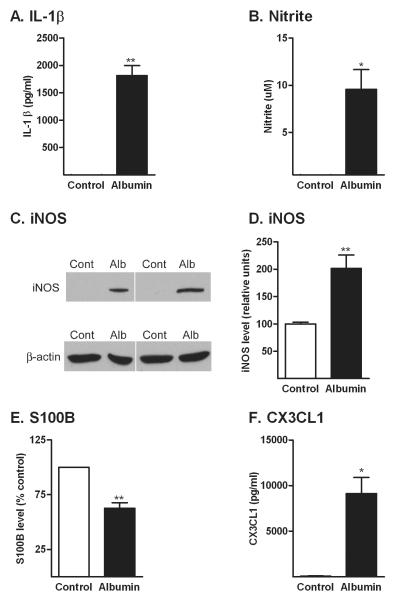
2.2. Albumin produces activation of astrocytes
We measured the level of the pro-inflammatory cytokine IL-1β, nitrite, S100B and the chemokine CX3CL1 in the conditioned media, and the level of inducible nitric oxide synthase (iNOS) in the astrocyte cell lysates after treatment with either PBS or albumin. First, we performed a time-course analysis of these endpoints over 24 hr exposure to albumin. At 0.5- and 1.5hr, there were no changes in the level of any of the factors measured compared to control cells (data not shown). At 6 hr, albumin induced an increase in the media levels of IL-1β (60.8 ± 18.5 pg/mL) compared to control cells, in which it was not detectable, and in the levels of CX3CL1 (517.6 ± 12.9 pg/mL) compared to controls (148.7 ± 12.9 pg/mL, P < 0.05 by ANOVA). However, levels of nitrite, iNOS, and S100B were unchanged at 6 hr.
Treatment with albumin for 24 hr resulted in a significant increase in the level of IL-1β (Fig. 2A). IL-1 β levels were undetectable in the culture media of control cells. Similarly, treatment with albumin for 24 hr produced significant increases in nitrite released in the media (Fig. 2B), which corresponded with an increase in the level of iNOS in the cells lysates (Fig. 2C and D).
Figure 2. Albumin induces a change in the production of inflammatory mediators by astrocytes.
Treatment of astrocytes with albumin (Alb) induced an increase in the level of IL-1β (A), nitrite (B) iNOS protein levels (C and D), a decrease in the level of S100B (E), and an increase in CX3CL1 (F). The data are representative of mean ± SEM of three independent experiments. All endpoints were measured after 24 hr exposure to albumin. *P<0.05, **P<0.01 compared to control group.
The glial protein S100B is released by activated astrocytes and may contribute both to the mechanisms of injury or repair (Van Eldik and Wainwright, 2003). Albumin induced a decrease in the level of S100B in the media compared to control cells (Fig. 2E).
The chemokine CX3CL1 is constitutively expressed by neurons in the CNS, is neuroprotective in vivo, regulates activation of microglia and is expressed in glia in response to chronic neuroinflammation (Re and Przedborski, 2006). Treatment with albumin induced an increase in the level of the CX3CL1 compared to the control cells in which it was undetectable (Fig. 2F).
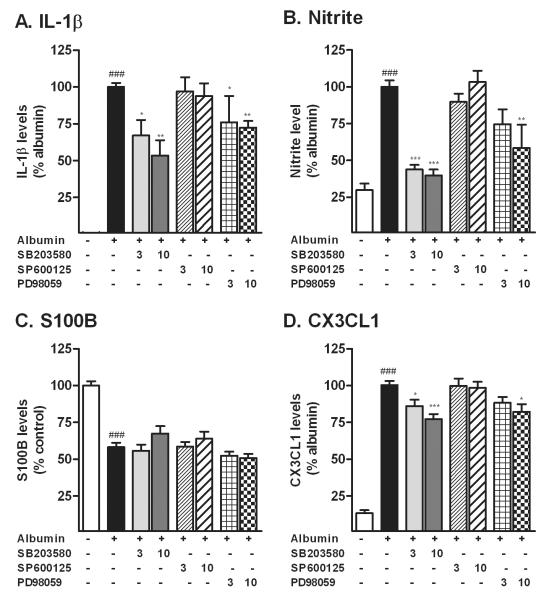
2.3. Inhibition of p38 MAPK and ERK suppresses the production of IL-1β, nitrite and CX3CL1 but does not change the decrease in S100B in astrocytes exposed to albumin
Next, we investigated whether the increase in the production of inflammatory factors induced by albumin was dependent on the activation of the MAPK pathway (Fig. 3). We treated astrocytes with two concentrations of either a p38 MAPK inhibitor (SB203580), a JNK inhibitor (SP600125), or the ERK pathway inhibitor (PD98059) before treatment with albumin. Albumin-induced increases in both IL-1β (Fig. 3A) and nitrite (Fig. 3B) measured after 24 hr exposure were attenuated by inhibition of p38MAPK and ERK. Inhibition of JNK activation did not prevent activation of either endpoint. The decrease in S100B produced by albumin was not altered by inhibition of MAPK activation (Fig. 3C). The increase in the release of CX3CL1 produced by albumin was partially prevented by inhibition of p38MAPK and ERK activation (Fig. 3D) but not by inhibition of JNK.
