Abstract
Ca2+/calmodulin dependent protein kinase II (CaMKII), the most abundant kinase at the postsynaptic density (PSD), is expected to be involved in activity-induced regulation of synaptic properties. CaMKII is activated when it binds calmodulin in the presence of Ca2+ and, once autophosphorylated on T-286/7, remains active in the absence of Ca2+ (autonomous form). In the present study we used a quantitative mass spectrometric strategy (iTRAQ) to identify sites on PSD components phosphorylated upon CaMKII activation. Phosphorylation in isolated PSDs was monitored under conditions where CaMKII is: (1) mostly inactive (basal state), (2) active in the presence of Ca2+ and (3) active in the absence of Ca2+. The quantification strategy was validated through confirmation of previously described autophosphorylation characteristics of CaMKII. The effectiveness of phosphorylation of major PSD components by the activated CaMKII in the presence and absence of Ca2+ varied. Most notably, autonomous activity in the absence of Ca2+ was more effective in the phosphorylation of three residues on SynGAP. Several PSD scaffold proteins were phosphorylated upon activation of CaMKII. The strategy adopted allowed the identification, for the first time, of CaMKII-regulated sites on SAPAPs and Shanks, including three conserved serine residues near the C-termini of SAPAP1, SAPAP2 and SAPAP3. Involvement of CaMKII in the phosphorylation of PSD scaffold proteins suggests a role in activity-induced structural re-organization of the PSD.
Keywords: PSD, CaMKII, SAPAP, GKAP, Shank, iTRAQ
INTRODUCTION
The postsynaptic density (PSD) is a multiprotein complex lining the plasma membrane on the dendritic side of the synapse. The PSD contains numerous receptors and signal transduction elements organized in a tight array by specialized scaffold proteins (review [1]). Certain types of activity-dependent changes in synaptic strength, including long-term potentiation (LTP), require an increase in postsynaptic Ca2+-levels and activation of CaMKII, an abundant component of the PSD (review [2]). CaMKII-mediated phosphorylation of PSD components is likely to underlie changes in synaptic strength. In the present study we sought to identify those phosphorylation events at the PSD that are regulated through CaMKII.
PSDs are rigidly organized complexes with components attached to one-another, presumably at defined protein-protein interaction sites. This type of organization makes enzymatic reactions essentially different from those in soluble systems where all components can have access to each other via diffusion. Thus, phosphorylation of purified proteins by purified CaMKII in a soluble system does not necessarily imply that the reaction would occur at the PSD, even if both kinase and substrate were present. We therefore chose to observe endogenous phosphorylation in isolated, intact PSDs where native appositions of components of the PSD are maintained.
CaMKII exhibits unique activation characteristics in its response to Ca2+ (review [3]). The holoenzyme is composed of ~12 identical/similar subunits, each with catalytic and regulatory domains that allow activation upon binding of Ca2+/calmodulin. Organization within a multimeric unit allows intra-holoenzyme phosphorylation (autophosphorylation) of one subunit by another on multiple residues. Autophosphorylation of a particular residue -T286 on the alpha subunit, T287 on the beta subunit- confers autonomous activity, that is, CaMKII maintains the capacity to phosphorylate in the absence of Ca2+. We took advantage of the unique activation properties of the kinase to identify CaMKII-mediated phosphorylation events in isolated PSDs.
In recent years, implementation of mass spectrometric techniques in conjunction with an enrichment strategy for phosphopeptides yielded an extensive catalogue of phosphorylation sites at the PSD [4–9]. While these approaches identified sites phosphorylated in vivo, information on the regulation of phosphorylation, including identities of the kinases involved, is, in many instances, still lacking. A previous study [10,11] reported CaMKII-mediated phosphorylation of several PSD proteins, but in this case, the residues phosphorylated could not be identified by the methods employed. In the present study we applied a mass spectrometric strategy combined with the iTRAQ method for relative quantification to monitor the relative levels of phosphorylation at specific residues in isolated PSDs during different activity states of CaMKII.
MATERIAL AND METHODS
Preparation of PSD Fraction from rat cerebral cortex, as well as procedures following in vitro phosphorylation, including protein digestion, iTRAQ labeling, phosphopeptide enrichment, LC/MS/MS, and data analysis are detailed in the Supplementary Material section.
In Vitro Phosphorylation Experiments
Prior to phosphorylation, PSD fractions were treated with 0.1 M dithiothreitol and let to stand on ice for 2–4 hours. Reactions were started by the addition of PSD fraction on phosphorylation media as described below and stopped by the addition of an equal volume of ice-cold stop solution containing 100μg/ml leupeptin, 2 mM NaF, 1μM Microcystin-LR and 100 mM EDTA. Samples (100 μg protein each) were centrifuged (11,700g, 40min) to obtain a PSD pellet.
