Abstract
Background
Previous PET imaging studies in non-human primates have shown that striatal dopamine type 2/3 (D2/3) receptors correlate with social hierarchy in monkeys, and that dominant animals exhibit higher levels of D2/3 receptor binding. The goal of the present study was to examine this phenomena in human subjects using Positron Emission Tomography (PET) and the radiotracer [11C]raclopride.
Methods
Fourteen healthy volunteers were scanned with [11C]raclopride to measure D2/3 receptor binding potential. Social status was assessed using the Barratt Simplified Measure of Social Status. In addition, participants were asked to assess their level of social support using the Multidimensional Scale of Perceived Social Support (MSPSS).
Results
A correlation was seen between social status and dopamine D2/3 receptors, where volunteers with the higher status had higher values for [11C]raclopride BP. A similar correlation was seen with the perceived social support, where higher [11C]raclopride BP correlated with higher scores on the MSPSS.
Conclusions
The results of this study support the hypothesis that social status and social support is correlated with D2/3 receptor binding.
Keywords: dopamine 2/3 receptor, [11C]raclopride, PET imaging, social status
Introduction
Previous studies in animals have shown a correlation between dopamine transmission in the brain and social hierarchy (1). In monkeys, dominant and subordinate social rank is determined by physical and social triumph and defeat, in which the dominant animal wins more physical confrontations and receives more social attention, such as grooming or huddling. Two PET imaging studies have investigated the relationship between social status and D2/3 receptors in the striatum in monkeys, and both showed that social dominance was associated with higher D2/3 receptor binding compared to subordinate animals (2, 3).
In humans, social hierarchy is a more subtle phenomenon, which can be approximated by measuring social status and social support (4). Thus, the goal of the present study was to examine the correlation between these factors and dopamine D2/3 receptor binding in human subjects. Given the known effect of disease states on striatal D2/3 receptors, including substance dependence, schizophrenia, and anxiety disorders (5-7), only healthy control volunteers were included in this study. Social status was measured using the Barratt Simplified Measure of Social Status (8) and social support was measured using the Multidimensional Scale of Perceived Social Support (9). Our hypothesis was that low social status and low levels of social support would correlate with low D2/3 receptor binding in the striatum measured with [11C]raclopride.
Methods and Materials
The study was approved by the Institutional Review Board of the New York State Psychiatric Institute and all subjects provided written informed consent. Study participants were non-smoking healthy controls, and were required to have no Diagnostic and Statistical Manual of Mental Disorders-IV Axis I disorder (including substance abuse or dependence), no significant medical conditions, and no use of medications prior to the scan (6 months for medications that could affect dopamine; 2 weeks for all others). Subjects (9 men and 5 women) were recruited from the New York City metropolitan area. Participant screening included a psychiatric assessment with Structured Clinical Interview for DSM Disorders (10), physical exam, electrocardiogram, and laboratory tests. All subjects were asked for data to complete the Barratt’s Simplified Measure of Social Status (BMSSS) and to complete the Multidimensional Scale of Perceived Social Support (MSPSS). The scans performed on female subjects were not controlled for menstrual cycle phase.
[11C]Raclopride was prepared as previously described (11), and PET studies were acquired using a bolus injection of the radiotracer. The PET scans were obtained on the ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN) in 3D mode. Emission data were obtained as 15 frames of increasing duration up to 60 minutes. The PET images were reconstructed by filtered backprojection with attenuation correction using the data from a 10 min transmission scan and a Shepp 0.5 filter.
All image analysis was performed in MEDx (Sensor Systems, Inc., Sterling, Virginia). Each subject underwent a transaxial T1 MRI scan, acquired on the GE Signa EXCITE 3T/94 cm scanner (GE Medical Systems, Milwaukee, WI), for delineation of the regions of interest. The regions of interest outlined on the MRI included the subdivisions of the striatum, which have been previously described (12). Briefly, these included the ventral striatum (VST), the dorsal caudate rostral to the anterior commissure (AC) (pre-commissural dorsal caudate, preDCA), the dorsal putamen rostral to the AC (pre-commissural dorsal putamen, preDPU), the caudate caudal to the AC (post-commissural caudate, postCAU), and the putamen caudal to the AC (post-commissural putamen, postPUT). The subdivisions were derived based on their cortical and subcortical connections, as described previously (12).
The PET scans were registered to the MRI scans in MEDx (Sensor Systems, Inc., Sterling, Virginia) as previously published (11, 13). The activity measured in the left and right regions were averaged. The activity in the striatum as a whole (STR) was derived as the spatially weighted average of the five ROIs.