Figure 3. Inhibition of p38 and ERK suppresses the production of IL-1β, nitrite and CX3CL1 but does not affect the level of S100B.
Astrocytes were treated with PBS or albumin in presence (+) or absence (-) of p38 MAPK inhibitor SB203580, JNK inhibitor SP600125, or ERK pathway inhibitor PD98059, at 3 or 10μM. The p38 MAPK and ERK pathway inhibitors attenuated the increases in the level of IL-1β (A), nitrite (B), without affecting the levels of S100B (C) induced by albumin. The p38 MAPK and ERK pathway inhibitors also partially suppressed the increase in the levels of CX3CL1 (D) induced by albumin. The inhibition of JNK did not modify any of these endpoints. All endpoints were measured after 24 hr exposure to albumin. The data are representative of mean ± SEM of three to five independent experiments. ### P<0.001 compared to control group. *P<0.05, **P<0.01, ***P<0.001 compared to albumin group.
2.4. Different preparations of albumin activate astrocytes
In the studies for this report we used an albumin preparation, fatty acid- and globulin free-BSA, shown in other published studies to modulate calcium uptake in astrocytes (Nadal et al., 1995; Nadal et al., 1996). Cellular responses to albumin may vary with the purity of the preparation and the species of origin (Calvo et al., 2005; Nadal et al., 1995; Nadal et al., 2001; Si et al., 1997). To determine whether the astrocyte responses to BSA were affected by albumin type, we exposed primary astrocytes to different albumin preparations and quantified the activation responses (Table 1). Compared to BSA, the fraction V formulation showed a similar increase in the release of IL-1β, but a lesser increase in nitrite and CX3CL1, and a lesser decrease in S100B. Next, we examined whether the albumin effects are species-dependent. The effects of rat serum albumin (RSA) were also comparable to the BSA on the increase in IL-1β, nitrite and the decrease in S100B. RSA induced a slightly lesser increase in CX3CL1 compared to BSA. Human serum albumin (HSA) induced a comparable increase in of IL-1β but showed a greater increase in nitrite and a lesser decrease in S100B compared to BSA.
Table 1. Comparative effects of four albumin preparations on astrocyte activation.
Enriched astrocytes cultures were incubated for 24 hours in the presence of either PBS (Control), 0.1mM fatty acid free and globulin free Bovine Serum Albumin (BSA), BSA fraction V (FrV), Rat Serum Albumin (RSA) or Human Serum Albumin (HSA). For IL-1β, nitrite and CX3CL1, data are expressed as a percentage of the results obtained for BSA. For S100B, data are expressed as a percentage of the control group. Data are shown as mean ± SEM of two experiments.
| Albumin Type | Species of Origin | ||||
|---|---|---|---|---|---|
| Control | BSA | BSA-FrV | RSA | HSA | |
| IL-1β (% ) | 1.52±0.9 | 100±2.62 | 100.1±20.5 | 94.7±32.8 | 104.6±0.5 |
| Nitrite (%) | 22.4±5.6 | 100±2.9 | 50.1±1.5*** | 83.3±7.6 | 142.1±12.1*** |
| CX3CL1 (%) | 12.9±3.9 | 100 ±2.5 | 38.1±5.2*** | 68.4±8.8** | 87.8±10.1 |
| S100B (%) | 100±3.8 | 52.2±3.3 | 87.7±8.6*** | 66.4±3.1 | 80.6±10.8** |
p<0.001
p<0.001 compared to BSA group. (Il-1β, Interleukin-1β)
The production of IL-1β, NO and CX3CL1 induced by albumin in astrocyte cultures was not altered by treatment with polymyxin B (data not shown). This suggests that the effects of albumin observed in the astrocyte preparation were not due to contamination by endotoxin.
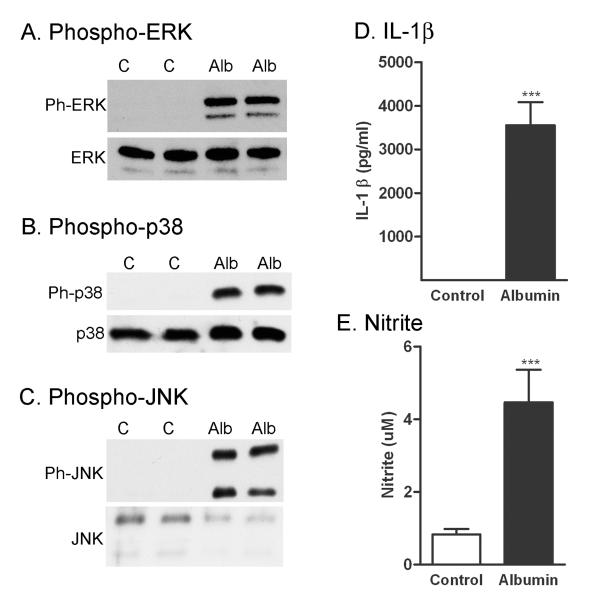
2.5. Albumin activates MAPKs and increases IL-1β and nitrite levels in microglia
We exposed microglia to albumin using the same conditions as those used for the astrocyte experiments (Fig. 4). After 1 hour exposure to albumin, there were significant increases in levels of phosphorylated ERK, p38 MAPK, and JNK compared to controls (Fig. 4A-C). Similar to the response we observed in astrocytes, there were also significant increases in both IL-1β (Fig. 4D) and nitrite (Fig. 4E) after 24 hours exposure. There was no S100B or CX3CL1 detected in the microglia in any experimental groups (data not shown).
