Abstract
The lacto-series gangliosides 3'-isoLM1 and 3',6'-isoLD1 have been identified as tumor-associated antigens whose formation is initiated by the Lc3 synthase. Until now, high- affinity IgG monoclonal antibodies (mAbs) against 3'-isoLM1 and 3',6'-isoLD1, which are highly expressed in gliomas, have not been developed, although mAbs against lacto-series gangliosides are powerful tools for functional studies. We recently produced the Lc3-synthase gene β3Gn-T5 knockout mice. In this study, we immunized β3Gn-T5 knockout mice with 3'-isoLM1/3',6'-isoLD1 and produced the anti-3'-isoLM1/3',6'-isoLD1 mAb GMab-1, of the IgG3 subclass, which should be useful for functional analysis of lacto-series gangliosides and for antibody-based therapy of gliomas.
Keywords: Lacto-series ganglioside; 3'-isoLM1; 3',6'-isoLD1; monoclonal antibody; Lc3-synthase; knockout mice
Introduction
Gangliosides are sialic acid-containing glycosphingolipids highly enriched in the vertebrate nervous system. Although their functions have not been fully clarified, they are thought to mediate neural cell-cell recognition and modulate intracellular signaling [1]. Gangliosides are implicated in various neural disorders, primarily the autoimmune neuropathies, but also in storage disorders, e.g. Tay-Sachs disease or Sandhoff disease [2–4]. High-affinity IgG anti-ganglioside antibodies may be used to develop animal models of autoimmune neuropathies and probe normal ganglioside functions. However, it has been difficult to produce high-affinity IgG antibodies against major brain gangliosides, especially GM1, GD1a, and GT1b [5]. This unresponsiveness has been attributed to poor immunogenicity, T-cell independence, and tolerance [5–7]. Advances in genetics provide a potential solution to this problem. Several studies have shown that mice genetically engineered to lack the glycosyltransferase gene for ganglioside synthesis do not express complex gangliosides [8–12]. These mice, immunologically naive to complex gangliosides, have been used for raising new antibodies against complex gangliosides [2, 6, 13, 14].
The Lc3-synthase gene (β1,3-N-acetylglucosaminyltransferase-V: β3Gn-T5) enzyme initiates the formation of the lacto-/neolacto-series ganglioside by transferring GlcNAc in a β1,3-linkage to lactosylceramide (Fig. 1A) [15]. The β3Gn-T5 is detected in mouse development and then again later mainly in the spleen and placenta in adult mice. Additionally, Lc3-synthase transcripts are found in cerebellar Purkinje cells of the adult mouse brain. On the other hand, lacto-series gangliosides such as 3'-isoLM1 and 3',6'-isoLD1 have been reported to be major mono- and oligo-sialogangliosides, respectively, of human gliomas [16–18]. In those studies, monoclonal antibodies (mAbs) such as SL-50, DMab-14, or DMab-22 recognizing lacto-series gangliosides were successfully produced; however, those antibodies proved to be of the low-affinity IgM subclass. In this study, we immunized β3Gn-T5 knockout mice with purified 3'-isoLM1 and 3',6'-isoLD1 to overcome these problems and generated an anti-lacto-series ganglioside IgG antibody with high affinity.
Fig. 1.
