Abstract
Cocaine binds with the dopamine transporter (DAT), an effect that has been extensively implicated in its reinforcing effects. However, persisting adaptations in DAT regulation after cocaine self-administration have not been extensively investigated. Here, we determined the changes in molecular mechanisms of DAT regulation in the caudate-putamen (CPu) and nucleus accumbens (NAcc) of rats with a history of cocaine self-administration, followed by three weeks of withdrawal under extinction conditions (i.e., no cocaine available). DA uptake was significantly higher in the CPu of cocaine-experienced animals as compared to saline-yoked controls. DAT Vmax was elevated in the CPu without changes in apparent affinity for DA. In spite of elevated CPu DAT activity, total and surface DAT density and DAT-PP2Ac (protein phosphatase 2A catalytic subunit) interaction remained unaltered, although p-Ser- DAT phosphorylation was elevated. In contrast to the CPu, there were no differences between cocaine and saline rats in the levels of DA uptake, DAT Vmax and Km values, total and surface DAT, p-Ser-DAT phosphorylation, or DAT-PP2Ac interactions in the NAcc. These results show that chronic cocaine self-administration leads to lasting, regionally specific alterations in striatal DA uptake and DAT-Ser phosphorylation. Such changes may be related to habitual patterns of cocaine-seeking observed during relapse.
Keywords: addiction, cocaine, dopamine transport, phosphorylation, trafficking, transporter
Introduction
Most evidence suggests that cocaine produces its primary reinforcing effects by binding to the dopamine transporter (DAT) and blocking presynaptic DA reuptake, thereby potentiating DA neurotransmission in mesocorticolimbic reward pathways [1]. DAT has been well characterized as a constitutively phosphorylated protein in the central nervous system [2; 3]. Increased DAT phosphorylation by activation of PKC, CAMKII, or inhibition of PP2A and PP1, and the DAT substrate amphetamine (AMPH) have been suggested for inhibition of DAT activity concomitant with transporter sequestration and/or DA efflux [2; 4]. Furthermore, DAT-associated proteins including PP2Ac, syntaxin 1A, PICK1, Hic-5, and CaMKII have been implicated in the regulation of DAT trafficking, uptake, efflux, and membrane stabilization [5]. We recently reported that activation of presynaptic DA D2 (D2s) and D3 receptors stimulates DAT function [6; 7]. Together, these studies suggest that DATs are subject to multiple, interacting modes of kinase-dependent modulation following receptor/kinase activation.
Previous studies using animal models and human cocaine dependent users have reported that DAT expression/activity is increased, decreased, or showed no changes after repeated cocaine administration [8; 9; 10]. However, most studies that have examined DAT function have utilized limited, noncontingent cocaine administration, an approach that does not appropriately model the self-administration of drugs in human addicts. Cocaine self-administration and withdrawal in rats provides a more homologous model for determining whether DAT regulation is persistently compromised as a consequence of chronic cocaine intake and whether DAT dysregulation leads to a greater risk for relapse to cocaine-seeking. In a previous study [3], we found that chronic cocaine self-administration followed by three weeks of abstinence produced elevated DAT activity in the CPu and NAcc of cocaine-experienced animals. However, higher levels of surface DAT, DAT-PP2Ac association, and decreased serine phosphorylation of DAT were only observed in the CPu, and not in the NAcc.
Most animal model studies of relapse have utilized an extinction-reinstatement model after chronic cocaine self-administration [11], whereby animals undergo daily extinction sessions to reduce responding to low levels prior to reinstatement of extinguished responding. The process of extinction invokes new learning and results in profound neuroadaptive changes over time, as seen in animals that have experienced extinction rather than abstinence following chronic cocaine self-administration [12]. In the present study, we investigated DA transport, functional expression of DAT protein at the plasma membrane, phospho-status of DAT, and DAT-PP2Ac association using rats with a history of prior cocaine self-administration followed by daily extinction for 3 weeks.
Materials and Methods
Subjects
Male, Sprague-Dawley rats (Charles-River, Wilmington, MA), weighing 275–300 g, were individually housed in a temperature and humidity controlled vivarium on a 12-h light-dark cycle. Rats were maintained on ad libitum water and 25 g of rat chow (Harlan, Indianapolis, IN) per day. The housing and care of the rats followed the guidelines of the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996). Rats were given a minimum of 4 days for adaptation and handling before the start of the experiment.