Figure 4. Albumin activates MAPK pathways and increases IL-1β and nitrite in microglial cells.
Representative blots of the levels of phosphorylated and total ERK1/2 (A), p38 MAPK (B), and JNK (C) in astrocytes treated with either PBS control (C) or albumin (Alb) for 1 hour. Similar results were obtained in two other experiments. Treatment of microglial cells with albumin for 24 hours induced an increase in the level of IL-1β (D), nitrite (E) in the conditioned media. The data are representative of mean ± SEM of 3 independent experiments. ***P<0.001 compared to level in the control groups.
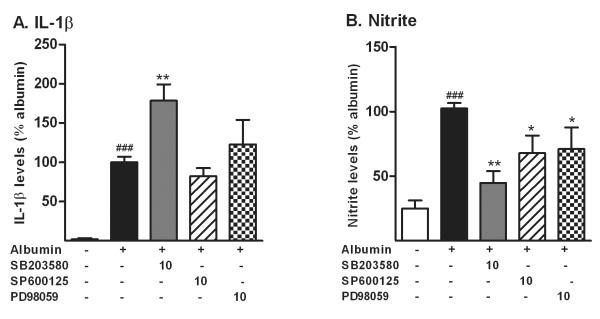
We then investigated whether the production of inflammatory mediators released by microglial cells stimulated by albumin required activation of MAPKs (Fig. 5). Microglia were pre-treated with 10μM of MAPK inhibitors described above, before activation with albumin. Inhibition of p38 MAPK enhanced the increase in IL-1β in the microglia-conditioned media. Inhibition of ERK and JNK did not affect the increase in IL-1β caused by albumin. In contrast, the inhibition of P38 MAPK, ERK and JNK all significantly suppressed the increase in nitrite induced by albumin (Fig. 5B).
Figure 5. MAPKs partially mediate the increase in IL-1β and nitrite induced by albumin in microglial cells.
Microglial cells were treated with PBS or albumin in presence (+) or absence (-) of p38 MAPK inhibitor SB203580, JNK inhibitor SP600125, or ERK pathway inhibitor PD98059, at 10μM. Inhibition of p38 MAPK enhanced the increase in the level of IL-1β (A), while the inhibition of ERK and JNK did not modify the response to albumin. However, the inhibition of each MAPK pathway partially suppressed the increase in the levels nitrite (B) induced by albumin. Both endpoints were measured after 24 hr exposure to albumin. The data are representative of mean ± SEM of four independent experiments. ###P<0.001 compared to control group *P<0.05, **P<0.01 compared to albumin group.
2.6. Albumin exposure does not alter astrocyte viability
We visualized apoptotic nuclei with the fluorescent dye Hoescht 33342. Treatment of enriched astrocytes with albumin for 24 hours did not change the number of apoptotic cells (Suppl. Fig.1A and B) while the nuclei of cells exposed to the positive control staurosporine (100nM) became shrunken and dense. To determine the contribution of necrotic cell death to albumin-induced astrocyte injury, we stained the cells with propidium iodide (PI) which identifies permeable cells by red fluorescence of the nuclei. Albumin did not induce any increase in the number of cell nuclei stained with PI (Suppl. Fig.1A and B). Again, cells treated with staurosporine showed a greater number of cells nuclei stained red by PI compared to controls.
3. Discussion
The principal finding of this report is the demonstration that albumin can activate astrocytes and microglia, producing increases in nitric oxide and the pro-inflammatory cytokine IL-1β. In astrocytes, these responses are mediated by MAPKs, of which ERK is the most rapidly activated. The glial-derived protein S100B has been implicated both in the mechanisms of repair and injury in response to neurologic insults (Van Eldik and Wainwright, 2003). Our data show a decrease in S100B produced by exposure to albumin and an increase in the chemokine CX3CL1, which is thought to be neuroprotective (Re and Przedborski, 2006). In microglia, the albumin-induced increase in nitrite also involves MAPK pathways, while the increase in IL-1β is not MAPK-dependent. Taken together, these data show a combination of pathologic, inflammatory responses to albumin as well as responses which may be considered protective. The absence of cell death produced by albumin suggests that these responses are physiologically relevant rather than the result of irreversible cell injury. This study highlights possible new mechanisms in glial cells response after brain injury leading to blood brain barrier injury and extravasation of albumin into the brain parenchyma.
The activation of MAPKs in astrocytes exposed to albumin has not previously been reported. MAPKs play a crucial role in signal transduction, regulating both cell death and survival following neurologic insults (Harper and Wilkie, 2003). In vitro studies have shown that MAPKs are activated in response to traumatic injuries. ERK is activated in response to trauma, while studies showing p38 and JNK activation are more variable (Huang et al., 2009; Mori et al., 2002; Neary et al., 2003). ERK inhibition increases cell survival in the scratch model of trauma (Mori et al., 2002). JNK has been reported to mediate astrocyte apoptosis following traumatic injury (Huang et al., 2009). Our data indicate that the activation of these pathways in glia is an early event after exposure to albumin.
