Abstract
Background
A valine158methionine polymorphism in Catechol-O-methyltransferase (COMT) modulates cortical dopaminergic catabolism and has been associated with schizophrenia. Consistent with schizophrenia itself, during cognitive tasks, the risk (val) allele predicts less efficient prefrontal cortex (PFC) physiology and worse performance, while during aversive stimuli viewing, this allele predicts less limbic activation. Task-independent effects of this polymorphism in schizophrenia have not yet been characterized.
Methods
Twenty-five medication-free patients (28±6 years; 19 male) and forty-seven healthy individuals (29±8 years; 33 male) were genotyped for the COMT val158met polymorphism and underwent two 60-second [15O]H2O regional cerebral blood flow (rCBF) positron emission tomography (PET) scans (10 mCi/scan) during rest. Data were analyzed with a random-effects general linear model using COMT genotype as a covariate.
Results
In patients, but not healthy individuals, val (risk) allele load predicted less regional cerebral blood flow (rCBF) in the right dorsolateral PFC (DLPFC), right superior temporal gyrus, and left precuneus, but greater rCBF in the amygdala and parahippocampal gyrus.
Conclusions
In schizophrenia, brain structures important for executive and affective processing show activity that is differentially predicted by COMT allelic variation in an opposing manner even at rest, providing evidence for the salience of prefrontal dopaminergic tone in task-independent, basal-level neural activity.
Keywords: schizophrenia, COMT, PET, rest, prefrontal, limbic
Introduction
The val158met single-nucleotide polymorphism (SNP) in the catechol-O-methyltransferase (COMT) gene, which regulates dopamine catabolism, has garnered considerable attention in schizophrenia research (1). The high-activity (val) allele is weakly associated with illness risk (2) and results in inefficient prefrontal functioning and poor working memory performance in healthy and patient subjects (1;3). Conversely, the low-activity (met) allele is associated with anxiety and pain phenotypes and with greater limbic reactivity during viewing of aversive images (4). Remarkably complementary results have been described in COMT genetically-engineered mice (5).
Notably, previous imaging studies have used cognitive and affective tasks to demonstrate schizophrenia-associated abnormal dorsolateral prefrontal (DLPFC) and limbic physiology, respectively (6;7). Given continued evidence for aberrant prefrontal and limbic brain function in schizophrenia during rest (8;9) and the lack of data regarding COMT effects on resting neurofunctional topography in schizophrenia, we studied medication-free patients using [15O]H2O PET, hypothesizing that COMT genotype is also relevant to PFC and limbic systems function in the resting schizophrenic brain.
Methods and Materials
Twenty-five patients (ages 18-43, mean 27.5±6.2; 19 male, 23 Caucasian/1 African-American/1 Hispanic) meeting DSM-IV criteria for schizophrenia and 47 healthy individuals (ages 20-50, mean 28.7±7.5, 33 male, 47 Caucasian) participated after providing informed consent as per the NIMH IRB and NIH Radiation Safety Committee. COMT genotyping at the val158met locus employed the TaqMan 5′ exonuclease assay (10). Patients discontinued antipsychotic medications four weeks before PET scanning and Positive and Negative Symptom Scale (PANSS) ratings (11). Caffeine and nicotine were not permitted for four hours prior to scanning.
An Advance PET scanner (GE, Milwaukee, WI) in 3-dimensional mode was used to measure rCBF. After a transmission scan, participants were asked to quietly rest in the scanner with eyes closed during two sixty-second scans, six minutes apart, each following intravenous injection of [15O]H2O (10 mCi/injection). This PET method has the advantage of providing an unequivocal, absolute measurement of resting rCBF. After attenuation-correction and reconstruction, images were background-activity corrected, spatially normalized to Montreal Neurological Institute space, smoothed (10 mm3 full-width half-maximum Gaussian kernel), and proportionally scaled. Subsequently, each subject's scans were averaged then analyzed with general linear modeling using SPM99 (Wellcome Department of Cognitive Neurology, London, England). Using random-effects, regression analysis, we examined voxel-wise correlations between rCBF and a covariate defined as the number of COMT met alleles carried. Direct between-groups comparisons of genotype-rCBF relationships were accomplished by ANCOVA, as implemented in SPM, in which beta weights for two regressors – one representing genotypes of patients and the other genotypes of healthy volunteers – were contrasted. We used a p<0.001 voxel-wise height threshold and extent threshold of 10 voxels. Additionally, we applied a cluster-level correction of p<0.05 to results in neocortical regions (12). Finally, we conducted a confirmatory region of interest (ROI) analysis (see Supplement).