Phosphorylation Protocol I
PSD fractions (150 μl) were added on 120 μl of phosphorylation media containing Ca2+/calmodulin (samples 1,2,3) or Ca2+/calmodulin and EGTA (sample 4). Whenever indicated EGTA was added at a concentration 50X that of Ca2+. After 5 min incubation on ice, reaction in sample 1 was stopped. To the other samples, either 30 μl of H2O (samples 2 and 4) or 30 μl of EGTA (sample 3) were added and, after an additional minute on ice, these were transferred to 37°C and incubated for another 10 min. Final concentrations during incubations at 37°C were as follows: 0.4 mg/ml PSD protein, 0.1 mM ATP, 5 mM MgCl2, 1 mM CaCl2, 40μg/ml calmodulin, 50 mM EGTA (samples 3 and 4 only), 50μg/ml leupeptin, 0.6 μM Microcystin-LR in 20 mM HEPES pH 7.4
Phosphorylation Protocol II
PSD fraction 0.4 mg/ml protein was phosphorylated for 10 min at 37°C in media containing 0.1 mM ATP, 5 mM MgCl2, 50μg/ml leupeptin in 20 mM HEPES pH7.4 and, when indicated, 1 mM CaCl2+40μg/ml calmodulin, or 2 mM EGTA, 20μM [Ala286]281-301 peptide, 0.6 μM Microcystin-LR+1 mM NaF.
RESULTS
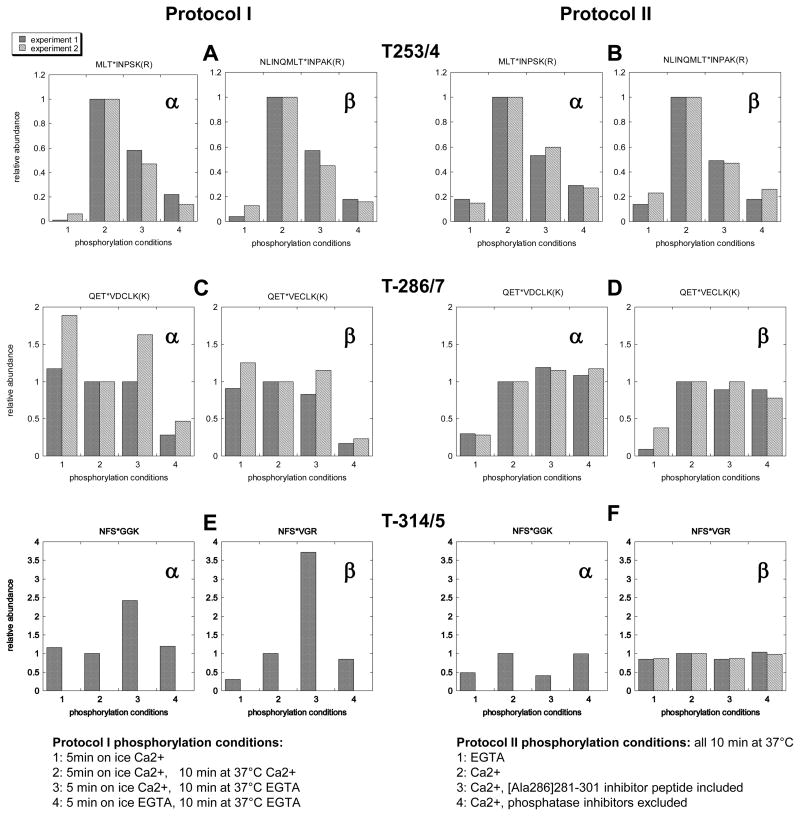
Autophosphorylation characteristics of CaMKII in the PSD fraction were studied using two sets of protocols comparing a group of samples subjected to four different incubation conditions each (Figure 1). Under each protocol, samples 1–4 were differentially labelled with iTRAQ reagents for comparison of phosphopeptide levels. Protocol I was designed to produce different activity states of CaMKII. Incubation for 5 min on ice in the presence of Ca2+/calmodulin was intended to switch on autonomous activity (Sample 1). Phosphorylation was then continued for another 10 min at 37 °C, either in the presence of Ca2+ (Ca2+/calmodulin bound state -Sample 2), or in the presence of EGTA (autonomous state –Sample 3), while in a fourth group incubation was in EGTA throughout (inactive state –Sample 4). Protocol II was designed to assess the effects of the peptide CaMKII inhibitor [Ala286]281-301 and of phosphatase inhibitors.
Figure 1. Phosphorylation at homologous residues on α-CaMKII and β-CaMKII under different incubation conditions.