Albumin produces a diverse range of effects on astrocytes including altered calcium signaling and DNA synthesis (Nadal et al., 1995), and increase in production of the inflammatory chemokine MCP-1 (Calvo et al., 2005). Furthermore, in vivo studies show that exposure of the brain to albumin induces an increase genes associated with astrocyte activation (Cacheaux et al., 2009). Our data show that p38 and ERK, but not JNK, partially mediate the increase in IL-1β, NO and CX3CL1 produced by albumin. Consistent with these results, p38 MAPK and ERK are linked to the production of IL-1β, NO and CX3CL1 in activated astrocytes (Bhat et al., 1998; Lee et al., 2000; Sheng et al., 2009).
In primary microglia cultures, albumin has been previously shown to activate the ERK pathway leading to an increase in NO, but not IL-1β (Hooper et al., 2009). In contrast, studies using the N9 microglial cell line demonstrated an increase in IL-1β in response to albumin (Zhao et al., 2009), which is consistent with our findings. The discrepant responses between these studies may reflect the differences in treatment regimens and dosage of albumin used to treat the microglia.
IL-1β and NO increased in both astrocytes and microglia in response to albumin but this increased was accentuated in microglia by the inhibition of p38 MAPK. It is possible that this response is due to an inhibitory pathway stimulated by albumin that is dependent on p38 MAPK. The increase in nitrite in microglia was dependent on the all three MAPK pathways, concordant with a previous report showing that increase in nitrite induced by albumin in microglia is dependent on ERK pathway (Hooper et al., 2009). Alternatively, the extracellular signaling pathways activated by albumin are likely to differ between cell types, and this may account for different cell-specific responses. For example, astrocytes internalize albumin in vesicle-like structures by a receptor-mediated process (Tabernero et al., 2002). Other studies using astrocytes have identified megalin (Bento-Abreu et al., 2008) or the TGFβ receptor (Cacheaux et al., 2009; Ivens et al., 2007) as receptors for albumin..
We found a reduction in the release of the astrocyte-derived protein S100B following exposure to albumin, which was not dependent on MAPK activation. At low concentrations (nM) S100B is neurotrophic and neuroprotective, while at higher concentrations (μM) it may cause excess glial activation and neuronal injury (for review, see Van Eldik and Wainwright, 2003). Other studies have identified a restorative role for S100B after TBI. In the cell stretch model of TBI of combined neuronal glial cultures, exogenous S100B decreases delayed neuronal injury (Ellis et al., 2007). In our experiments, the level of S100B that were measured in the media of the cells was in the range of 1-30 ng/mL (0.05-1.5 nM). Therefore, the decrease that we saw in response to albumin might impair the neurotrophic role of S100B.
The chemokine CX3CL1 is constitutively expressed by neurons in the CNS, is neuroprotective in vivo, and regulates activation of microglia (Re and Przedborski, 2006). Our data indicate that the increase in CX3CL1, like the increase in IL-1β and nitric oxide, induced by albumin was partially dependent on the activation of p38 MAPK and ERK but not JNK. Thus, the increase in CX3CL1 we observed in response to albumin might act as a negative feedback on the production of IL-1β by both astrocytes and microglia.
The in vivo functional consequences of the activation of astrocytes and microglia in response to albumin are unclear, as activation of glia may produce both deleterious and protective effects after injury (Chen and Swanson, 2003; Laird et al., 2008; Wyss-Coray and Mucke, 2002). Factors derived from albumin-treated microglia induce neuronal apoptosis in vitro (Hooper et al., 2009). The increase in IL-1β released both by astrocytes and microglia also suggests a potential mechanism for neurologic injury. Multiple lines of evidence support a role for IL-1β in neuronal injury (Allan et al., 2005) and epileptogenesis; Ravizza et al., 2008). In contrast, IL-1β may also attenuate cell injury by increasing the levels of trophic growth factors (Friedman, 2005; Laird et al., 2008).
Administration of albumin in clinical practice has been associated with no effect on mortality in critically ill patients (SAFE Study Investigators, 2004), increase in mortality after TBI (SAFE Study Investigators, 2007) and improved functional outcome 2 years after ischemic stroke (Palesch et al., 2006). While the increase in mortality in TBI may be attributed to differences in fluid management (Rodling Wahlström et al., 2008), the effect of albumin on outcome after TBI remains unresolved. Interestingly, pre-clinical studies in models of both TBI (Belayev et al., 1999a) and stroke (Belayev et al., 1999b) show that albumin attenuates neurologic injury by maintaining integrity of the blood brain barrier (Belayev et al., 2005) and improving microvascular blood flow (Nimmagadda et al., 2008). The contribution of albumin-induced effects on glial activation to neurologic outcome after TBI or stroke is not known.