The simplified reference tissue model (SRTM, 14) was used for derivation of the binding potential (BP) implemented in MATLAB (The Math Works, Inc., South Natick, MA), using the cerebellum as the reference region. The outcome measure for the PET studies was binding potential, defined as the ratio of specifically bound to non-displaceable radioligand at equilibrium (BPND) (15). BPND can also be described as
where Bmax is the concentration of D2/3 receptors, KD is the inverse of the affinity of the radiotracer for the receptor, and fND is the free fraction in the nonspecific distribution volume of the brain (16). [11C]Raclopride has a similar affinity for D2 and D3 receptors (17), and the signal from these receptors cannot be distinguished.
Relationships between BPND and the scores on the BSMSS and MSPSS were analyzed with the Pearson product moment correlation coefficient. A two tailed probability value of p < 0.05 was chosen as the level of significance. Since age is known to affect D2/3 receptor BP, this factor was included in the regression analysis. Given that previous studies had shown a correlation between social status and the striatum measured as a whole (2, 3), there were no specific hypothesis regarding the striatal subregions. Therefore, the primary analysis was restricted to the striatum, with post-hoc analyses of the individual subregions.
Results
The research volunteers included 9 men and 5 women with an average age of 30 ± 4 years (range 25 to 37). The average BSMSS score was 33.2 ± 4.8 (range 24.3 to 44.0). One subject declined to complete the MSPSS. The average MSPSS score was 19.0 ± 9.5 (range 11.5 to 20.8). The average decay corrected injected dose was 8.0 ± 1.0 mCi and the average specific activity was 1533 ± 140 Ci/mmoles.
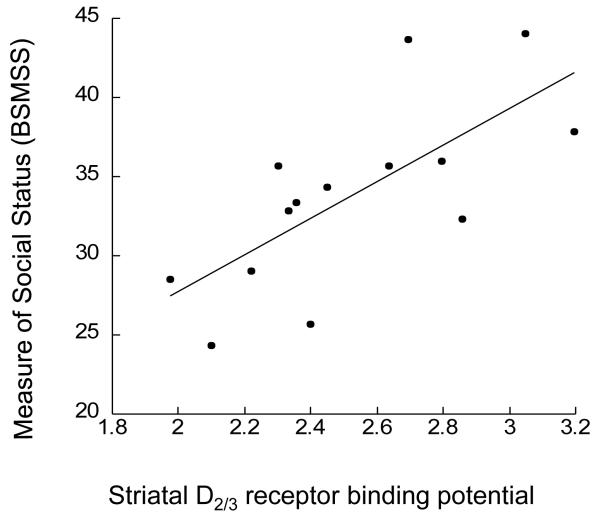
A positive correlation was seen between [11C]raclopride BPND and social status for the striatum (r = 0.71, p = 0.004, age corrected p = 0.007), as shown in figure 1. A post-hoc analysis was performed with the striatal subregions, and a positive correlation was seen in the ventral striatum (r = 0.73, p = 0.003, age corrected p = 0.004), pre-commissural caudate (r = 0.63, p = 0.015, age corrected p = 0.018), and post-commissural putamen (r = 0.85, p = 0.001, age corrected p = 0.003). Correlations did not reach significance in the pre-commissural putamen (r = 0.48, p = 0.08), or post-commissural caudate (r = 0.20, p = 0.5).
Figure 1.
Correlation between [11C]raclopride BP (x axis) and social status, measured with the Barrat Simplified Measure of Social Status (BSMSS). A positive correlation was seen, where higher BP correlated with higher BSMSS (r = 0.71, p = 0.004, age corrected p = 0.007).