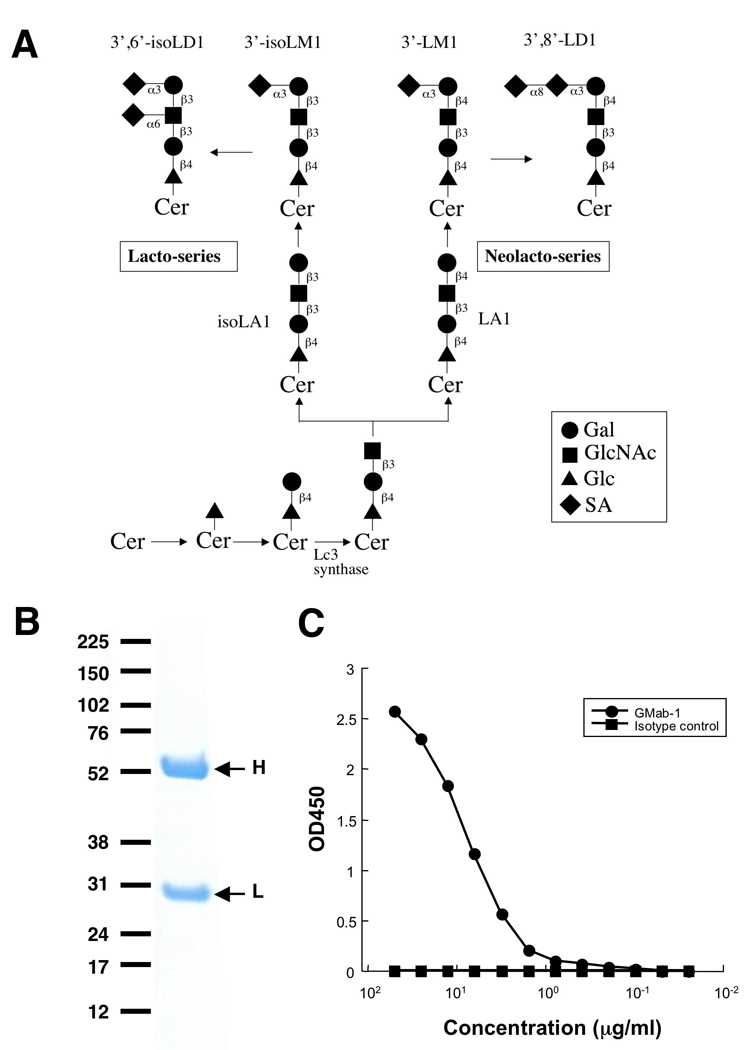
Production of an anti-3'-isoLM1/3',6'-isoLD1 ganglioside antibody. (A) Biosynthesis of lacto-/neolacto-series ganglioside. (B) Electrophoresis of 2 µg of GMab-1 under reducing conditions on 4–10% NuPAGE gel. (C) ELISA of GMab-1 against 3'-isoLM1. The 3'-isoLM1 conjugated with BSA was immobilized. After blocking, the plates were incubated with GMab-1 and isotype control at several concentrations.
Materials and methods
Animals, cell lines, xenograft, and tissues
The β3Gn-T5 knockout mice were recently developed at Duke University Medical Center (Kuan et al., manuscript submitted). P3U1 cells were obtained from the American Type Culture Collection (Manassas, VA), and we established a D54MG glioblastoma cell line at Duke [16]. P3U1 and D54MG cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air in RPMI 1640 medium including 2 mM l-glutamine (Invitrogen Corp., Carlsbad, CA) and 1% of penicillin-streptomycin solution (Invitrogen Corp.) or Zinc Option medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma, St. Louis, MO), respectively. We established and maintained a D54MG xenograft at Duke, derived from cultured D54MG cells. Human tissues slides from anonymous donors were obtained from the Tissue Bank at the Preston Robert Tisch Brain Tumor Center at Duke University
Antibodies and gangliosides
Anti-ganglioside antibodies SL-50, DMab-14, and DMab-22 were previously produced at Duke and the University of Gothenburg [16–18]. Isotype control of mouse IgG3 was purchased from eBioscience, Inc. (San Diego, CA). All gangliosides used for immunization or enzyme-linked immunosorbent assay (ELISA) were isolated and characterized at the University of Gothenburg as described previously [16, 17, 19].
Hybridoma production
The β3Gn-T5 knockout mice were immunized by neck s.c. injections of 20 µg of purified 3'-isoLM1 and 3',6'-isoLD1 coupled to Salmonella minnesota with Imject Freund's Complete Adjuvant (Thermo Scientific Inc., Rockford, IL). One week later, secondary i.p. immunization of 20 µg of purified gangliosides was performed. After additional immunization of 20 µg of purified gangliosides, a booster injection was given i.p. 2 days before spleen cells were harvested. The spleen cells were fused with mouse myeloma P3U1 cells by using Sendai virus (hemagglutinating virus of Japan: HVJ) envelope: GenomONE-CFEX (Cosmo Bio USA, Inc., Carlsbad, CA) according to the manufacturer’s instructions. The hybridomas were grown in RPMI medium including hypoxanthine, aminopterin, and thymidine selection medium supplement (Sigma), 2 mM l-glutamine (Invitrogen Corp.), 10% heat-inactivated FBS (Sigma), 5% BriClone (QED Bioscience Inc., San Diego, CA), and 1% of penicillin-streptomycin solution (Invitrogen Corp.). The culture supernatants were screened by ELISA for the binding to 3'-isoLM1 conjugated with fatty-acid free-bovine serum albumin (BSA). Single cell cloning was performed with ClonaCell-HY Hybridoma Selection Medium (Medium D; StemCell Technologies Inc., Vancouver, BC, Canada). The IgG subclass was determined by IsoStrip Mouse Monoclonal Antibody Isotyping Kit (Roche Diagnostics Corp., Indianapolis, IN).