PSD fractions, incubated under phosphorylating conditions as summerized above, were digested with trypsin, the four samples within each group were differentially labeled with iTRAQ reagents. Phosphopeptides were separated by TiO2 chromatography and analyzed by LC/MS/MS. Relative abundance of phosphopeptides were estimated by normalizing corresponding total counts with respect to sample 2. Two experiments were carried out for each protocol. Values from both experiments are shown when available.
Several phosphorylation sites were identified on α- and β-CaMKII. Quantitative data were evaluated for only a subset of these. Data for certain phosphopeptides, especially those containing multiple phosphorylation sites with differing phosphorylation properties (e.g. a peptide containing T-305/6 on the α-subunit) were judged to be uninterpretable and hence not selected for analysis.
Pairs of homologous residues on α- and β-CaMKII show similar phosphorylation patterns (compare adjacent panels for α- and β-CaMKII, Figure 1). On the other hand, patterns of phosphorylation for the three sites on a subunit are distinct (compare three panels top to bottom, Figure 1).
One pair of residues, T-253 on αCaMKII and T-254 on βCaMKII are substantially phosphorylated upon 10 min incubation at 37°C in the presence of Ca2+ (Figure 1A, Sample 2), but not in the presence of EGTA (Figure 1A, Sample 4). However, if incubation in cold in the presence of Ca2+ (which in itself does not promote significant phosphorylation at T-253/4 -Figure 1A, Sample1) precedes 10 min EGTA incubation at 37°C, a higher level phosphorylation is obtained (Figure 1A, Sample 3). Inclusion of the peptide inhibitor [Ala286]281-301, as well as exclusion of phosphatase inhibitors, significantly lower phosphorylation at T-253/4 (Figure 1B, Samples 3 and 4 respectively).
Phosphorylation on T-286 on αCaMKII and T-287 on βCaMKII (autonomy sites) is activated in the presence of Ca2+ and is maximal after 5 min incubation in cold (Figure 1C, Sample 1). Inclusion of [Ala286]281-301 peptide or omission of phosphatase inhibitors have little or no effect on the phosphorylation of T-286/7 (Figure 1D, Samples 3 and 4). A third set of sites, T-314 on αCaMKII and T-315 on β-CaMKII are most efficiently phosphorylated in the absence of Ca2+, after previous incubation in cold in the presence of Ca2+ (Figure 1E, Sample 3). Inclusion of phosphatase inhibitors does not appear to have any impact on the phosphorylation levels at these residues (Figure 1F, Sample 4).
Phosphorylation of core PSD proteins under conditions described in Protocol 1 were evaluated. The experimental design in this series allows for the identification of those phosphorylation events involving CaMKII. Phosphorylation via CaMKII can be in the presence of Ca2+, mediated by the Ca2+/calmodulin bound form of the kinase (Sample 2), or in the absence of Ca2+ mediated by the autonomous form of the kinase (Sample 3). Thus, involvement of CaMKII was decided only if an increase in phosphorylation could be observed in both Samples 2 and 3. Control Sample 4 reflects phosphorylation levels in the absence of Ca2+, when the autonomous activity has not been turned on.
The above criteria for involvement of CaMKII are met for all of the phosphorylation sites reported in Table 1 and Figure 2. Indeed, for all of these sites: (1) relative phosphorylation levels in Sample 2 (Ca2+/calmodulin throughout) are higher compared to Sample 4 (EGTA throughout), indicating activation by Ca2+; (2) relative phosphorylation levels in Sample 3 (Ca2+/calmodulin in cold then EGTA at 37°C) are higher compared to Sample 4 (EGTA throughout), indicating phosphorylation when the autonomous form of CaMKII is switched on. The fact that in all cases phosphorylation levels in Sample 1 (incubation in cold in the presence of Ca2+/calmodulin) is less than those in Sample 3 (continued incubation at 37 °C in the presence of EGTA) is evidence that phosphorylation is occurring after the addition of EGTA. In Table 1 and Figure 2 we report a total of 13 sites whose phosphorylation is promoted by CaMKII. Of these sites, 5 fall in the consensus CaMKII phosphorylation motif RXXS and another 2 show a KXXS motif.
TABLE 1.