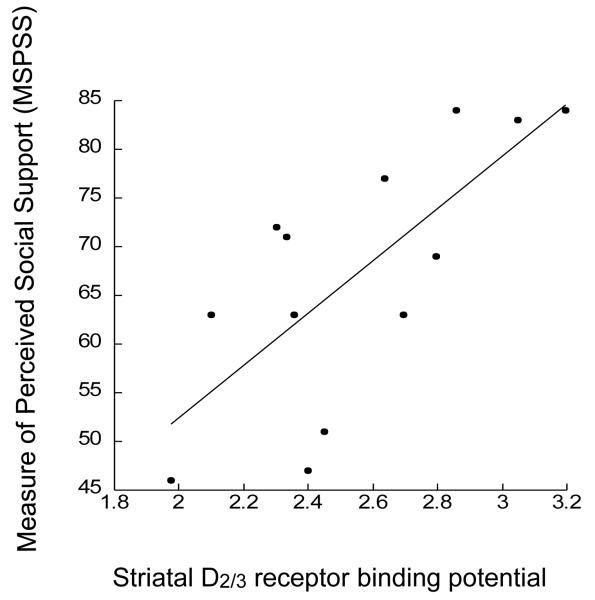
A positive correlation was seen between [11C]raclopride BPND and the MSPSS for the striatum (r = 0.73, p = 0.005, age corrected p = 0.02) , as shown in figure 2. A post-hoc analysis was performed with the striatal subregions, and a positive correlation was seen in the ventral striatum (r = 0.63, p = 0.02, age corrected p = 0.05), pre-commissural putamen (r = 0.78, p = 0.002, age corrected p = 0.09), pre-commissural caudate (r = 0.67, p = 0.02, age corrected p = 0.05), and post-commissural putamen (r = 0.55, p = 0.05, age corrected p = 0.15). Correlation did not reach significance in the post-commissural caudate (r = 0.28, p = 0.35). Thus, within the subdivisions of the striatum, this correlation was seen in most, but not all, of the subdivisions. A correlation was seen between the BSMSS and MSPSS (r = 0.53, p = 0.05), showing that these are scales measure factors that are related, but not identical.
Figure 2.
Correlation between [11C]raclopride BP (x axis) and score on the Multidimensional Scale of Perceived Social Support (MSPSS). A positive correlation was seen, where higher BP correlated with higher score on the MSPSS (r = 0.73, p = 0.005, age corrected p = 0.02).
Discussion
In this study, a positive correlation was seen between D2/3 receptor binding potential and measures of social status and perceived social support. These results are similar to those reported previously in non-human primates which showed that striatal D2/3 receptors were higher in rhesus monkeys who were dominant in a social hierarchy compared to subordinate monkeys (2, 3). However, to our knowledge, this is a first demonstration of this type of association in human volunteers.
Striatal D2/3 receptor availability has also been shown to correlate with measures of social detachment in healthy human volunteers and is low in patients with social phobia (7, 18-20). These studies have shown that low D2/3 receptor availability is associated with personal detachment and aloofness, measured with the Detachment Subscale of the Karolinska Scales of Personality. In the present study, we found a correlation with the volunteer’s score on the Multidimensional Scale of Perceived Social Support (9), a scale that assesses three sources of social support: family, friends, and significant other. While the MSPSS and Karolinska detachment scales ask about different aspects of social behavior, they can both be viewed as measuring the extent of social interaction. Taken together, these data suggest that striatal D2/3 receptor binding is associated with an individual’s social capital, which can be thought of as a balance of social rank and stress offset by social support and attachment (21). Overall, these data suggest that the higher social status, a greater sense of perceived social support, and lower levels of social avoidance are associated with higher D2/3 receptor binding.
It is interesting to note that the study in non-human primates by Morgan et al (2) showed that D2/3 receptor binding was modulated by the environment. In that study, D2/3 receptors did not differ between the animals prior to the establishment of a social hierarchy, but once the social structure was established, the animals that became dominant developed higher D2/3 receptor binding. At this point it is unknown if D2/3 receptor binding in human beings can be modulated by changes in the environment. Nader et al (22) have recently reported that rearrangement of the social hierarchy, such that some previously subordinate monkeys became dominant (and some dominant became subordinate) did not produce significant differences in D2/3 receptor binding, suggesting that this neurobiological marker, once established, may become unchangeable.
A number of previous studies have investigated the behavioral significance of high and low striatal D2/3 receptor availability in humans. This has been of particular interest to the field, given that low D2/3 receptor BP is the most replicated finding in imaging studies of drug and alcohol addiction (for review see (6)). In addition, the study of rhesus monkeys showed that low D2/3 receptor binding predicted increased cocaine self-administration (2) and similar results have been shown in rodents (23). Taken together, these data suggest that D2/3 receptor binding may provide a molecular marker that reflects the interaction between genes and environment and the predisposition to drug abuse (24).
A potential significant limitation of this study is the use of the BSMSS, which provides an estimate of social status across society, but does not provide an accurate measure of social prominence with respect to one’s peers nor does it provide a measure of socioeconomic status. However, while there is a clear consensus in the literature regarding the importance of these factors on health and disease, there is a lack of consensus of how to measure social status. Many studies investigating the effects of social status on health have used the Hollingshead index, which provides a composite score of social status based on the subject’s occupation and level of education (25). However, this scale uses a list of occupations generated from the 1970 census data, and many of our subject’s occupations were not included in this list. The BSMSS is based on the Hollingshead scale, in that it generates a composite score based on level of education and occupation, but uses an updated list of occupations. In addition, the Barratt scale includes the subject’s parent’s education/occupation scores, which is weighted to lesser degree that the subject’s own educational and occupational achievement, but recognizes that social status is partly determined the opportunities provided by one’s background. Another more simple determination of social status that is sometimes used is years of education. In this dataset, a post hoc analysis of years of education and [11C]raclopride binding in the striatum also showed a significant positive correlation (r = 0.54, p = 0.04), suggesting that this correlation was not simply a function of error within the BSMSS.