Results
Among patients, genotyping yielded five val/val, 12 val/met, and eight met/met; five val/val, eight val/met, and six met/met were male. Among healthy participants, there were 15 val/val, 21 val/met, and 11 met/met; 12 val/val, 13 val/met, and eight met/met were male. No significant deviation from Hardy-Weinberg equilibrium existed. Age did not significantly vary between genotype groups within patients, comparison subjects, or all participants.
PANSS ratings were available for all but one patient lost due to a transcription error. Average ratings (±SD) on the total PANSS (maximum 210), and Negative (maximum 49), Positive (maximum 49), and General Psychopathology (maximum 112) subscales were 74.7±25.9, 21.4±8.0, 18.2±8.5, and 35.1±12.6, respectively. These did not significantly vary between genotypes.
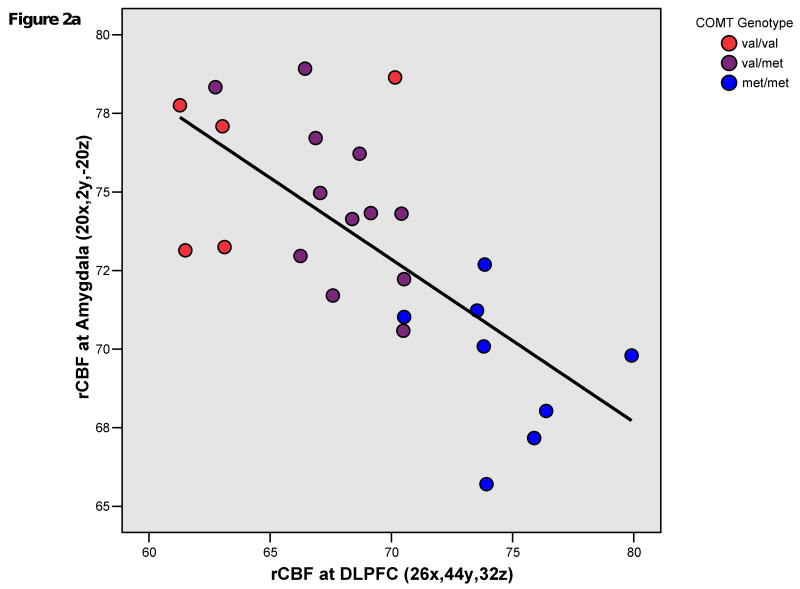
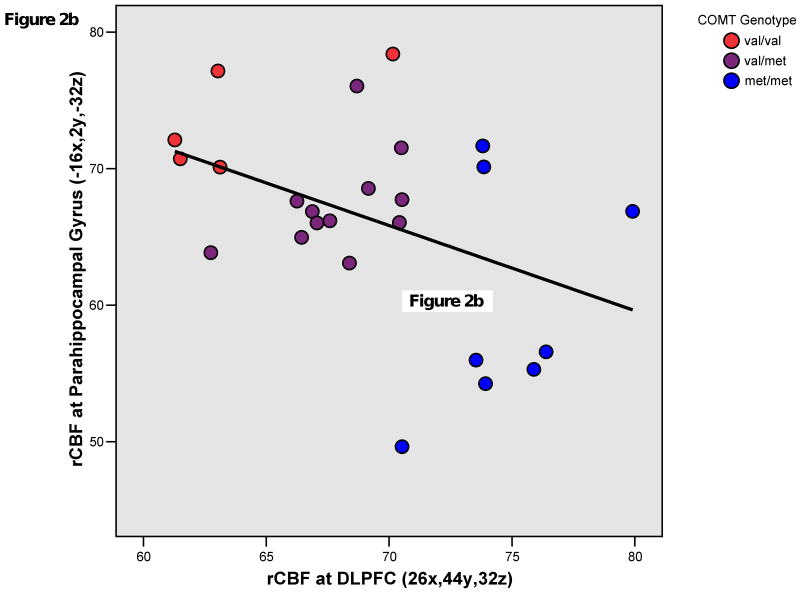
In patients, COMT met allelic load robustly and positively correlated with rCBF in a right middle frontal gyrus region (BA9/46; 26x,44y,32z). COMT met allelic load also positively correlated with rCBF in right superior temporal gyrus (BA22; 56x,-48y,16z) and precuneus (BA7; -2x,-70y,48z) regions. These findings were all significant after cluster-level correction. COMT val allelic load positively correlated with rCBF (p<0.001,k>10) at the right amygdala (BA34; 20x,2y,-20z) and left parahippocampal gyrus (BA28; -16x,2y,-32z). (Figures 1,2a,2b)
Figure 1.