Enzyme-Linked Immunosorbent Assay (ELISA)
For evaluation by ELISA, gangliosides conjugated with BSA were immobilized on 96-well plates at 1 µg/ml for 30 min. After blocking with 1% BSA in PBS, the plates were incubated with primary antibodies at several concentrations, followed by 1:1000 diluted horseradish-peroxidase-conjugated anti-mouse IgG (GE Healthcare UK Ltd., Buckinghamshire, England). The enzymatic reaction was conducted with a substrate solution containing 3,3′,5,5′-tetramethylbenzidine (TMB; Thermo Scientific Inc.). After the reaction was stopped with 2 M H2SO4, the optical density was measured at 450 nm with a microplate reader (Bio-Rad Laboratories, Inc., Philadelphia, PA). These reactions were performed with a volume of 50 µl at room temperature.
SDS-PAGE
For sodium dodecyl sulfate polyacrylamide gel electrophoresis, 2 µg of each protein was electrophoresed under reducing conditions on 4–10% NuPAGE gel (Invitrogen). The gel was stained with Bio-Safe Coomassie (Bio-Rad Laboratories, Inc.).
Flow cytometry
D54MG or D54MG xenograft cells, which were collected by 0.6 mM EDTA treatment, were incubated with GMab-1 or isotype control (10 µg/ml) for 1 h at 4°C. Then the cells were incubated with the Oregon green-conjugated anti-mouse antibody (1/200 dilution; Invitrogen Corp.) for 30 min. Flow cytometry was performed with a FACS Calibur instrument (Becton Dickinson, Franklin Lakes, NJ).
Affinity constant determination by surface plasmon resonance
To determine the affinity constant (KA), purified GMab-1 was immobilized on the surface of biosensor chips for analysis by using the BIAcore 3000 system (BIAcore, Piscataway, NJ). Coupling of GMab-1 was achieved by using N-ethyl-N'-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide according to the instructions of the manufacturer. The running buffer was 10 nM HEPES, 150 mM NaCl, and 3.4 mM EDTA (pH 7.4). The gangliosides conjugated with BSA and control BSA were passed over the biosensor chip and affinity rate constants (association rate constant, kassoc, and disassociation rate constant, kdiss) were determined by nonlinear curve-fitting using the Langmuir one-site binding model of the BIAevaluation software (BIAcore). KA at equilibrium was calculated as KA = kassoc / kdiss.
Immunohistochemical analysis
All procedures were performed with the Ventana Discovery XT system (Ventana Medical Systems, Inc., Tucson, AZ). Briefly, acetone-fixed 5- to 8-µm frozen tissue sections of human glioblastoma tissues were incubated with GMab-1 (10 µg/ml) or isotype control (10 µg/ml) for 30 min. The universal biotin-conjugated secondary antibody was incubated with tissue sections for 20 min followed by the horseradish-peroxidase-conjugated streptavidin for 16 min. Color was developed by using 3,3′-diaminobenzidine tetrahydrochloride for 8 min, and hematoxylin was used for counterstained.