Lever response training
Rats were initially trained to lever press along a fixed ratio (FR) 1 schedule of food reinforcement (45 mg pellets; Noyes, Lancaster, NH) in sound-attenuating operant chambers (30 × 20 × 24 cm high; Med Associates Inc., St. Albans, VT) during a 16-h overnight training session. The chambers were equipped with two retractable levers, a stimulus light above each lever, a food pellet dispenser between the levers, a house light on the wall opposite to the levers, and a tone generator. During the session, each lever press on the active lever resulted in the delivery of a food pellet only. Lever presses on the inactive lever had no programmed consequences. Following lever response training, food pellet dispensers were removed from the chambers.
Surgery
Forty-eight h after lever response training, rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 mg/kg and 1.33 mg/kg, respectively, IP) followed by equithesin (0.5 ml/kg, IP). Catheters was inserted into the right jugular vein and secured to surrounding tissue with suture and maintained as described previously [3; 13].
Cocaine self-administration
Following surgery, rats were randomly divided into active cocaine self-administration or yoked saline groups (final N=30/group). Rats self-administered cocaine for 2-h daily sessions for 10 days along a FR 1 schedule of cocaine reinforcement (cocaine hydrochloride provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA). These parameters of cocaine self-administration have been widely used in previous cocaine self-administration experiments, and we chose them for comparability with prior studies [3; 13]. At the start of each session, the rat’s catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA) via polyethylene 20 tubing that was connected to an infusion pump (Model PHM-100, Med Associates Inc., St. Albans, VT). Lever presses on the active lever resulted in a 2-s activation of the infusion pump and a 5-s presentation of the white stimulus light above the active lever. Cocaine hydrochloride was dissolved in sterile saline, filtered using a 0.45 μm Ultracleaning Filter Unit (Fisher Scientific), and delivered at a dose of 0.2 mg/50 μl infusion. After each infusion, responses on the active lever had no consequences during a 20-s time out period. During the sessions, responses on the inactive (non cocaine-paired) lever were recorded, but had no programmed consequences. Yoked-saline rats were placed in test chambers in the same manner as the cocaine self-administration rats, but received a 2-s infusion of saline (50 μl) and 5-s presentation of the white stimulus light, contingent upon the cocaine infusion received by the self-administering rat in the adjacent chamber.
Extinction
Following chronic cocaine self-administration or yoked saline infusions, rats from each group underwent daily extinction sessions for 21 days. For extinction, animals were placed back into the self-administration chambers for 2-h daily sessions and responses were recorded on both levers, but had no programmed consequences (i.e., neither infusions nor stimuli were presented). We chose a period of 21 days, since this represents a time well beyond active cocaine reinforcement, when long lasting changes would be apparent. Animals at this time period show robust reinstatement of extinguished cocaine-seeking in relapse models [13].
DAT activity measurements using synaptosomes
Rats were rapidly decapitated, and the CPu and NAcc were dissected and synaptosomes were prepared and used for DA uptake measurements as described previously [3]. Synaptosomes (30 μg) were incubated in 250 μl of KRH buffer pH 7.4 containing 0.1 mM ascorbic acid and 0.1 mM pargyline and 40 nM [3H]DA for 5 min. For DAT kinetic analysis, [3H]DA was mixed with unlabelled DA so that total DA concentration ranges from 0.01 to 2 μM. Synaptosomes were preincubated with the DAT inhibitor nomifensine (100 μM) at 37° C for 10 min followed by the addition of [3H]DA to determine the nonspecific DA uptake. Uptake was terminated with the addition of 1 ml ice-cold KRH buffer containing 100 μM nomifensine followed by rapid filtration over GF-B filters on a Brandel Cell Harvester. Radioactivity bound to filter was counted by liquid scintillation counter. Nonspecific uptake was defined as the uptake in the presence of 100 μM nomifensine and subtracted from the total accumulation to yield specific DAT mediated DA uptake.