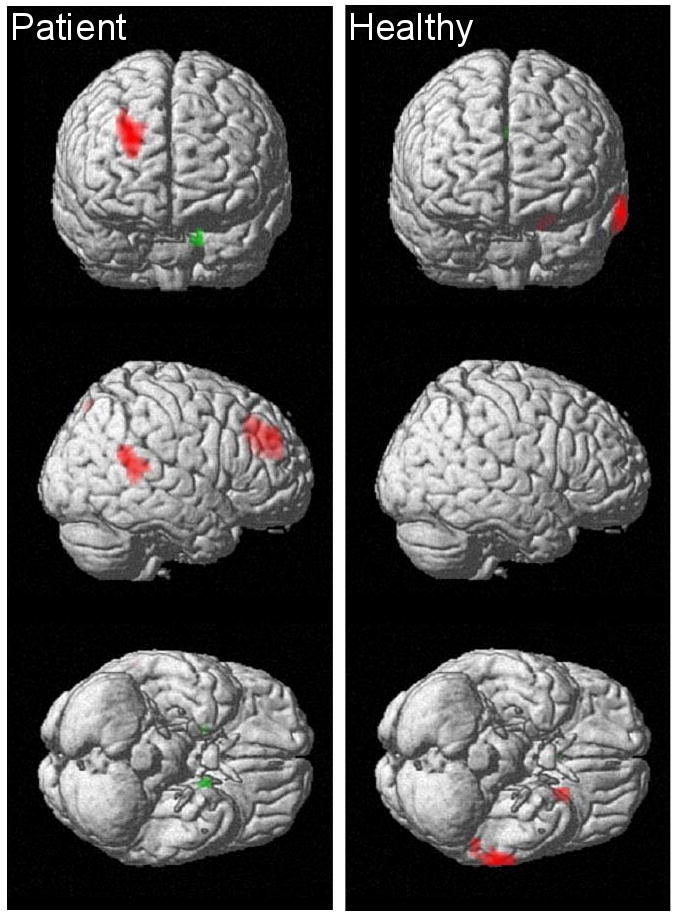
Statistical PET maps showing areas of significant correlation between COMT genotype and mean resting regional cerebral blood flow (rCBF) in twenty-five, medication-free patients with schizophrenia (right images) and forty-seven healthy volunteers (left images). Red indicates a correlation with the number of met alleles carried and green indicates a correlation with the number of val alleles carried. All regions shown meet an uncorrected voxel-wise height threshold of p<0.001 and extent threshold of 10 voxels. Additionally, neocortical regions meet a cluster-level correction of p<0.05. The right frontal, right superior temporal, precuneus, and left parahippocampal regions indicated in patients also showed significant diagnostic group interactions, indicating the absence of these COMT effects in healthy participants. None of the regions indicated in healthy volunteers showed significant between-diagnostic group differences.
Figure 2.
Figure 2a. Plot of mean resting regional cerebral blood flow (rCBF) in right dorsolateral prefrontal cortex (DLPFC) and amygdala at the representative peak voxels of clusters showing significant correlation with COMT genotype in patients with schizophrenia. Pearson's r = -0.701; p<0.001; R Sq Linear = 0.491.
Figure 2b. Plot of mean resting regional cerebral blood flow (rCBF) in right dorsolateral prefrontal cortex (DLPFC) and left parahippocampal gyrus at the representative peak voxels of clusters showing significant correlation with COMT genotype in patients with schizophrenia. Pearson's r = -0.418; p = 0.038; R Sq Linear = 0.175.
In healthy participants, COMT met allelic load positively correlated with rCBF in left orbital cortex (BA47; -22x,14y,-16z) and middle temporal gyrus (BA21; -66x,-30y,-12z), and COMT val allelic load positively correlated with rCBF in a cingulate gyrus (14x,8y,28z) region. No other regions showed significant COMT effects in this group. In parametric analyses comparing rCBF-genotype relationships between patients and healthy participants, only the right middle frontal gyrus (BA9/46; 26x,42y,32z), adjacent inferior frontal gyrus (BA45; 60x,22y,16z), superior temporal gyrus (BA22; 60x,-48y,20z), left precuneus (BA7; -6x,-68y,48z), and parahippocampal gyrus (BA28; -16x,0y,-32z) showed significant between-diagnostic-group differences, reflecting the robust COMT effects in these regions in patients but not comparison subjects.