Phosphorylation of PSD scaffold elements under different activity states of CaMKII.
| Protein Accession number |
Phosphorylated Peptide | Residue | Relative phosphorylation | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
|
Densin-180 UniProtKB: P70587 |
R.IVGVPLELEQ[ST]*HR.H | S-831 or T-832 | 0.02 0.22 |
1.00 1.00 |
0.47 0.66 |
0.35 0.08 |
|
SAPAP1 UniProtKB: P97836 |
K.ATQPS*LTELTTLK.I | S-389 | 0.60 | 1.00 | 1.14 | 0.44 |
|
SAPAP1 UniProtKB: P97836 |
K.FQS*VGVQVEEEK.C | S-696 | 0.11 | 1.00 | 0.62 | 0.24 |
|
SAPAP1 UniProtKB: P97836 |
R.ERS*LESSQR.Q | S-947 | 0.62 | 1.00 | 0.88 | 0.33 |
|
SAPAP2 UniProtKB: P97837 |
R.EKS*LDLPDR.Q | S-1012 | 0.81 | 1.00 | 0.96 | 0.26 |
|
SAPAP3 UniProtKB: P97838 |
K.ERS*LDSVDR.Q | S-930 | 0.51 | 1.00 | 0.95 | 0.37 |
|
Shank1 UniProtKB:Q9WV48 |
K.RLPPPAIS*LR.S | S-783 | 0.41 0.36 |
1.00 1.00 |
0.86 0.81 |
0.29 0.14 |
|
Shank3 UniProtKB: Q9JLU4 |
R.SKS*MTAELEELASIR.R | S-769 | 0.20 0.39 |
1.00 1.00 |
0.63 0.72 |
0.09 0.12 |
|
Shank3 UniProtKB: Q9JLU4 |
R.S*LGEEPVGGLGSLLDPAK.K | S-1586 | 0.68 0.34 |
1.00 1.00 |
1.13 0.90 |
0.65 0.11 |
|
Spinophilin (PP1) UniProtKB: O35274 |
R.A[SS]*LNENVDHSALLK.L | S-99 or S-100 | 0.30 0.49 |
1.00 1.00 |
0.74 1.05 |
0.38 0.42 |
PSD samples were phosphorylated under four different conditions as follows:
1: 5min on ice in Ca2+; 2: 5min on ice in Ca2+,10 min at 37°C in Ca2+; 3: 5 min on ice in Ca2+, 10 min at 37°C in EGTA; 4: 5 min on ice in EGTA, 10 min at 37°C in EGTA. Relative abundances of phophopeptides under each phosphorylation condition (1–4) were evaluated in two experiments. Both values are listed when available. Phosphorylated residues are depicted by an asterisk (*). Sequences carrying homologous phosphorylation sites on the C-terminals of SAPAPs are underlined. Comparison of columns 2 and 3 indicate differences in the efficiencies of phosphorylation of a particular residue by the calmodulin-bound form of CaMKII in the presence of Ca2+ (2) and by the autonomous form of CaMKII in the absence of Ca2+ (3).
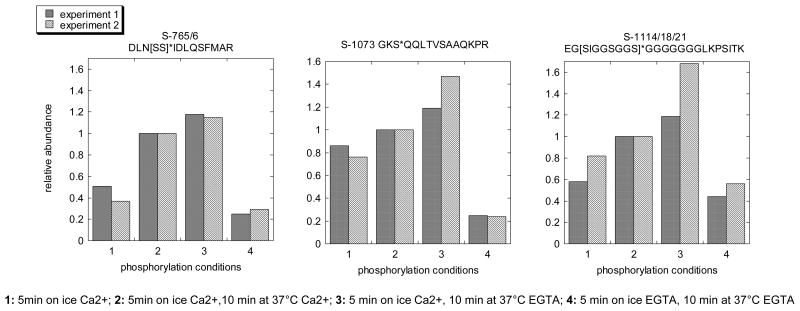
Figure 2. SynGAP is most efficiently phosphorylated by the autonomous form of CaMKII in the absence of Ca2+.
PSD samples ware phosphorylated under four different conditions as follows: Sample 1: 5min on ice in Ca2+; Sample 2: 5min on ice in Ca2+,10 min at 37°C in Ca2+; Sample 3: 5 min on in ice Ca2+, 10 min at 37°C in EGTA; Sample 4: 5 min on ice in EGTA, 10 min at 37°C in EGTA. Relative phosphorylation levels for all three residues indicate highest levels of phosphorylation in Sample 3, corresponding to autonomous activity in the absence of Ca2+.
The majority of phosphorylation sites regulated by CaMKII are on scaffolds, proteins that form the structural backbone of the PSD (Table 1). Most notable is phosphorylation on three homologous sequences, R.ERS*LESSQR.Q, R.EKS*LDLPDR.Q, and K.ERS*LDSVDR.Q on SAPAP1, SAPAP2 and SAPAP3respectively. Two of these correspond to the consensus CaMKII phosphorylation sequence RXXS and the third has a KXXS sequence.
Another major PSD protein, SynGAP, is phosphorylated on multiple residues upon activation of CaMKII (Figure 2). The levels of phosphorylation of SynGAP, on all three sites reported are higher in Sample 3 (autonomous kinase in the absence of Ca2+) than in Sample 2 (Ca2+/calmodulin bound kinase).