Surface biotinylation, immunoprecipitations and immunoblotting
Synaptosomes (500 μg) were treated with sulfo-NHS-SS-Biotin (1 mg/1 mg protein) for 30 min at 4° C in cold Krebs-bicarbonate buffer as described previously [3]. Subsequently, the samples was extracted in RIPA lysis buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate) supplemented with protease inhibitors and phosphatase inhibitors and centrifuged at 40,000 × g for 20 min. The biotinylated proteins were separated using monomeric avidin beads. Aliquots from total extracts (50 μl) and entire eluted fractions were separated by SDS-PAGE, transferred to membrane, and probed with DAT specific antibody. DAT reactive proteins were visualized using ECL plus reagent followed by exposure to Hyperfilm-ECL (GE Health Care). Subsequently, the blots were stripped and reprobed with anti-calnexin antibody to validate the surface biotinylation of plasma membrane proteins. DAT densities from total, and biotinylated (representing the surface pool) fractions were normalized using levels of calnexin in the total extract [3]. DAT immunoprecipitations and DAT-PP2Ac immunoblotting were performed from detergent extracts of synaptosomes as described previously [3]. To test specificity, additional experiments were carried out with irrelevant IgG, preimmune serum or Protein A Sepharose alone as described recently [3].
Statistical analyses
All values are expressed as mean ± SEM of at least three separate experiments. For both cocaine self-administration and biochemical data, statistical differences between the means of the two groups were determined by Student’s t test or analysis of variance (ANOVA). A value of p < 0.05 was considered statistically significant.
Results
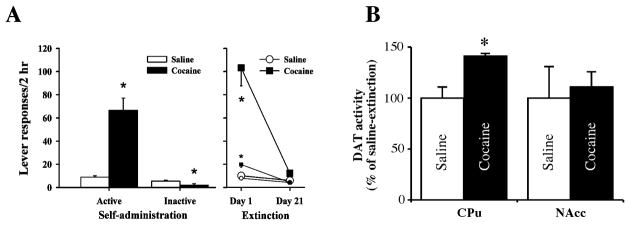
Rats with daily access to contingent cocaine showed consistent and robust cocaine self-administration. The number of i.v. infusions (cocaine or yoked saline) calculated for the last three days of self-administration was 35.97 ± 2.19 per session, and the cocaine group self-administered an average of 22.23 ± 1.26 mg/kg/session. Figure 1A (left panel) shows active and inactive lever responding averaged over the last 3 days of self-administration for both experimental groups. As expected, animals that self-administered cocaine showed significantly higher active lever responding than yoked saline treated animals (t58 = −5.5, p < 0.001), and selectively higher responding on the active lever, with saline-yoked animals exhibiting higher pressing on the inactive lever relative to the cocaine group (t58 = 2.28, p < 0.05).
Figure 1. Lever responding and DA transport.
A. Left panel in A: Responses on the active and inactive levers exhibited by rats averaged for the last three days of cocaine self-administration or yoked saline infusions (N=30/group). Right panel in A: Responses on the active (large symbols) and inactive (small symbols) levers exhibited by rats with a history of cocaine self-administration or yoked saline infusions on the first (day 1) and last (day 21) extinction session. Significant differences are indicated between cocaine and saline groups (*p < 0.05). B. DA transport in synaptosomes from CPu and NAcc following 3 weeks of extinction after chronic cocaine self-administration. Synaptosomal DA uptake was measured as described in “Materials and Methods”. The results are expressed as percentage of saline and data represent mean ± SEM of three experiments. *p <0.001 compared with saline (CPu) by a two-tailed Student’s t-test.
Figure 1A (right panel) shows responding on day 1 and day 21 of extinction. Two way repeated measures ANOVA of responding on the active lever across the first and last days of extinction revealed a significant group (F1,58 = 37.13, p < 0.001), session (F1,58 = 39.39, p < 0.001), and group × session interaction (F1,58 = 32.89, p < 0.001). Animals with a history of cocaine self-administration showed a typically high level of responding on the first day of extinction, with responding dropping rapidly over the first week of extinction (data not shown). Post hoc comparisons showed significantly higher responding for the cocaine self-administration group on day 1 as compared to day 21 and as compared to the saline-yoked group (ps < 0.05), but no differences between the two groups on day 21, or for responding in the saline yoked animals on day 1 vs. day 21 (ps > 0.05). Analysis of responding on the inactive lever across extinction also revealed a significant group (F1,58 = 10.93, p < 0.005), session (F1,58 = 46.52, p < 0.001), and group × session interaction (F1,58 = 18.46, p < 0.001). Post hoc comparisons showed the same pattern for inactive lever responding as seen for active lever responding, with no differences between groups on day 21.