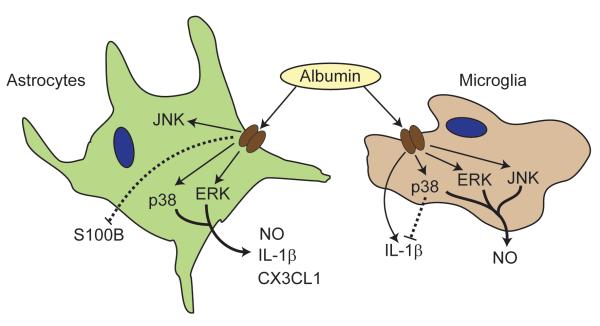
This study advances the previous findings on the effects of albumin on astrocytes (Cacheaux et al., 2009; Ivens et al., 2007) and microglia (Hooper et al., 2009). Here, we identify a mechanism involving activation of MAPKs, by which astrocytes and microglia respond to albumin, and show that albumin activates opposing pathways in the two cell types (Fig. 6). In astrocytes, albumin-induced activation of the p38 MAPK and ERK pathways leads to the production both of pro- (IL-1β, nitric oxide) and anti- inflammatory (CX3CL1) markers in addition to reducing the levels of S100B, a molecule with a complex role in the mechanisms of both injury and repair. In microglia, p38 MAPK is activated and acts as an inhibitory pathway on the release of IL-1β. Conversely, p38 MAPK, ERK and JNK are involved in the release of nitric oxide in microglia. The mechanisms by which albumin attenuates acute cerebral injury in pre-clinical studies (Belayev et al., 1999b; Belayev et al., 2001), and its potential as a therapeutic for clinical treatment of ischemic stroke (Ginsberg et al., 2006) may therefore involve enhancement of glial activation. The multiple pathways activated by albumin, and the complex divergent roles for glia in the mechanisms of cellular repair and injury, underscore the potential of glia as a therapeutic target in brain injury, and the need for greater understanding of the mechanisms which regulate the contributions of glia to injury and repair in the central nervous system.
Figure 6. Schema of astrocyte and microglia responses to albumin.
The activation of p38 MAPK and ERK pathways in astrocytes leads to the increase in IL-1β, nitric oxide and CX3CL1. Albumin induced a decrease (dashed line) in S100B via an undetermined pathway. In microglia, p38 MAPK acts as an inhibitory pathway (dashed line) on the release of IL-1β while the release of nitric oxide involves activation of p38 MAPK, ERK and JNK.
4. Experimental procedures
4.1. Reagents
Sprague Dawley rats were obtained from Charles River (Wilmington, MA). Ca2+ and Mg2+ free Hank’s balanced buffered salt solution (HBSS), Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum penicillin, streptomycin and fungizone, Trypsin/EDTA solution were obtained from Hyclone (Logan, UT). Tissue culture plasticware and coverslips, anti-inducible nitric oxide synthase (iNOS) were from BD Biosciences (San Jose, CA). N2 supplement was from Invitrogen (Carlsbad, CA). SB203580, PD98059, SP600125 were from Calbiochem (Gibbstown, NJ). Griess assay kit was from Promega (Madison,WI). Bicinchoninic acid protein assay was from Pierce (Rockford, IL). IL-1 and CX3CL1 ELISA, streptavidin-HRP are from R&D Systems (Minneapolis, MN). Maxisorp 96-well plates were from Nunc (Rochester, NY). Rabbit anti-S100B was from Abcam (Cambridge, MA). Biotinylated rabbit anti-S100B and rabbit anti-GFAP were from Dako (Carpinteria, CA). Rabbit anti anti-IBA-1 was from Wako (Richmond, VA). Antibodies against -phospho P38 MAPK, phospho ERK1/2, phospho JNK, p38MAPK, ERK1/2 and JNK were from Cell Signaling Technology (Danvers, MA), horse radish perodixase (HRP)-conjugated secondary antibodies were from Jackson Immunoreserach (West Grove, PA). Paraformaldehyde was from Electron Microscopy Sciences (Hatfield, PA). Normal goat serum was from Vector Laboratories (Burlingame, CA). Hoescht 33342, goat anti-rabbit IgG conjugated with Alexa Fluor 488 and Prolong gold anti-fade were from Invitrogen (Carlsbad, CA). All other chemicals and reagents, including all albumin preparations were from Sigma, (St Louis, MO).
4.2. Isolation and culture of primary cells
All experiments were performed following a protocol approved by the Institutional Animal Care and Use Committee of Children’s Memorial Research Center, Chicago, Illinois. Primary cortical astrocyte cultures were prepared from 1-3 day old Sprague Dawley rat pups. Cortices were isolated and cleaned of meninges in HBSS. After trypsin digestion, the cell suspension was filtered through a 40 μm filter, centrifuged, resuspended in DMEM supplemented with 10% fetal bovine serum and 1% of penicillin, streptomycin and fungizone. Cells were then plated onto 75 cm2 flasks and cultured in 5% CO2 humidified incubator at 37°C with media changes every 2-3 days.