Results and discussion
Production of an anti-3'-isoLM1/3’,6'-isoLD1-specific antibody
Monoclonal antibodies recognizing lacto-series gangliosides such as 3'-isoLM1 and 3',6'-isoLD1 were previously produced and fully characterized [16, 17]. The 3'-isoLM1 and 3',6'-isoLD1 gangliosides are highly expressed in human glioblastomas and are rarely detected in the normal adult brain. Therefore, 3'-isoLM1 and 3',6'-isoLD1 are ideal molecular targets for immunotherapy against glioblastomas using specific mAbs. However, those established mAbs have relatively low-affinity against 3'-isoLM1 and 3',6'-isoLD1 because they are of the IgM subclass. In several studies, anti-ganglio-series ganglioside IgGs with high affinity have been developed by immunizing GalNAcT−/− mice with ganglio-series gangliosides [2, 6, 13, 14]. Similarly, in this study, we immunized β3Gn-T5 knockout mice with purified 3'-isoLM1 and 3',6'-isoLD1 coupled to Salmonella minnesota to generate high-affinity anti-lacto-series ganglioside mAbs (Fig. 1A). Because the hybridomas that we previously produced from β3Gn-T5 knockout mice using the conventional polyethylene glycol fusion method had low viability (data not shown), for this study, we selected a low-toxicity, high-efficiency fusion method using a Sendai virus envelope [20]. After single cell cloning, one of the clones, GMab-1 (IgG3 subclass) was established (Fig. 1B). As shown in Fig. 1C, GMab-1 reacted with 3'-isoLM1 in a dose-dependent manner in ELISA. Furthermore, both D54MG cells and D54MG xenograft cells, which express lacto-series gangliosides, were recognized by GMab-1 as demonstrated by flow cytometry (Fig. 2). To determine the association and dissociation rate constants (kassoc and kdiss) and to calculate the affinity constants (KA), we next performed a kinetic analysis of the interaction of GMab-1 with 3'-isoLM1 by surface plasmon resonance (BIAcore). Determination of the association and dissociation rates from the sensorgrams revealed a kassoc of 5.07 × 104 (mol/L-s)−1 and a kdiss of 2.74 × 10−4 s−1. The KA at binding equilibrium, calculated as KA = kassoc / kdiss, was 1.85 × 108 (mol/L)−1, indicating that GMab-1 has high affinity against 3'-isoLM1. Likewise, the KA of the interaction of GMab-1 with 3',6'-isoLD1 was calculated as 6.58 × 107 (mol/L)−1, indicating that GMab-1 also has high affinity against 3',6'-isoLD1.
Fig. 2.
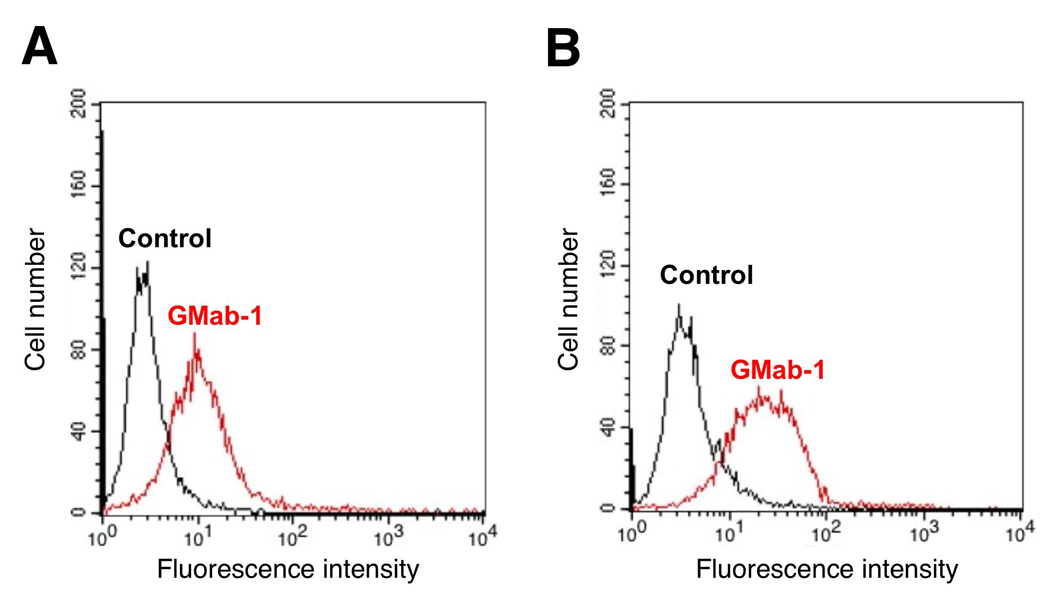
GMab-1 recognition of lacto-series ganglioside on the cell surface of glioblastoma cells by flow cytometry. D54MG cell line (A) or D54MG xenograft cells (B), which were collected by 0.6 mM EDTA treatment, were incubated with GMab-1 or isotype control.