Phosphorylation at most of the identified sites showed some sensitivity to the [Ala286]281-301 peptide inhibitor (Supplementary Table 1). However, as explained above, because the peptide did not block CaMKII autophosphorylation at the autonomy site, we presume that a certain level of CaMKII-mediated phosphorylation can still proceed in the presence of the peptide.
Omission of phosphatase inhibitors in one group of samples allowed the assessment of phosphatase action. Relative levels of phosphorylation in the absence of phosphatase inhibitors are generally lower, indicating action of PSD-associated phosphatases. Differences in percent change observed for different sites point out to variable turnover rates. Variability between the two independent experiments was somewhat greater in this series, possibly due to combined effect of the variability in kinase and phosphatase rates.
DISCUSSION
In vitro protein phosphorylation in intact organelles offers two advantages: retention of the native appositions of kinases and phosphatases vis a vis their substrates and the ability for precise manipulation of phosphorylation conditions which allows the tracking of particular enzymatic pathways. The present study demonstrates that application of quantitative mass spectrometry by the iTRAQ technique for the analysis of differentially phosphorylated intact organelles can lead to the global identification of those residues regulated by specific kinases and/or phosphatases. The analysis of in vitro phosphorylated PSD fractions identified phosphorylation at several residues on CaMKII as well as on core PSD proteins under different incubation conditions designed to promote distinct activity states of CaMKII.
Regulation of CaMKII phosphorylation
Our results on differential modulation of phosphorylation at specific residues of CaMKII by Ca2+ are in agreement with previous studies using other strategies for the identification of phosphorylated residues [12–17]. Moreover, The regulation of phosphorylation on homologous residues on α- and β-CaMKII are found to be strikingly similar, conferring confidence to the iTRAQ quantification strategy employed.
We show that phosphorylation at the autonomy site, T-286/7, is promoted by Ca2+/calmodulin whereas phosphorylation at T-314/5 is maximal when a short incubation in cold to switch on autonomous activity is followed by incubation in the absence of Ca2+ at 37°C. Indeed residues T-314/5 are thought to become occluded upon binding of calmodulin in the presence of Ca2+ [15]. Phosphorylation at T-253/4 requires prior induction of autonomous activity, but as observed previously, can proceed in the presence or absence of Ca2+ [17]. Ca2+-dependence of phosphorylation at T-253/4 at the PSD is different from that observed for purified soluble enzyme which requires continued presence of Ca2+ [18]. As the above authors commented, specific protein interactions within the PSD may be responsible for the change in phosphorylation characteristics.
Dephosphorylation on CaMKII by endogenous phosphatase activity at the PSD was tested by comparing phosphopeptide levels in the presence and absence of phosphatase inhibitors. We show that T-253/4 sites are effectively dephosphorylated by endogenous phosphatase activity. On the other hand, the observed lack of effect of phosphatase inhibitors on T-286/7 and S-314/5 are in agreement with previous reports showing failure of dephosphorylation of T-286/7 by PSD-associated PP1[19] and the relative resistance of S-314/5 to PP2A in a soluble reaction [16]. However, it should be pointed out that our experiments do not provide information on the potential effects of the Ca2+/calmodulin regulated phosphatase calcineurin, since a specific inhibitor for this phosphatase was not included.
The peptide inhibitor [Ala286]281-301 had differing effects on the phosphorylation of different residues on CaMKII. While inclusion of the peptide significantly lowered the phosphorylation at T-253/4, it did not result in a decrease in phosphorylation of the autonomy site, T-286/7. The observed lack of effect of the peptide on the phosphorylation of some residues on CaMKII is in agreement with a previous study [20] showing relatively low efficiency of inhibiton of overall CaMKII autophosphorylation by the peptide compared to its effect on the phosphorylation of Syntide 2. Lack of effect on phosphorylation at T-286/7 implies that, under the conditions employed, the autonomous activity is switched on despite the presence of the peptide and, therefore, CaMKII-mediated phosphorylation of substrates can proceed. Thus, when analyzing phosphorylation of PSD proteins, sensitivity to this inhibitor could not be interpreted as a criterion for CaMKII involvement.
Regulation of the phosphorylation of PSD proteins
Relative phosphorylation levels of several residues on PSD proteins were compared under different activity states of CaMKII as well as in the presence or absence of inhibitors that block endogenous phosphatase activity. As explained above, incubation conditions were manipulated so that CaMKII is either in (1) mostly inactive, basal state in the absence of Ca2+, (2) active, calmodulin- bound state in the presence of Ca2+, or (3) active, autonomous state in the absence of Ca2+. Demonstration of maximal phosphorylation on T-286/7 upon incubation on ice in the presence of Ca2+ indicates that autonomous state has indeed been switched on under these conditions.