To obtain microglial cultures, the flasks were shaken at 200 rpm for 2 hours after 9-10 days in culture. The medium containing floating microglia was removed and filtered with a 40um cell strainer. The cells were centrifuged, resuspended in DMEM supplemented with 10% fetal bovine serum and 1% of penicillin, streptomycin and plated onto 12 well plates at a density of 2 × 105 cells/well. 30 min after plating, the media was replaced with media containing half filtered media from confluent astrocytes and half fresh supplemented DMEM. Media was changed after 3 days and the cells were used within 3-6 days after plating. The enriched microglia cultures were composed of >95% of microglia as determined by staining using an anti-IBA1 primary antibody and the nuclear staining dye DAPI (results not shown). Enriched astrocytes cultures were prepared by shaking the flasks at 200 rpm for an additional 22 hours after shaking off the microglia, and the media containing floating microglia cells and oligodendrocytes was removed and replaced. Cells were lifted from the flask with 0.05% Trypsin/0.2 % EDTA and plated onto 6 or 12 well plates. Cells were cultured to confluency in 5% CO2 humidified incubator at 37°C with media changes every 3-4 days. The enriched astrocyte cultures were composed of >95% of astrocytes as determined by staining using an anti-GFAP primary antibody and the nuclear staining dye DAPI (results not shown).
The media was changed to serum-free, phenol red free DMEM supplemented with 1% of N2 supplement 24 hours before treatment. Cells were treated with either phosphate buffered saline (PBS, control) or bovine serum albumin (BSA, >99% fatty acid and globulin free) at the same concentration (0.1mM) used in other studies (Cacheaux et al., 2009; Ivens et al., 2007). The p38 MAPK inhibitor SB203580, MEK/ERK pathway inhibitor PD98059, JNK inhibitor SP600125, polymyxin B (10μg/mL), or diluent were administered to the cells 30 min prior to the treatment with PBS or albumin.
4.3. Western blot analyses
Cells were washed with cold PBS and scraped in a lysis buffer containing 20mM Tris pH 8, 2mM EDTA, 1% Triton X, 1μg/mL aprotinin, 1mM phenylmethanesulphonylfluoride, 2mM sodium orthovanadate and 1μg/ml leupeptin. The cell suspension was then sonicated and centrifuged at 10,000 × g for 5 min. The supernatant was added to 5X Laemmli sample buffer, then heated at 90°C for 5 min. Equal amounts of protein, determined by the bicinchoninic acid protein assay were separated on SDS-PAGE gels and transferred to a polyvinylidene fluoride membrane. Membranes were blocked with Tris-buffered saline containing 0.1% Tween-20 and 5% non-fat dry milk for 1 hour at room temperature. Membranes were then incubated overnight at 4°C with either anti-phospho P38 MAPK, anti-phospho ERK1/2, anti-phospho JNK, or anti-inducible nitric oxide synthase (iNOS), followed by an incubation with horse radish perodixase (HRP)-conjugated secondary antibodies for 1 hour at room temperature. Immunodetection was performed using chemiluminescent substrate. Autoradiography films were scanned and analyzed for relative densitometry with OpenLab 5.5.0 (Improvision, Waltham, MA). To measure the expression of the total MAPK proteins, the membranes were incubated with antibodies to total p38MAPK, ERK1/2 and JNK respectively. To control for equal protein loading, blots were stripped and re-probed with an anti-β-actin antibody.
4.4. ELISA
Levels of IL-1β and CX3CL1 in cell culture media were measured by ELISA according to the manufacturer’s instructions. Levels of S100B were measured using an in-house ELISA. Briefly, medium samples were incubated in Maxisorp 96-well plates previously coated with a polyclonal rabbit anti-S100B, followed by an incubation with biotinylated rabbit anti-S100B. Detection was performed by adding a solution of streptavidin-HRP. Addition of the substrate tetramethylbenzidine leads to blue color that is proportional to the amount of antigen in the well. The reaction is stopped by adding 1N sulfuric acid solution and the intensity of the resulting yellow color is measured spectrophotometrically at 450nm. The levels of S100B in the unknown samples were calculated from a standard curve made with 0.6-40 ng/mL of S100B protein.
4.5. Griess assay for nitrite
The levels of nitrite in medium samples were measured using a Griess assay kit.
4.6. Cell viability assay
After the incubation period, astrocytes were rinsed with PBS and incubated with 1μM Hoechst 33342 (Molecular Probes) to quantify apoptotic cells or 1μg/ml propidium iodide (PI) (Sigma, St. Louis, MO) to quantify necrotic cells in serum-free media for 20 min. Cell cultures were rinsed with PBS and were examined under a fluorescent microscope (Leica DMIL) attached to a Retica 1300i camera. Images were acquired using OpenLab 5.5 (Improvision, Waltham, MA). Apoptotic cells were identified by the presence of shrunken nuclei with dense blue staining by Hoescht 33342. Cells with compromised membrane integrity were identified by the presence of nuclei that stained positive with PI. Cell counts were performed in 3-4 random fields in each well with 4 wells per group using Image J for microscopy software (National Institutes of Health, Bethesda, MD).
4.7 Immunocytochemistry
Cells cultures were washed with cold PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. After permeabilization, non-specific binding was blocked using PBS containing 10% of normal goat serum for 1 hour. The cells were then incubated with anti-GFAP or anti-IBA-1 antibody overnight at 4°C. Cells were washed with PBS and incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Molecular Probes, Invitrogen) at 1:500 for 1 hour. Coverslips were mounted using Prolong Gold Antifade mounting solution containing DAPI. Fluorescence was visualized with a Leica DM-IRB inverted microscope coupled with a QImaging Retiga 4000R camera and images were acquired using using a Hamamatsu ORCA ER camera and pseudocolored using OpenLab 5.5 (Improvision, Waltham, MA). Routine cell counting were performed in the cell cultures by counting GFAP or IBA-1 positive cells in 5 areas per well, 2 wells per staining to obtain percentage of either astrocytes or microglia cells.