Specificity of GMab-1 against gangliosides
To determine the epitope of GMab-1, we performed ELISA using 15 different gangliosides, including lacto-series (3'-isoLM1, 3',6'-isoLD1, isoLA1, and Fuc-3'-isoLM1), neolacto-series (3'-LM1 and 3',8'-LD1), globo-series (Gb3), asilalo-series (GA1), a-series (GM2 and GM1), and b-series (GD3, GD2, GD1b, and GT1b) (Fig. 3). As previously reported [16, 17], SL-50 reacted with 3'-isoLM1 and not with 3',6'-isoLD1 (Fig. 3C), whereas DMab-22 reacted with 3',6'-isoLD1 and not with 3'-isoLM1 (Fig. 3D). We have observed no cross-reaction with the other gangliosides using SL-50 and DMab-22. DMab-14 reacted with both 3'-isoLM1 and 3',6'-isoLD1; however, there is slight reactivity with the other gangliosides (Fig. 3B). On the other hand, we did observe activity that distinguished GMab-1 from SL-50, DMab-22, and DMab-14. GMab-1 recognized both 3'-isoLM1 and 3',6'-isoLD1, and the reactivity with 3',6'-isoLD1 was stronger than with 3'-isoLM1 (Fig. 3A). Although DMab-14 reacted with both 3'-isoLM1 and 3',6'-isoLD1, the reactivity with 3'-isoLM1 was stronger than with 3',6'-isoLD1 (Fig. 3B), which indicates that the epitope of GMab-1 is slightly different from that of DMab-14. Furthermore, there is no cross-reaction with the other gangliosides using GMab-1 (Fig. 3A). These results indicate that GMab-1 defines the epitope NeuAcα2-3Galβ1-3GlcNAc, the terminal sequence of 3'-isoLM1 and 3',6'-isoLD1.
Fig. 3.
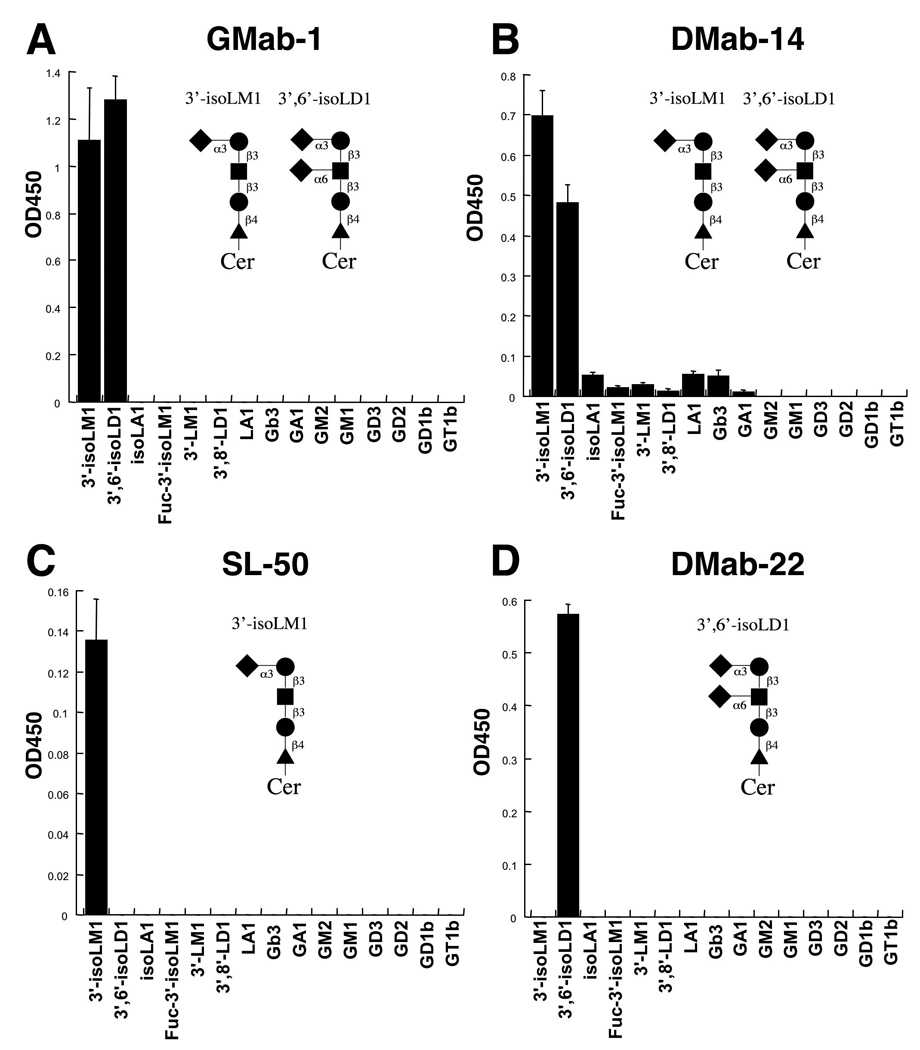
Epitope determination of GMab-1 in ELISA. Fifteen gangliosides conjugated with BSA were immobilized. After blocking, the plates were incubated with GMab-1 (A), DMab-14 (B), SL-50 (C), and DMab-22 (D) at a concentration of 10 µg/ml.
