Abstract
Objectives
To evaluate whether serum RBP4 correlates with gestational diabetes mellitus (GDM) in a cohort of borderline obese (BMI>30) pregnant women.
Design and methods
Serum RBP4 and retinol were measured in pregnant women with (n=12) and without (n=10) GDM.
Results
RBP4, retinol and RBP4:retinol molar ratio were not different between the groups and were not associated with markers of insulin resistance.
Conclusions
GDM is not associated with RBP4 or retinol among borderline obese pregnant women.
Keywords: Retinol-Binding Protein 4 (RBP4), retinol, gestational diabetes, BMI, obesity
INTRODUCTION
Retinol-binding protein (RBP or RBP4) is a key protein in the metabolism of retinoids (vitamin A and its derivatives), as it is the sole specific carrier for retinol (vitamin A alcohol) in the bloodstream (1). RBP4 is a 21 kDa protein with a single binding site for one molecule of all-trans-retinol. It is mainly, but not exclusively, synthesized within the hepatocytes (1), and it circulates in the bloodstream as a 1:1 molar complex with another serum protein, transthyretin (TTR) (1). Blood levels of retinol-RBP4 in both humans and animals are maintained very constant, except in extreme cases of nutrition and in certain disease states (1). The best-characterized function of RBP4 is to mobilize hepatic vitamin A stores and deliver retinol to peripheral tissues, especially in times of insufficient dietary intake (1). Other adult organs and tissues, like eye, brain, and adipose, are reported to express RBP4 (1). However, the function(s) of the protein synthesized in these extrahepatic tissues is still not clear. An intriguing answer to the biological significance of extrahepatically synthesized RBP4 emerged in 2005, with the pioneering studies of Yang et al. (2). By using animal models, the authors, demonstrated that RBP4 serves as an adipocyte derived “signal” (adipokine) that can play a role in the development of insulin resistance. Subsequent investigations have extended this research to demonstrate the association between elevated levels of serum RBP4 and the magnitude of insulin resistance in adult human subjects with obesity, impaired glucose tolerance and/or type 2 diabetes (3). This association remains still controversial (4).
Gestational diabetes mellitus (GDM) is glucose intolerance with onset or first diagnosis during the second half of pregnancy (5). Although the etiology of the disease has not been fully clarified, main risk factors for the development of GDM include advanced maternal age, specific ethnic backgrounds, BMI greater than 27 and family history of type 2 diabetes (5). Very recently, a few investigations showed that either elevated levels of RBP4 (6–8) or the RBP4:retinol molar ratio (9) correlate with GDM status. However, in these studies, the average BMI of the control and GDM groups ranged from normal to obese, and/or the BMI of the control group was lower than that of the GDM group and/or within the same group the BMI of the subjects ranged from normal to obese values.
Given the reported association between serum RBP4 levels and BMI (3, 10, 11), we analyzed borderline obese women with and without mild gestational diabetes.
METHODS
Subjects
Data for this analysis was obtained from a larger cohort of women who were recruited from the Women’s Ambulatory Clinic at Saint Peter’s University Hospital, New Brunswick, NJ for a prospective study on taste and its hormonal correlates in GDM. The original study was approved by the institutional review board at St. Peter’s Hospital and subjects gave their informed consent to participate in the study. Exclusion criteria for all women included pre-existing medical conditions (including Type 1 or Type 2 diabetes, hypertension or impaired renal function), GDM in a previous pregnancy, and use of medications that interfere with appetite or metabolism. Routine screening for GDM (1-hr, 50-g glucose challenge) was conducted at 24–28 wk gestational age. A positive screen (glucose > 7.7 mmol/L) was followed by a 3-hr, 100-g oral glucose tolerance test to confirm the diagnosis, according to Carpenter & Coustan (12). Data were available for 12 women with GDM and 10 pregnant controls who matched the GDM group in age, ethnicity and body mass index (BMI; kg/m2). The ethnic breakdown of the GDM group was: Hispanic (n=7); Caucasian (n=2); African American (n=2); other (n=1). The ethnic breakdown of the pregnant control group was: Hispanic (n=8); Caucasian (n=1); African American (n=1). Subject characteristics are shown in Table 1.
Table 1.