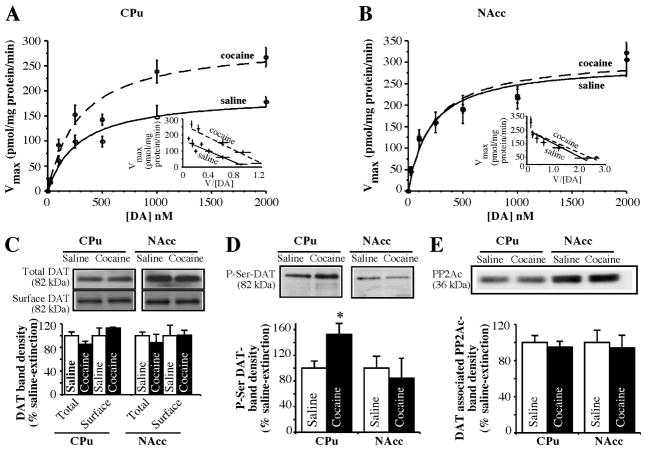
As shown in Figure 1B, DAT mediated DA uptake was significantly elevated in the CPu, but not in the NAcc, in rats with a history of cocaine self-administration as compared with saline-yoked control rats (141.2 ± 9.7%, 110.7 ± 15.3% respectively). The kinetic parameters of CPu and NAcc DAT (Michaelis Menten constant, Km, and maximal velocity, Vmax) are shown in Figure 2. Fig. 2A shows that in rats with a history of cocaine self-administration, CPu DAT Vmax was significantly increased (saline = 194.5 ± 9.85 pmol/mg protein/min; cocaine = 279.8 ± 13.84 pmol/mg protein/min, p <0.01), with no significant changes in the Km (saline = 319.3 ± 81.95 nM; cocaine = 362.7 ± 66.49 nM). However, chronic cocaine self-administration did not alter NAcc DAT Vmax (saline = 301.0 ± 32.02 pmol/mg protein/min; cocaine = 311.5 ± 10.24 pmol/mg protein/min) or Km (saline = 231.7 ± 45.7 nM; cocaine = 220.6 ± 19.75 nM) (Fig. 2B).
Figure 2. DA uptake kinetics, DAT-sub-cellular distribution, DAT Ser-phosphorylation and DAT-PP2 interactions in synaptosomes from CPU and NAc 3 following 3 weeks of extinction after chronic cocaine self-administration.
DA uptake was measured over a range of 0.01–2.0 μM DA using CPu (A) or NAc (B) synaptosomes as described in “Materials and Methods”. Nonlinear curve fits of data for uptake used the generalized Michaelis-Menten equation. Insets show Eadie-Hofstee plots of transformation of the data. Measurements of surface DAT (C), DAT-Ser phosphorylation (D) and DAT-PP2Ac association (E) were performed as described in “Materials and Methods”. Representative immunoblots are shown in C, D and E. Averaged protein band densities from three separate experiments are shown under the immunoblots (each experiment used CPu or NAcc tissue pooled from 3 or 4 rats). Data are presented as mean ± S.E.M percent change from the saline group. *p <0.02 compared with saline (CPu) by a two-tailed Student’s t-test.
Active DA transport is a property of DAT protein at the cell surface. The cocaine-induced elevation of Vmax for DA transport found in the CPu may be due to alterations in trafficking events, whereby more functional DAT is available on the neuronal cell surface for DA uptake. Alternatively, an increase in total DAT biosynthesis could increase both total and surface cell membrane DAT. To investigate these possibilities, we measured cell surface DAT by surface biotinylation and immunoblotting. Figure 2C illustrates the total expression of DAT and the levels of surface DAT in cocaine and saline rats. No significant differences were seen in the level of total, surface, and intracellular DAT protein expression in either the CPu or NAcc between cocaine and saline rats (Fig. 2C). In addition, there were no significant alterations in the total amount of calnexin (intracellular marker), and less than 4% of total calnexin was present in avidin bound fractions (data not shown).