Immunohistochemical analysis by GMab-1 against glioblastoma tissues
Since lacto-series gangliosides such as 3'-isoLM1 and 3',6'-isoLD1 are known to be expressed in glioblastoma tissues [18], immunohistochemistry was performed using 33 glioblastoma frozen tissue specimens. GMab-1 immunoreactivity was detected in 26 of 33 (78.8%) glioblastomas. Representative staining by GMab-1 in glioblastoma samples is shown in Fig. 4. Immunostaining by GMab-1 demonstrated predominantly both cell-surface patterns (Fig. 4A) and cell-surface/cytoplasmic patterns (Fig. 4C) in glioblastoma cells. Proliferating endothelial cells were negative for GMab-1 (Fig. 4A). The concentration-matched isotype control of mouse IgG3 did not stain any tumor cells (Figs. 4B and 4D). These results further suggest that GMab-1 specifically reacted with lacto-series gangliosides in glioblastoma tissues.
Fig. 4.
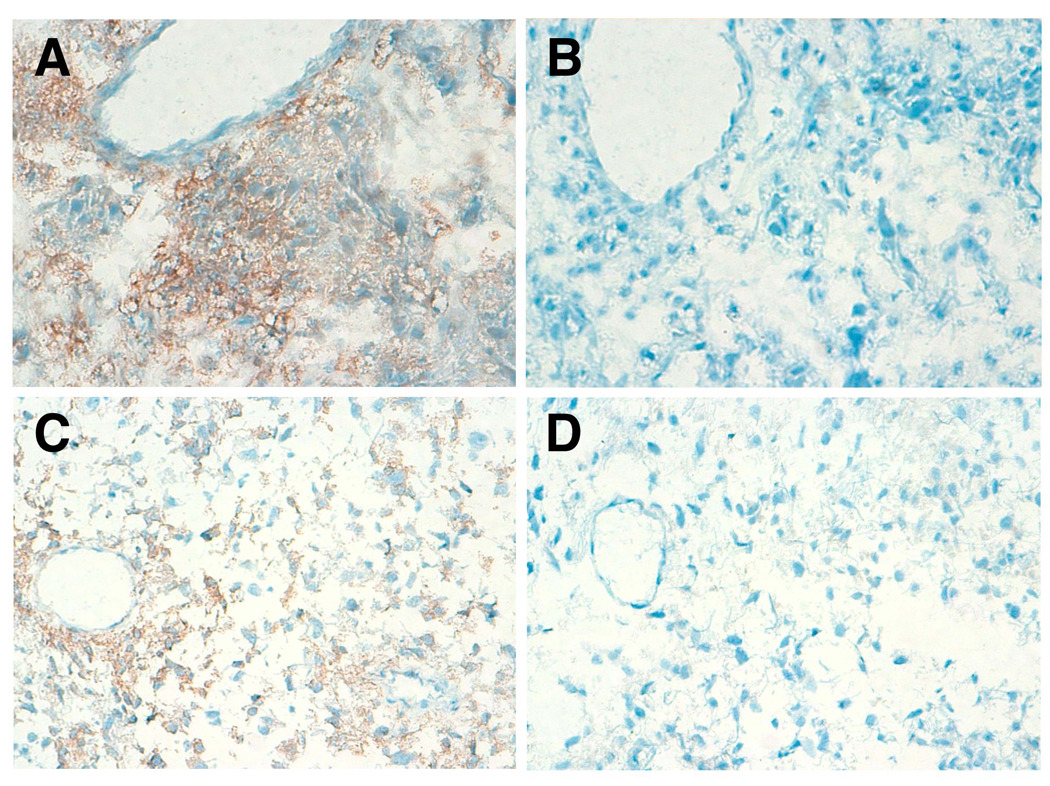
Immunohistochemical analysis by GMab-1 against glioblastoma tissues. Glioblastoma tissues were stained by GMab-1 (A, C) or isotype control (B, D). Magnification: × 200.