Subject characteristics, clinical measures, RBP4 and retinol levels in women with gestational diabetes and pregnant controls1
| Gestational Diabetes (n=12) |
Pregnant Controls (n=10) |
|
|---|---|---|
| Age (yrs) | 28.6 ± 4.9 (21–39) | 28.8 ± 6.2 (20–40) |
| Maternal BMI (kg/m2) | 31.1 ± 0.6 (28.3–34.4) | 31.1 ± 0.9 (28.3–34.6) |
| Maternal weight gain (kg) | 8.7 ± 1.6 (0.9–16.4) | 13.8 ± 2.7 (2.3–28.2) |
| Infant birth weight (g) | 3469 ± 222 (2010–4410) | 3443.3 ± 211 (2340–4290) |
| Gestational age at birth (wk) | 40.4 ± 0.6 (39.0–45.2) | 38.8 ± 0.5 (37.0–41.0) |
| Fasting glucose (mg/dL) | 82.0 ± 3.2 (63–111) | 70.2 ± 3.7 (61–82) * |
| Glucose 1-hr after 50-g glucose (mg/dL) | 143.4 ± 8.1 (88–187) | 105.3 ± 8.9 (54–142) ** |
| Fasting insulin (µU/mL) | 27.4 ± 4.7 (10.1–79.9) | 15.4 ± 5 .2 (3.9–39.8) |
| Insulin 1-hr after 50-g glucose (µU/mL) | 111.8 ± 10.4 (34.52–192.3) | 61.6 ± 11.4 (17.87–134.3) ** |
| HOMA-IR | 5.6 ±1.0 (2.6–17.8) | 3.0 ± 1.2 (1.41–6.5) |
| Fasting Leptin (ng/mL) | 29.7 ± 4.8 (15.2–77.0) | 29.6 ± 5.3 (19.2–40.0) |
| Retinol (µmol/L) | 1.6 ± 0.1 (1.3–2.2) | 1.7 ± 0.1 (1.1–2.9) |
| RBP4 (µmol/L) (EIA) | 1.2 ± 0.1 (0.6–1.7) | 1.2 ± 0.1 (0.8–1.8) |
| RBP4 (µmol/L) (Western blot) | 2.1 ± 0.4 (1.4–3.4) | 1.8 ± 0.6 (1.1–2.9) |
| RBP4:retinol (Western blot)2 | 1.3 ± 0.2 (1.0–1.7) | 1.1 ± 0.3 (0.5–1.3) |
Values are means (±SEM); range of values for each measurement is indicated in parenthesis.
Molar ratio of RBP4:retinol according to the amount of RBP4 determined by Western blot and the amount of retinol determined by HPLC.
p<0.05
p<0.01
Measurements of serum glucose, insulin and leptin
Blood samples collected during the screening test (24–28 wk gestation) were assayed for serum insulin, glucose and leptin. Serum insulin was measured by double-antibody RIA, and serum leptin was measured by RIA using commercial kits (Linco Research, St. Charles, MO). RIAs were performed in duplicate by the Diabetes Research Center of the University of Pennsylvania. Serum glucose was analyzed with a Hitachi 717 analyzer using hexokinase reagents from Amresco (Solon, OH). Analysis of glucose was performed by Accumed Diagnostic Laboratory (South Amboy, NJ)
Homesostasis model assessment
The degree of insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR), that was calculated as follows: fasting glucose (mmol/L) × fasting insulin (mU/L)/22.5.
Measurements of serum retinol and RBP4
Reverse phase HPLC analysis was performed to measure serum retinol levels (13). Serum RBP4 (21 kDa) levels were measured by two different methods, western blot analysis and enzyme immunometric assay (EIA). For western blot, a rabbit polyclonal anti-human RBP antiserum (13) was used for immunodetection. Signals were detected by using a Biorad Chemidoc XRS Molecular Imager System. Albumin, detected upon treating the membranes with either Coomassie or Ponceau S stain, was used as a loading control. A reference sample was loaded on each gel to allow for normalization between gels. In addition, we also generated an RBP standard curve, as previously described (14). Purified human RBP protein was a generous gift of Dr. F. Mancia, Columbia University. Quantification of the membranes was completed by densitometry analysis with Quantity One Program (Biorad). The EIA was performed by using the RBP4 EIA kit, Pheonix Pharmaceutical (Blemont, CA, USA), as previously described (8, 9).