4.8. Statistical analysis
Data are expressed as mean ± SEM. Statistical differences between two groups were determined by Student’s unpaired t-test. Comparisons between multiple groups were performed with analysis of variance followed by Newman-Keuls post-hoc test. The criterion for statistical significance was P<0.05. Prism 5.0 (GraphPad Software, Inc., San Diego, CA) was used for statistical analyses.
Supplementary Material
Acknowledgements
We are grateful for the technical assistance of Fatima Patel. Dr. Wainwright is supported by the Davee Foundation, Medical Research Junior Board Foundation, the Lyndsey Whittingham Foundation, and by NIH grant KO8 NS044998.
Abbreviations
- IL-1
Interleukin-1
- NO
Nitric oxide
- TBI
Traumatic brain injury
- TGF-β
Transforming growth factor-β
- MAPK
Mitogen activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: Disease-Related Neuroscience
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–40. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Huh PW, Zhao W, Busto R, Ginsberg MD. Posttreatment with high-dose albumin reduces histopathological damage and improves neurological deficit following fluid percussion brain injury in rats. J Neurotrauma. 1999a;16:445–53. doi: 10.1089/neu.1999.16.445. [DOI] [PubMed] [Google Scholar]
- Belayev L, Saul I, Huh PW, Finotti N, Zhao W, Busto R, Ginsberg MD. Neuroprotective effect of high-dose albumin therapy against global ischemic brain injury in rats. Brain Res. 1999b;845:107–11. doi: 10.1016/s0006-8993(99)01952-6. [DOI] [PubMed] [Google Scholar]
- Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–60. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- Belayev L, Saul I, Busto R, Danielyan K, Vigdorchik A, Khoutorova L, Ginsberg M. Albumin treatment reduces neurological deficit and protects blood-brain barrier integrity after intracortical hematoma in the rat. Stroke. 2005;36:326–331. doi: 10.1161/01.STR.0000152949.31366.3d. [DOI] [PubMed] [Google Scholar]
- Bento-Abreu A, Velasco A, Polo-Hernandez E, Perez-Reyes PL, Tabernero A, Medina JM. Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J Neurochem. 2008;106:1149–59. doi: 10.1111/j.1471-4159.2008.05462.x. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne KD, Iwata A, Putt ME, Smith DH. Chronic ibuprofen administration worsens cognitive outcome following traumatic brain injury in rats. Exp Neurol. 2006;201:301–7. doi: 10.1016/j.expneurol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–35. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo CF, Amigou E, Tence M, Yoshimura T, Glowinski J. Albumin stimulates monocyte chemotactic protein-1 expression in rat embryonic mixed brain cells. J Neurosci Res. 2005;80:707–14. doi: 10.1002/jnr.20511. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–49. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, Fernandes J, Gogichaisvili T, Golden N, Hartzenberg B, Husain M, Ulloa MI, Jerbi Z, Khamis H, Komolafe E, Laloe V, Lomas G, Ludwig S, Mazairac G, Sanchez Mde L. Munoz, Nasi L, Olldashi F, Plunkett P, Roberts I, Sandercock P, Shakur H, Soler C, Stocker R, Svoboda P, Trenkler S, Venkataramana NK, Wasserberg J, Yates D, Yutthakasemsunt S. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–9. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Willoughby KA, Sparks SA, Chen T. S100B protein is released from rat neonatal neurons, astrocytes, and microglia by in vitro trauma and anti-S100 increases trauma-induced delayed neuronal injury and negates the protective effect of exogenous S100B on neurons. J Neurochem. 2007;101:1463–70. doi: 10.1111/j.1471-4159.2007.04515.x. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Interactions of interleukin-1 with neurotrophic factors in the central nervous system: beneficial or detrimental? Mol Neurobiol. 2005;32:133–44. doi: 10.1385/MN:32:2:133. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Palesch YY, Hill MD. The ALIAS (ALbumin In Acute Stroke) Phase III randomized multicentre clinical trial: design and progress report. Biochem Soc Trans. 2006;34:1323–6. doi: 10.1042/BST0341323. [DOI] [PubMed] [Google Scholar]
- Grande P. Time out for albumin or a valuable therapeutic component in severe head injury. Acta Anaesthesiol Scand. 2008;52:738–741. doi: 10.1111/j.1399-6576.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- Harper SJ, Wilkie N. MAPKs: new targets for neurodegeneration. Expert Opin Ther Targets. 2003;7:187–200. doi: 10.1517/14728222.7.2.187. [DOI] [PubMed] [Google Scholar]
- Hooper C, Taylor DL, Pocock JM. Pure albumin is a potent trigger of calcium signalling and proliferation in microglia but not macrophages or astrocytes. J Neurochem. 2005;92:1363–76. doi: 10.1111/j.1471-4159.2005.02982.x. [DOI] [PubMed] [Google Scholar]
- Hooper C, Pinteaux-Jones F, Fry VA, Sevastou IG, Baker D, Heales SJ, Pocock JM. Differential effects of albumin on microglia and macrophages; implications for neurodegeneration following blood-brain barrier damage. J Neurochem. 2009;109:694–705. doi: 10.1111/j.1471-4159.2009.05953.x. [DOI] [PubMed] [Google Scholar]