Since altered phosphorylation of DAT may underlie the altered DAT kinetics observed in the CPu after cocaine self-administration, DAT proteins were immuno-isolated followed by immunoblotting with anti-phosphoserine. In the cocaine self-administration group, we found a marked increase in the amount of Ser-phosphorylated DAT in the CPu, whereas there was no observable change in the level of serine phosphorylation of DAT in the NAcc (Fig. 2D). We have previously validated the specificity of our DAT antibody for DAT immunoprecipitation using irrelevant IgG, or preimmune serum, or Protein A Sepharose as well as the nature of P-Ser DAT [3]. In the current study, immunoblotting of DAT immuno-complex with anti-PP2Ac detected PP2Ac in both the CPu and NAcc (Fig. 2E). The level of PP2Ac from DAT immuno complex was not altered in either the CPu or NAcc in cocaine rats as compared with saline rats, and there were no differences in the total expression of PP2Ac between groups (data not shown).
Discussion
Collectively, the current results reveal a region-specific dysregulation of DAT as a consequence of cocaine self-administration followed by extinction of responding over three weeks of withdrawal. In addition, our data also provide evidence that DAT-phosphorylation may be one among many critical molecular mechanisms to account for elevated DAT activity that was selectively found in the CPu, but not the NAcc. These altered properties of striatal DAT are of particular interest in light of growing evidence for dorsal striatal-based changes as an important mechanism underlying compulsive drug-seeking in addiction [14; 15]. Furthermore, the current results indicate that differential experience during withdrawal (abstinence vs. extinction) from cocaine self-administration differentially impacts DAT function [3].
Our finding that elevated DAT activity in the CPu in animals with a history of cocaine self-administration following 3 weeks of extinction with no significant changes in the surface DAT density indicates trafficking independent adaptations in intrinsic DAT activity. It has been suggested that biogenic amine transporters exhibit several modes of DA transport along with Na+ and Cl− [16]. Supportive of these observations, recent microdialysis experiments have shown that the higher DAT Vmax resulted in concomitant decreased level of extracellular DA found in rats that had 24 hr cocaine access followed by withdrawal [17]. It is important to note that different levels of DA uptake and Vmax values have been described in the CPu and NAcc [17; 18; 19]. Consistent with our previous studies [3], we found higher Vmax in NAcc when compared to CPu. In agreement with higher Vmax in NAcc, our DAT immunoblot showed higher level of total and surface DAT protein in NAcc than in CPu (Figure 2C). The discrepancy of variously reported Vmax values in CPu and NAcc across different studies remains to be understood.
Altered DAT basal phosphorylation has been implicated in trafficking dependent and independent transporter functional expression [2]. In this regard, the current study has identified the perturbation of normal DAT-serine phosphorylation in CPu, but not in NAcc following extinction withdrawal from chronic cocaine self-administration as a possible marker of long-lasting DAT upregulation that shows selectivity to the dorsal striatum. DAT is a substrate for several kinases, including PKC, CAMKII and MAPK [20]. Our current findings suggest that decreased DAT-serine phosphorylation could lead to increased DA uptake in CPU. Previously, we demonstrated that DAT, NET, and SERT are associated with PP2Ac [21]. Regulated association of PP2Ac with DAT may dictate DAT transporter phosphorylation state and/or may regulate DAT associated protein’s phopho-state by regulating the rate of dephosphorylation. However, we found that a history of cocaine self-administration followed by prolonged extinction did not produce long lasting influences on DAT association with PP2Ac. It is important to point out that decreased DAT-serine phosphorylation was not limited, but may also involve DAT associated proteins and other unknown cellular mechanisms to increase DA-uptake following extinction withdrawal from chronic cocaine self-administration. Differential activation and/or inhibition of several kinase cascades have been implicated in the neuronal plasticity occurring during cocaine addictive processes (reviewed in [22; 23]. Such changes in kinase activities may influence DAT phosphorylation and/or other mechanisms regulating DA-transport and hence DA neurotransmission. Pre-synaptic D2, D3 and TrkB receptors modulate DAT function [6; 7; 24]. The potential roles of D2, D3 and TrkB receptors and their down-stream synaptic signaling cascades have been well documented in cocaine addiction and other drugs of abuse [22; 23; 25; 26; 27]. It is reasonable to suggest that, in cocaine experienced animals, withdrawal may result in altered and/or compromised presynaptic receptor(s) and their downstream signaling cascade(s) that may target DAT differently, with subsequent influences on synaptic DA transmission.