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA). Associations between measures were examined using Pearson correlation coefficients. Statistical analyses were conducted using SAS version 9.1 for the personal computer (SAS Institute Inc, Cary, NC) with α= 0.05 for all comparisons.
RESULTS
As shown in Table 1, both the pregnant control and the GDM group had an average BMI=31 at 24–28 wk gestational age. A variety of indicators also established that the women with GDM had a mild form of the disease. The GDM group showed higher fasting glucose levels than pregnant controls. However, values were within the normal range, which is typical of mild GDM (5). Although serum glucose and insulin were more elevated after oral glucose in the GDM group as compared to pregnant controls, serum glucose remained within a clinically acceptable range for women with GDM (5). HOMA–IR was only marginally higher in the GDM group relative to controls. Additionally, the GDM group had good pregnancy outcomes. Maternal weight gain was marginally lower in the GDM group relative to pregnant controls and infant birth weight and the rate of cesarean delivery did not differ between the two groups of women. Finally, women with GDM began nutritional therapy soon after diagnosis and continued it to term. None of the patients required insulin or hypoglycemic agents to control their disease.
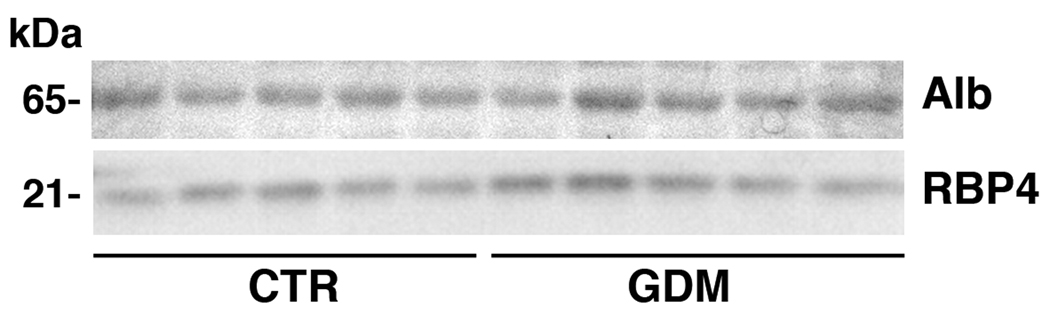
No difference was observed between the GDM and the pregnant control group with respect to serum RBP4 levels, as measured by both EIA (Table 1) and western blot analysis (Table 1 and Figure 1), and serum retinol levels (Table 1). The molar ratio RBP4:retinol was also not different between the two groups (Table 1).
Figure 1. Representative western blot analysis of serum RBP4.
Serum RBP4 from 5 pregnant GDM and 5 pregnant control women is shown. Albumin (Alb) was used as a loading control as described in Methods. The molecular weight of each detected protein is indicated on the left side of the panels.
Correlations between RBP4, retinol or RBP4:retinol molar ratio and insulin measures, glucose measures, HOMA-IR and maternal BMI were also performed. None of these correlations was statistically significant. In particular, no associations were observed between RBP4 by western blot and fasting insulin, fasting glucose, HOMA-IR or BMI (r= −0.16 – +0.22; p= 0.31–0.46). Similarly, no associations were observed between RBP4 EIA and these same variables (r=0.09–0.31; p=0.22–0.71)
DISCUSSION
This work investigated the correlation between RBP4 and GDM in a cohort of obese pregnant women. Four previous reports have shown a positive association between elevated levels of serum RBP4 (6–8) or the RBP4:retinol molar ratio (9) and GDM status. However, in all these studies, either the BMI within a group was not homogenous (7), or control and GDM groups had different BMI (8), or the average BMI ranged from normal to slightly overweight values (5–7).
Here we analyzed, for the first time, borderline obese groups of women with and without gestational diabetes. These two groups, which showed very homogenous characteristics (Table 1), had BMI values exclusively toward the overweight-obese range before pregnancy and at the time the analyses were performed (24–28 wk gestation). The average BMI value was essentially identical in the two groups. We applied two methods, western blot analysis and EIA, to assess RBP4 levels, to eliminate possible concerns raised about the accuracy of the RBP4 measurements (14). The concentration values of RBP4 obtained with the two methods are different within the same group (Table 1), as seen also by Krzyzanowska et al. (9). Nevertheless, the conclusions of our study are unaffected by the methodology used, as RBP4 levels in control and GDM group are always similar, regardless of the measurement technique (Table 1 and Figure 1). Notably, the same commercial RBP4 EIA kit utilized in our study was used by Krzyanowska et al. (9) and by Lewandowski et al. (8). However, the range of values obtained in these three studies is quite different, further pointing to the fact that a comparison between studies is always troublesome, even when the same measurement method for RBP4 is used.