We took advantage of the unique activation properties of CaMKII to identify CaMKII-mediated phosphorylation events at the PSD. Indeed, while other kinases besides CaMKII are also activated by Ca2+, CaMKII when autophosphorylated on T-286/7 becomes autonomous, that is retains activity in the absence of Ca2+. Thus, phosphorylation in the absence of Ca2+ by the autonomous CaMKII can be taken as a signature of CaMKII involvement.
Several sites on PSD proteins including those on major scaffolds (Table 1) and on SynGAP, a Ras GTPase-activator (Figure 2), fulfill the criteria for CaMKII mediated phosphorylation: an increase in the relative levels of phosphorylated peptide in the presence of Ca2+/calmodulin, and in the absence of Ca2+, following induction of the autonomous activity. CaMKII-mediated phosphorylation on the same residues on SynGAP [21] and, on spinophilin [22] have previously been documented.
Comparison of the levels of phosphorylation by the Ca2+/calmodulin-bound and the autonomous forms of CaMKII is expected to reveal differences in substrate specificity of the kinase in the presence and absence of Ca2+. For the majority of the identified sites, the Ca2+/calmodulin bound form of CaMKII appears to be more effective than the autonomous form in promoting phosphorylation (Table 1, columns 2 and 3), in agreement with the notion that T-286/7 phosphorylation confers only partial activity. On the other hand, relative phosphorylation levels in the presence and absence of Ca2+ are different for different sites indicating site-specificity in the effectiveness of the calmodulin-bound and autonomous forms of CaMKII. For example Densin-180 is more efficiently phosphorylated in the presence of the Ca2+/calmodulin while phosphorylation on three different sites on SAPAP1, show differing sensitivities, with relative phosphorylation levels by the autonomous versus Ca2+/calmodulin bound forms (column 3) ranging from 1.14 to 0.62 (Table 1). Interestingly, all three sites on SynGAP show higher levels of phosphorylation in the absence of Ca2+, when autonomous activity is switched on (Figure 2).
Preferential phosphorylation by the autonomous CaMKII may be due to changes in the juxtaposition of proteins within the PSD in the presence of Ca2+/calmodulin or to the occlusion of phosphorylation sites by bound calmodulin. Indeed, an early study [23] describes CaMKII-mediated phosphorylation at certain sites on cyclic nucleotide phosphodiesterase that are phosphorylated only by the autonomous form of the kinase and blocked by calmodulin binding. Occlusion by calmodulin also accounts for the differential autophosphorylation of CaMKII obseved previously [15,16], and in this study on S-314/5, as discussed above.
An additional factor that may contribute to differential phosphorylation in the presence and absence of Ca2+ is phosphatase activity. Because a phosphatase, calcineurin, would be activated in the presence of Ca2+, differing levels of phosphorylation in the presence and absence of Ca2+ may reflect differences in calcineurin sensitivity.
It is generally accepted that acquisition of autonomous activity upon autophosphorylation of CaMKII on T-286 prolongs CaMKII-mediated phosphorylation after the cessation of the Ca2+ signal. Differences in the effectiveness of phosphorylation of different residues on CaMKII, and on CaMKII substrates during and after a Ca2+ rise, may confer an additional dimension to CaMKII-mediated signaling.
The structural organization of the PSD is determined by a group of specialized “scaffolds proteins” with several protein-protein interaction domains that can bind receptors, channels and signal transduction molecules. These scaffold proteins also bind to each other, thus forming the structural framework of the postsynaptic complex –the basic organization appears to be PSD-95 family of MAGUKs binding to SAPAPs (GKAPs) which in turn bind to Shanks (review [1]). In addition, PSDs contain other specialized scaffold proteins including Densin-180 which binds to alpha-actinin, CaMKII and Shank (review [24]) and spinophilin binds to protein phosphatase-1, actin, as well as to a number of receptors and signaling molecules (review [25]).
We show that group of conserved serine residues near the C-termini of SAPAP1, 2 and 3 (S-947, S-1012 and and S-930 respectively) are phosphorylated upon activation of CaMKII. The extreme C-termini of SAPAPs are involved in binding to Shanks [26]. An isoform of SAPAP1, called GKAP1b, (Q9D415-4) which does not contain a major portion of the C-terminal sequence, including the conserved serine residue, cannot bind to Shanks [26]. Thus, it is conceivable that phosphorylation of SAPAPs at the conserved C-terminal serines regulates SAPAP-Shank association.
We also observe involvement of CaMKII in the phosphorylation of two sites on Shank3 (S-769 and S-1586) and of one site on Densin-180 (S-831/T-832). Previous phosphoproteomic analyses of PSD fractions showed that the above-mentioned residues on SAPAPs, Shank3 and Densin 180-are at least partially phosphorylated in intact tissue [7, 8], thus indicating that the observed phosphorylation events are not an artifact of in vitro conditions. On the other hand, two other sites shown on Table 1, one on SAPAP1 (S-696) and the other on Shank1 (S-783) do not appear in a phosphoprotein database (PhosphoSite). Failure of detection of these sites in phosphoproteomic studies may be due low levels of in vivo phosphorylation or selective post-mortem dephosphorylation. Alternatively, phosphorylation at these sites may not occur in vivo.