In conclusion, we immunized β3Gn-T5 knockout mice with 3'-isoLM1 and 3',6'-isoLD1. GMab-1, of the IgG3 subclass, specifically recognized both 3'-isoLM1 and 3',6'-isoLD1 and showed high binding activity. GMab-1 was also reactive against glioblastomas in immunohistochemical analyses, which indicates that GMab-1 should be useful in antibody-based therapy of glioblastomas.
Acknowledgements
We thank Mr. Ling Wang of Duke University Medical Center and Mrs. Birgitta Dellheden of University of Gothenburg for immunohistochemical analysis and ganglioside isolation, respectively. We also thank Dr. Chifumi Kitanaka of Yamagata University School of Medicine for his helpful suggestion and Ms. Janet Parsons of Duke University Medical Center for editing the manuscript. This study was supported by NIH grants 5P50 CA108786, 5P50 NS20023, and R37 CA011898. Y.K. was supported by the Global COE Program for Medical Sciences, Japan Society for the Promotion of Science.
Abbreviations
- β3Gn-T5
β1,3-N-acetylglucosaminyltransferase-V
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- KA
affinity constant
- kassoc
association rate constant
- kdiss
disassociation rate constant
- mAb
monoclonal antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hakomori SI. Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochim. Biophys. Acta. 2008;1780:325–346. doi: 10.1016/j.bbagen.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowes T, Wagner ER, Boffey J, Nicholl D, Cochrane L, Benboubetra M, Conner J, Furukawa K, Furukawa K, Willison HJ. Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barre syndrome. Infect. Immun. 2002;70:5008–5018. doi: 10.1128/IAI.70.9.5008-5018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akeboshi H, Chiba Y, Kasahara Y, Takashiba M, Takaoka Y, Ohsawa M, Tajima Y, Kawashima I, Tsuji D, Itoh K, Sakuraba H, Jigami Y. Production of recombinant beta-hexosaminidase A, a potential enzyme for replacement therapy for Tay-Sachs and Sandhoff diseases, in the methylotrophic yeast Ogataea minuta. Appl. Environ. Microbiol. 2007;73:4805–4812. doi: 10.1128/AEM.00463-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawashima N, Tsuji D, Okuda T, Itoh K, Nakayama KI. Mechanism of abnormal growth in astrocytes derived from a mouse model of GM2 gangliosidosis. J. Neurochem. 2009;111:1031–1041. doi: 10.1111/j.1471-4159.2009.06391.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima I, Nakamura O, Tai T. Antibody responses to ganglio-series gangliosides in different strains of inbred mice. Mol. Immunol. 1992;29:625–632. doi: 10.1016/0161-5890(92)90199-8. [DOI] [PubMed] [Google Scholar]
- 6.Lunn MP, Johnson LA, Fromholt SE, Itonori S, Huang J, Vyas AA, Hildreth JE, Griffin JW, Schnaar RL, Sheikh KA. High-affinity anti-ganglioside IgG antibodies raised in complex ganglioside knockout mice: reexamination of GD1a immunolocalization. J. Neurochem. 2000;75:404–412. doi: 10.1046/j.1471-4159.2000.0750404.x. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa H, Kotani M, Kawashima I, Tai T. Generation of one set of monoclonal antibodies specific for b-pathway ganglio-series gangliosides. Biochim. Biophys. Acta. 1992;1123:184–190. doi: 10.1016/0005-2760(92)90110-h. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Wada R, Kawai H, Sango K, Deng C, Tai T, McDonald MP, Araujo K, Crawley JN, Bierfreund U, Sandhoff K, Suzuki K, Proia RL. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Invest. 1999;103:497–505. doi: 10.1172/JCI5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, Fukumoto S, Haraguchi M, Takeda N, Fujimura K, Sakae M, Kishikawa M, Shiku H, Furukawa K, Aizawa S. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. U S A. 1996;93:10662–10667. doi: 10.1073/pnas.93.20.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada M, Itoh Mi M, Haraguchi M, Okajima T, Inoue M, Oishi H, Matsuda Y, Iwamoto T, Kawano T, Fukumoto S, Miyazaki H, Furukawa K, Aizawa S, Furukawa K. b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 2002;277:1633–1636. doi: 10.1074/jbc.C100395200. [DOI] [PubMed] [Google Scholar]