We also measured serum retinol levels and analyzed the RBP4:retinol molar ratio as reported by Krzyzanowska et al. (9). Our data demonstrate that neither circulating RBP4 nor retinol levels nor the RBP4:retinol molar ratio are significantly different between GDM patients and pregnant controls. Also, these parameters are not significantly associated with markers of insulin resistance or GDM in this cohort of women.
These results seem to suggest that GDM is not associated with RBP4 or retinol among borderline-obese pregnant women. We speculate that one of the reasons for the discrepancy of our data with the previous published reports could be that the women with GDM in our study showed only a mild form of the disease (Table 1). This interpretation is supported by the data of Lewandowski and colleagues (8) who identified, within their cohort, a sub-group of pregnant women with impaired glucose tolerance that failed to show statistically significant differences in circulating RBP4 levels relative to normal pregnant controls. In addition, since the relationship of RBP4 with BMI, waist circumference, visceral fat and weight loss has been reported (3, 10, 11), we cannot exclude that the elevated BMI in our cohort (both the women in the GDM and the control group were obese) may mask the potential changes in RBP4 in the GDM group. Further experiments are needed to unequivocally confirm this possibility.
ACKNOWLEDGEMENTS
Supported by NRI Grant no. 2006-35200-16580 from the USDA, Bioactive Food Components for Optimal Health Program (31.0) to LQ and by grant HD057493 (LQ) and DC04702 (BJT) from the National Institutes of Health. We thank Lisa Belzer for collecting the primary data and St. Peter’s University Hospital for providing access to patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Quadro L, Hamberger L, Colantuoni V, Gottesman M, Blaner WS. Understanding the Physiological Role of Retinol-Binding Protein in Vitamin A Metabolism Using Transgenic and Knockout Mouse Models. Mol Aspect Med. 2003;24:421–430. doi: 10.1016/s0098-2997(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contibutes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 3.Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JG, Shand BI, Frampton CM, Elder PA, Scott RS. Plasma retinol-binding protein is not a marker of insulin resistance in overweight subjects: a three year longitudinal study. Clin Biochem. 2008;41:1034–1038. doi: 10.1016/j.clinbiochem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71:1256S–1261S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 6.Kim S-H, Choi H-J. Retinol-binding protein 4 responses during an oral glucose tolerance testing in women with gestational diabetes mellitus. Clin Chim Acta. 2007;391:123–125. doi: 10.1016/j.cca.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Chan TF, Chen HS, Chen YC, et al. Increased serum retinol-binding protein 4 concentrations in women with gestational diabetes mellitus. Reprod Sci. 2007;14:169–174. doi: 10.1177/1933719106298407. [DOI] [PubMed] [Google Scholar]
- 8.Lewandowski KC, Stojanovic N, Bienkiewicz M, et al. Elevated concentrations of retinol-binding protein-4 (RBP-4) in gestational diabetes mellitus: negative correlation with soluble vascular cell adhesion molecule-1 (sVCAM-1) Gynecol Endocrinol. 2008;24:300–305. doi: 10.1080/09513590802141052. [DOI] [PubMed] [Google Scholar]
- 9.Krzyzanowska K, Zemany L, Krugluger W, et al. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia. 2008;51:1115–1122. doi: 10.1007/s00125-008-1009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kloting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Tschoner A, Sturm W, Engl J, et al. Retinol-binding protein 4, visceral fat, and the metabolic syndrome: effects of weight loss. Obesity (Silver Spring) 2008;16:2439–2444. doi: 10.1038/oby.2008.391. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 13.Quadro L, Blaner WS, Hamberger L, et al. Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J Biol Chem. 2002;277:30191–30197. doi: 10.1074/jbc.M205046200. [DOI] [PubMed] [Google Scholar]
- 14.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 2007;50:814–823. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]