CaMKII-mediated phosphorylation of PSD scaffold proteins listed in Table 1 may be involved in the induction of LTP. Indeed, in many brain regions, LTP requires CaMKII activation. While CaMKII-mediated phosphorylation of a pivotal element, the GluR1 subunit of AMPA receptors, has been documented in conjunction to LTP induction [27], additional CaMKII mediated phosphorylation events are implied [28]. One postulated mechanism of LTP induction predicts creation of new postsynaptic “slots” for the anchoring of newly inserted AMPA receptors [29]. Phosphorylation-induced changes in the PSD scaffold could underlie formation of new slots for AMPA receptors. Demonstration of the action of PSD-associated phosphatases in reversing CaMKII-induced phosphorylation of scaffold proteins (Table 2) suggests that phosphorylation would be a transient step setting the stage for a permenant structural modification. Our results pave the way to future studies that would test phosphorylation of the identified residues on scaffold proteins during LTP and to further studies using phophorylation mutants to establish the role of individual residues in LTP induction.
TABLE 2.
Effect of endogenous phosphatase activity on the phosphorylation levels at identified residues at the PSD.
| Protein Accession number |
Phosphorylated Peptide | Residue | % Phosphorylation in the presence vs. absence of phosphatase inhibitor |
|---|---|---|---|
|
Densin-180 UniProtKB: P70587 |
R.IVGVPLELEQ[ST]*HR.H | S-831 or T-832 |
51% 64% |
|
SAPAP1 UniProtKB: P97836 |
K.ATQP[SLT]*ELTTLK.I | S-389 or T-391 |
60% |
|
SAPAP1 UniProtKB: P97836 |
K.FQS*VGVQVEEEK.C | S-696 | 45% |
|
SAPAP2 UniProtKB: P97837 |
R.EKS*LDLPDR.Q | S-1012 | 76% 80% |
|
SAPAP3 UniProtKB: P97838 |
K.ERS*LDSVDR.Q | S-930 | 53% 58% |
|
Shank1 UniProtKB: Q9WV48 |
K.RLPPPAIS*LR.S | S-783 | 31% 54% |
|
Shank3 UniProtKB: Q9JLU4 |
R.SK[SMT]*AELEELASIR.R | S-769 or T-771 |
85% 59% |
|
Shank3 UniProtKB: Q9JLU4 |
R.S*LGEEPVGGLGSLLDPAK.K | S-1586 | 89% 86% |
|
Spinophilin UniProtKB: O35274 |
R.A[SS]*LNENVDHSALLK.L | S-99 or S-100 |
41% |
|
SynGAP UniProtKB: Q9QUH6 |
R.DLN[SS]*IDLQSFMAR.G | S-765 or S-766 |
64% 81% |
|
SynGAP UniProtKB: Q9QUH6 |
R.GKS*QQLTVSAAQKPR.P | S-1073 | 83% 72% |
|
SynGAP UniProtKB: Q9QUH6 |
K.EG[SIGGS]*GGSGGGGGGGLKPSITK.Q | S1114 or S1118 |
116% 88% |
PSD samples were incubated at 37°C for 10 min in media containing Ca2+ with or without phosphatase inhibitors (MicrocystinLR and NaF).
In conclusion, comparative mass spectrometric analysis of PSD fractions following phosphorylation under different incubation conditions yielded information on the regulation of phosphorylation at multiple residues on PSD components. Results on the regulation of CaMKII autophosphorylation are in agreement with numerous previous studies and, as such, validate the present approach. Comparison of phosphorylation of the PSD components under different activity states of CaMKII suggests site-specific differences in the efficiencies of the autonomous and Ca2+/calmodulin bound forms of CaMKII. Finally, our study reveals several CaMKII-regulated phosphorylation sites on PSD scaffolds, most notably three homologous residues on the C-termini of SAPAPs. These results point out to an involvement of CaMKII in activity-mediated modification of PSD organization.
Supplementary Material
Acknowledgments
This work was supported by the intramural research program at NIH/NINDS. We would like to thank Dr Thomas Reese for support and discussion.