- 11.Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J. Biol. Chem. 2006;281:10230–10235. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 12.Kondo Y, Tokuda N, Fan X, Yamashita T, Honke K, Takematsu H, Togayachi A, Ohta M, Kotzusumi Y, Narimatsu H, Tajima O, Furukawa K, Furukawa K. Glycosphingolipids are not pivotal receptors for Subtilase cytotoxin in vivo: sensitivity analysis with glycosylation-defective mutant mice. Biochem. Biophys. Res. Commun. 2009;378:179–181. doi: 10.1016/j.bbrc.2008.10.163. [DOI] [PubMed] [Google Scholar]
- 13.Boffey J, Nicholl D, Wagner ER, Townson K, Goodyear C, Furukawa K, Furukawa K, Conner J, Willison HJ. Innate murine B cells produce anti-disialosyl antibodies reactive with Campylobacter jejuni LPS and gangliosides that are polyreactive and encoded by a restricted set of unmutated V genes. J. Neuroimmunol. 2004;152:98–111. doi: 10.1016/j.jneuroim.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Boffey J, Odaka M, Nicoll D, Wagner ER, Townson K, Bowes T, Conner J, Furukawa K, Willison HJ. Characterisation of the immunoglobulin variable region gene usage encoding the murine anti-ganglioside antibody repertoire. J. Neuroimmunol. 2005;165:92–103. doi: 10.1016/j.jneuroim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Henion TR, Zhou D, Wolfer DP, Jungalwala FB, Hennet T. Cloning of a mouse beta 1,3 N-acetylglucosaminyltransferase GlcNAc(beta 1,3)Gal(beta 1,4)Glc-ceramide synthase gene encoding the key regulator of lacto-series glycolipid biosynthesis. J. Biol. Chem. 2001;276:30261–30269. doi: 10.1074/jbc.M102979200. [DOI] [PubMed] [Google Scholar]
- 16.Wikstrand CJ, He XM, Fuller GN, Bigner SH, Fredman P, Svennerholm L, Bigner DD. Occurrence of lacto series gangliosides 3'-isoLM1 and 3',6'-isoLD1 in human gliomas in vitro and in vivo. J. Neuropathol. Exp. Neurol. 1991;50:756–769. doi: 10.1097/00005072-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Wikstrand CJ, Longee DC, McLendon RE, Fuller GN, Friedman HS, Fredman P, Svennerholm L, Bigner DD. Lactotetraose series ganglioside 3',6'-isoLD1 in tumors of central nervous and other systems in vitro and in vivo. Cancer Res. 1993;53:120–126. [PubMed] [Google Scholar]
- 18.Wikstrand CJ, Fredman P, Svennerholm L, Bigner DD. Detection of glioma-associated gangliosides GM2, GD2, GD3, 3'-isoLM1 3',6'-isoLD1 in central nervous system tumors in vitro and in vivo using epitope-defined monoclonal antibodies. Prog. Brain Res. 1994;101:213–223. doi: 10.1016/s0079-6123(08)61951-2. [DOI] [PubMed] [Google Scholar]
- 19.Månsson JE, Fredman P, Bigner DD, Molin K, Rosengren B, Friedman HS, Svennerholm L. Characterization of new gangliosides of the lactotetraose series in murine xenografts of a human glioma cell line. FEBS Lett. 1986;201:109–113. doi: 10.1016/0014-5793(86)80580-4. [DOI] [PubMed] [Google Scholar]
- 20.Kato Y, Jin G, Kuan CT, McLendon RE, Yan H, Bigner DD. A monoclonal antibody IMab-1 specifically recognizes IDH1R132H, the most common glioma-derived mutation. Biochem. Biophys. Res. Commun. doi: 10.1016/j.bbrc.2009.10.001. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