- Huang T, Solano J, He D, Loutfi M, Dietrich WD, Kuluz JW. Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma. 2009;26:1535–45. doi: 10.1089/neu.2008.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–47. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–64. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Drabik K, Van Wagoner NJ, Lee S, Choi C, Dong Y, Benveniste EN. ICAM-1-induced expression of proinflammatory cytokines in astrocytes: involvement of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. J Immunol. 2000;165:4658–66. doi: 10.4049/jimmunol.165.8.4658. [DOI] [PubMed] [Google Scholar]
- Mori T, Wang X, Jung JC, Sumii T, Singhal AB, Fini ME, Dixon CE, Alessandrini A, Lo EH. Mitogen-activated protein kinase inhibition in traumatic brain injury: in vitro and in vivo effects. J Cereb Blood Flow Metab. 2002;22:444–52. doi: 10.1097/00004647-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–72. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc Natl Acad Sci USA. 1995;92:1426–30. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, McNaughton PA. Albumin stimulates uptake of calcium into subcellular stores in rat cortical astrocytes. J Physiol. 1996;492(Pt 3):737–50. doi: 10.1113/jphysiol.1996.sp021342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, McNaughton PA. Glial cell responses to lipids bound to albumin in serum and plasma. Prog Brain Res. 2001;132:367–74. doi: 10.1016/S0079-6123(01)32088-5. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of Extracellular Signal-Regulated Kinase by Stretch-Induced Injury in Astrocytes Involves Extracellular ATP and P2 Purinergic Receptors. J. Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmagadda A, Park H, Prado R, Ginsberg M. Albumin therapy improves local vascular dynamics in a rat model of primary microvascular thrombosis: a two-photon laser-scanning microscopy study. Stroke. 2008;39:198–204. doi: 10.1161/STROKEAHA.107.495598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesch Y, Hill M, Ryckborst R, Tamariz D, Ginsberg M. A dose-escalation and safety study of albumin therapy for acute ischemic atroke—II: Neurologic outcome and efficacy analysis. Stroke. 2006;37:2107–2114. doi: 10.1161/01.STR.0000231389.34701.b5. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol Disease. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Re DB, Przedborski S. Fractalkine: moving from chemotaxis to neuroprotection. Nat Neurosci. 2006;9:859–61. doi: 10.1038/nn0706-859. [DOI] [PubMed] [Google Scholar]
- Rodling Wahlström M, Olivecrona M, Nyström F, Koskinen L, Naredi S. Fluid therapy and the use of albumin in the treatment of severe traumatic brain injury. Acta Anaesthesiologica Scand. 2008;53:18–25. doi: 10.1111/j.1399-6576.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- The SAFE Study Investigators A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- The SAFE Study Investigators Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiology of Disease. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Ni HT, Rock RB, Peterson PK. WIN55,212-2 inhibits production of CX3CL1 by human astrocytes: involvement of p38 MAP kinase. J Neuroimmune Pharmacol. 2009;4:244–8. doi: 10.1007/s11481-009-9147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si QS, Nakamura Y, Kataoka K. Albumin enhances superoxide production in cultured microglia. Glia. 1997;21:413–8. doi: 10.1002/(sici)1098-1136(199712)21:4<413::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Somera-Molina K, Robin B, Somera C, Anderson C, Stine C, Koh S, Behanna H, Van Eldik L, Watterson D, Wainwright M. Glial activation links early-life seizures and long-term neurologic dysfunction: evidence using a small molecule inhibitor of proinflammatory cytokine up-regulation. Epilepsia. 2007;48:1785–1800. doi: 10.1111/j.1528-1167.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- Somera-Molina K, Nair S, Van Eldik L, Watterson D, Wainwright M. Enhanced microglial activation and proinflammatory cytokine upregulation are linked to increased susceptibility to seizures and neurologic injury in a ‘two-hit’ seizure model. Brain Res. 2009;1282:162–172. doi: 10.1016/j.brainres.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero A, Velasco A, Granda B, Lavado EM, Medina JM. Transcytosis of albumin in astrocytes activates the sterol regulatory element-binding protein-1, which promotes the synthesis of the neurotrophic factor oleic acid. J Biol Chem. 2002;277:4240–6. doi: 10.1074/jbc.M108760200. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–9. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Van Eldik LJ, Wainwright MS. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci. 2003;21:97–108. [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease-a double-edged sword. Neuron. 2002;35:419–32. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Zhao TZ, Xia YZ, Li L, Li J, Zhu G, Chen S, Feng H, Lin JK. Bovine serum albumin promotes IL-1beta and TNF-alpha secretion by N9 microglial cells. Neurol Sci. 2009;30:379–83. doi: 10.1007/s10072-009-0123-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.