Abbreviations
- CaMKII
Ca2+/calmodulin dependent protein kinase II
- LTP
long-term potentiation
- PSD
postsynaptic density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 2.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Reviews Neuroscience. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 3.Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffe H, Vinade L, Dosemeci A. Identification of novel phosphorylation sites on postsynaptic density proteins. Biochem Biophys Res Commun. 2004;321:210–218. doi: 10.1016/j.bbrc.2004.06.122. [DOI] [PubMed] [Google Scholar]
- 5.Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SG. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem. 2005;280:5972–5982. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- 6.Trinidad JC, Thalhammer A, Specht CG, Schoepfer R, Burlingame AL. Phosphorylation state of postsynaptic density proteins. J Neurochem. 2005;92:1306–1316. doi: 10.1111/j.1471-4159.2004.02943.x. [DOI] [PubMed] [Google Scholar]
- 7.Trinidad JC, Specht CG, Thalhammer A, Schoepfer R, Burlingame AL. Comprehensive identification of phosphorylation sites in postsynaptic density preparations. Mol Cell Proteomics. 2006;5:914–922. doi: 10.1074/mcp.T500041-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Trinidad JC, Thalhammer A, Specht CG, Lynn AJ, Baker PR, Schoepfer R, Burlingame AL. Quantitative analysis of synaptic phosphorylation and protein expression. Mol Cell Proteomics. 2008;7:684–696. doi: 10.1074/mcp.M700170-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Munton RP, Tweedie-Cullen R, Livingstone-Zatchej M, Weinandy F, Waidelich M, Longo D, Gehrig P, Potthast F, Rutishauser D, Gerrits B, Panse C, Schlapbach R, Mansuy IM. Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol Cell Proteomics. 2007;6:283–293. doi: 10.1074/mcp.M600046-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura Y, Aoi C, Yamauchi T. Investigation of protein substrates of Ca(2+)/calmodulin-dependent protein kinase II translocated to the postsynaptic density. Brain Research Molecular Brain Research. 2000;81:118–128. doi: 10.1016/s0169-328x(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura Y, Shinkawa T, Taoka M, Kobayashi K, Isobe T, Yamauchi T. Identification of protein substrates of Ca(2+)/calmodulin-dependent protein kinase II in the postsynaptic density by protein sequencing and mass spectrometry. Biochemical & Biophysical Research Communications. 2002;290:948–954. doi: 10.1006/bbrc.2001.6320. [DOI] [PubMed] [Google Scholar]
- 12.Thiel G, Czernik AJ, Gorelick F, Nairn AC, Greengard P. Ca2+/calmodulin-dependent protein kinase II: identification of threonine-286 as the autophosphorylation site in the alpha subunit associated with the generation of Ca2+-independent activity. Proc Natl Acad Sci U S A. 1988;85:6337–6341. doi: 10.1073/pnas.85.17.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schworer CM, Colbran RJ, Keefer JR, Soderling TR. Ca2+/calmodulin-dependent protein kinase II. Identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J Biol Chem. 1988;263:13486–13489. [PubMed] [Google Scholar]
- 14.Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- 15.Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- 16.Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990;265:11204–11212. [PubMed] [Google Scholar]
- 17.Dosemeci A, Gollop N, Jaffe H. Identification of a major autophosphorylation site on postsynaptic density-associated Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1994;269:31330–31333. [PubMed] [Google Scholar]
- 18.Migues PV, Lehmann IT, Fluechter L, Cammarota M, Gurd JW, Sim AT, Dickson PW, Rostas JA. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J Neurochem. 2006;98:289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- 19.Mullasseril P, Dosemeci A, Lisman JE, Griffith LC. A structural mechanism for maintaining the ‘on-state’ of the CaMKII memory switch in the post-synaptic density. J Neurochem. 2007;103:357–364. doi: 10.1111/j.1471-4159.2007.04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- 21.Oh JS, Manzerra P, Kennedy MB. Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. Journal of Biological Chemistry. 2004;279:17980–17988. doi: 10.1074/jbc.M314109200. [DOI] [PubMed] [Google Scholar]
- 22.Grossman SD, Futter M, Snyder GL, Allen PB, Nairn AC, Greengard P, Hsieh-Wilson LC. Spinophilin is phosphorylated by Ca2+/calmodulin-dependent protein kinase II resulting in regulation of its binding to F-actin. J Neurochem. 2004;90:317–324. doi: 10.1111/j.1471-4159.2004.02491.x. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto Y, Sharma RK, Soderling TR. Regulation of Ca2+/calmodulin-dependent cyclic nucleotide phosphodiesterase by the autophosphorylated form of Ca2+/calmodulin- dependent protein kinase II. J Biol Chem. 1989;264:10884–10887. [PubMed] [Google Scholar]
- 24.Thalhammer A, Trinidad JC, Burlingame AL, Schoepfer R. Densin-180: revised membrane topology, domain structure and phosphorylation status. J Neurochem. 2009;109:297–302. doi: 10.1111/j.1471-4159.2009.05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–1113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 27.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation [see comments] Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 29.Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.