Importantly, differential experiences during the withdrawal phase impact the nature of the changes in dynamic DAT regulation. In rats with a history of repeated cocaine self-administration followed by three weeks of abstinence (i.e., no return to the drug-taking environment), as opposed to daily extinction trials, cocaine-experienced rats exhibited elevated DAT activity and Vmax in both the CPu and NAcc [3]. Furthermore, increased surface DAT density and a higher level of DAT-PP2Ac association with decreased level of p-Ser DAT phosphorylation were observed in the CPu, but not in the NAcc. However, increased DA affinity was evident only in the NAcc, but not in CPu [3]. Thus, chronic cocaine self-administration followed by very different environmental conditions during withdrawal can lead to persisting, yet differential alterations in normal DAT-trafficking and catalytic regulatory cascades in a brain-region specific manner.
A practical implication of the current results in conjunction with our previous study [3] is the apparent ability of altered environmental conditions to change DAT regulation and trafficking in critical striatal brain regions following cocaine self-administration. The identification of critical presynaptic receptor/signaling pathways and other targets involved in cocaine-induced, anatomically specific DAT neuroplasticity may help guide environmentally based interventions for addiction, such as extinction therapies, as well as pharmacotherapies that may help restore DAT function.
Acknowledgments
This work was supported by NIH Grant P50 DA015369, GM081054 and DA016753 (to L.D.J.) and C06 RR015455 to the Medical University of South Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–97. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan RA. Phosphorylation and regulation of psychostimulant-sensitive neurotransmitter transporters. J Pharmacol Exp Ther. 2004;310:1–7. doi: 10.1124/jpet.103.052423. [DOI] [PubMed] [Google Scholar]
- 3.Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. J Pharmacol Exp Ther. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- 4.Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–9. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- 5.Jayanthi LD, Samuvel DJ, Buck ER, Reith ME, Ramamoorthy S. Regulation of Biogenic Amine Transporters. In: Abel Lajtha RE, editor. Handbook of Neurochemistry and Molecular Neurobiology. Springer-Verlag press; 2007. pp. 363–386. [Google Scholar]
- 6.Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–32. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- 7.Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, Jaligam V, Oz M, Jayanthi LD, Samuvel DJ, Ramamoorthy S, Shippenberg TS. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282:35842–54. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- 8.Izenwasser S. The role of the dopamine transporter in cocaine abuse. Neurotox Res. 2004;6:379–83. doi: 10.1007/BF03033312. [DOI] [PubMed] [Google Scholar]
- 9.Chefer VI, Shippenberg TS. Changes in basal and cocaine-evoked extracellular dopamine uptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience. 2002;112:907–19. doi: 10.1016/s0306-4522(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 10.Cass WA, Gerhardt GA, Gillespie K, Curella P, Mayfield RD, Zahniser NR. Reduced clearance of exogenous dopamine in rat nucleus accumbens, but not in dorsal striatum, following cocaine challenge in rats withdrawn from repeated cocaine administration. J Neurochem. 1993;61:273–283. doi: 10.1111/j.1471-4159.1993.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 11.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 12.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–5. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 15.Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005;83:93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 16.DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- 17.Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–63. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- 18.Near JA, Bigelow JC, Wightman RM. Comparison of uptake of dopamine in rat striatal chopped tissue and synaptosomes. J Pharmacol Exp Ther. 1988;245:921–7. [PubMed] [Google Scholar]
- 19.Marshall JF, O’Dell SJ, Navarrete R, Rosenstein AJ. Dopamine high-affinity transport site topography in rat brain: major differences between dorsal and ventral striatum. Neuroscience. 1990;37:11–21. doi: 10.1016/0306-4522(90)90187-9. [DOI] [PubMed] [Google Scholar]
- 20.Gorentla BK, Moritz AE, Foster JD, Vaughan RA. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry. 2009;48:1067–76. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci. 2000;20:7571–8. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nestler EJ. Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–8. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem. 2007;101:1258–71. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- 25.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corominas M, Roncero C, Ribases M, Castells X, Casas M. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology. 2007;55:2–13. doi: 10.1159/000103570. [DOI] [PubMed] [Google Scholar]