Other potential limitations included the fact that baseline measures of D2/3 receptor binding potential do not account for occupancy of the receptor by endogenous dopamine, and studies using the acute depletion of dopamine would be needed to answer this question. Another potential limitation of the present study is the possibility of gender differences. This study included only 5 females, which does not allow for a full analysis of the effects of gender. However, a post-hoc analysis of the correlation between BSMSS and MSPSS and BPND within each sex showed that the correlation was not gender-specific (males: 1) BSMSS and BPND r = 0.69, p = 0.04; 2) MSPSS and BPND r = 0.81, p = 0.01; females: 1) BSMSS and BPND r = 0.74, p = .18; 2) MSPSS and BPND r = 0.99, p = .005). In addition, the scans obtained in the female participants did not control for menstrual cycle, and it is possible that this factor could have affected our results. However, previous studies that investigated this question and have not show a consensus with respect to the effect of cycle on D2/3 receptor binding (26-28). Lastly, in this study, we did not include measures of other factors that could affect social status, such as intelligence or anxiety. While attributes such as these may affect the BSMSS score, the degree of their impact is not known, and an investigation of these factors is needed in future studies.
Acknowledgements
The authors would like to thank Andrei Ignat, Kaitlin Greene, Stephanie Cook, Eva Kolb and the staff of the Kreitchman PET center for their excellent technical assistance. Supported by the Public Health Service (R01DA016788 and R01DA020855).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26(1):27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Morgan D, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 3.Grant KA, et al. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29(1):80–3. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Adler NE, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49(1):15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Frankle WG. Neuroreceptor imaging studies in schizophrenia. Harv Rev Psychiatry. 2007;15(5):212–32. doi: 10.1080/10673220701679812. [DOI] [PubMed] [Google Scholar]
- 6.Martinez D, et al. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17(4):539–55. x. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Schneier FR, et al. Striatal dopamine D(2) receptor availability in OCD with and without comorbid social anxiety disorder: preliminary findings. Depress Anxiety. 2008;25(1):1–7. doi: 10.1002/da.20268. [DOI] [PubMed] [Google Scholar]
- 8.Barratt W. The Barratt Simplified Measure of Social Status (BSMSS) measuring SES. 2006 available at http://wbarratt.indstate.edu/socialclass/Barratt_Simplifed_Measure_of_Social_Status.pdf.
- 9.Zimet GD, et al. The Multidimensional Scale of Perceived Social Support. J Pers Assess. 1988;52(1):30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 10.First M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) Biometrics Research Dept., New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 11.Mawlawi O, et al. Imaging human mesolimbic dopamine transmission with PET: I. Accuracy and precision of D2 parameter measurements in the ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21(9):1034–57. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Martinez D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 13.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J. Compu. Assist. Tomogr. 1993;17(4):536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 15.Innis RB, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 16.Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nuclear Medicine and Biology. 2001;28(5):595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 17.Sokoloff P, et al. Molecular cloning and characterization of a novel dopamine receptor D3 as a target for neuroleptics. Nature. 1990;347(13 Sep):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 18.Schneier FR, et al. Low dopamine D(2) receptor binding potential in social phobia. Am J Psychiatry. 2000;157(3):457–9. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- 19.Farde L, Gustavsson JP, Jonsson E. D2 dopamine receptors and personality traits. Nature. 1997;385(6617):590. doi: 10.1038/385590a0. [DOI] [PubMed] [Google Scholar]
- 20.Kestler LP, et al. The relation between dopamine D2 receptor density and personality: preliminary evidence from the NEO personality inventory-revised. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13(1):48–52. [PubMed] [Google Scholar]
- 21.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–84. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- 22.Nader MA, et al. Review. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3223–32. doi: 10.1098/rstb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thanos PK, et al. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62(7):481–6. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111(10):1444–51. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollingshead AB. Four factor index of social status. New Haven, Connecticut: 1975. Working paper published by the author. [Google Scholar]
- 26.Nordstrom AL, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res. 1998;83(1):1–6. doi: 10.1016/s0925-4927(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 27.Munro CA, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–74. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Czoty PW, et al. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34(3):548–54. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]