Discussion
In patients, we identified a relationship between COMT genotype and resting rCBF in brain structures that are important in schizophrenia pathophysiology and where COMT effects during executive and affective tasks have been previously demonstrated. These findings distinguished patients from healthy participants, suggesting that in schizophrenia, resting-state neurophysiology is differentially predicted by genetic variation in COMT-mediated dopaminergic catabolism. Unlike prior investigations, all patients were medication-free, ensuring that these results are not confounded by concurrent neuroleptic effects, a particular concern when assessing dopamine-related parameters, such as COMT genotype that directly impacts intrasynaptic dopamine levels.
In schizophrenic but not healthy participants, a robust correlation existed between DLPFC rCBF and COMT genotype, extending the substantial literature linking task-associated DLPFC physiology with the val158met SNP (3;13;14). Because of the relatively sparse concentration of the dopamine transporter in prefrontal cortex, COMT plays a specific and potent role in controlling prefrontal synaptic dopaminergic tone (1), suggesting that the present finding reflects variation in DLPFC dopamine signaling. Prefrontal cortical dysfunction and abnormal dopamine neurotransmission have long been implicated in schizophrenia pathophysiology and may explain this remarkable sensitivity of prefrontal rCBF to genotype-related differences in dopaminergic tone, even in the absence of an explicit task. Indeed, in line with prior PET studies demonstrating reduced DLPFC rCBF during challenging executive tasks or resting-state paradigms in individuals with schizophrenia (15), possession of the putative risk (val) allele was associated with lower DLPFC resting rCBF.
Again, in patients but not healthy subjects, the reverse association existed between limbic rCBF and COMT genotype; val carriers demonstrated greater rCBF in the parahippocampal gyrus and amygdala. Notably, previous research has documented COMT effects in these regions during fMRI affective activation paradigms in healthy participants (4;14). The opposing genotype effects on rCBF in limbic regions and DLPFC (Figures 2a,b) not only are consistent with a dual (affective versus cognitive) role for COMT, as further substantiated by behavioral experiments in genetically-engineered mice (5), but also suggest that regionally-specific COMT regulation of catecholaminergic tone is important in understanding illness-associated abnormalities of reciprocal frontolimbic interactions (16) as a genetically-determined trait.
The DLPFC and limbic genotype effects in this resting study – in opposite directions from cognitive and affective task experiments, respectively – highlight the context-dependent nature of COMT influences on neural activity and suggest that during rest, patients are not simply in an attenuated cognitive or emotional state akin to previous experimental task paradigms, which would likely result in attenuated but directionally congruent effects of COMT. During explicit cognitive or emotional tasks, in which external demands require increased dopamine-dependent neural activity, lower dopaminergic tone in val carriers restricts prefrontal efficiency (requiring greater activation at a given performance level) during executive challenge (1;3) but limits limbic reactivity during exposure to emotionally evocative stimuli (4). However, at rest, an internally-driven and necessarily uncontrolled state, it may be that val-carrying patients bias neural activity, and perhaps even mental processes, toward those systems that are more functionally robust to challenge (i.e. greater limbic and less prefrontal signaling), providing a potential, mechanistic explanation for how dopaminergic tone could impact rCBF differentially in disparate brain structures even at rest.
Though our study is not powered to confirm negative findings, the lack of genotype effects in the DLPFC and limbic regions of healthy individuals suggests that in the neuropsychiatrically intact brain, COMT-determined dopaminergic tone is not as critical a determinant of resting neural activity in these regions. We speculate that in schizophrenia, frontolimbic and/or dopaminergic system dysfunction – and perhaps associated psychopathology – allows for greater impact of COMT effects on interindividual differences in resting brain physiology. In healthy individuals, who have at their disposal a greater arsenal of neural resources to maintain cognitive flexibility and affective resilience, the above postulated neural biases might be less apt to develop. Additionally, the resting state in healthy individuals is unencumbered by psychotic thought processes that could also potentially contribute to the observed genotype-diagnosis interaction.
This is the first report of a relationship between the val158met SNP and activity in the superior temporal lobe (STL) in schizophrenia, a finding that merits future investigation, given substantial literature linking STL abnormalities with schizophrenia, particularly in morphometric experiments (17) and fMRI studies of formal thought disorder (18). Consistent with these results, a SNP (rs2097603) in the P2 promoter region of COMT is associated with right STL gray matter volume variability in schizophrenia spectrum illness (19), inviting speculation that medication-free, resting-state rCBF may be a sensitive measure for detecting functional COMT genotype STL effects in schizophrenia.
Given male-female differences in COMT gene expression (1), future work powered to examine sex-specific effects on COMT-resting rCBF relationships in schizophrenia is needed. Additionally, because nicotine interacts with central dopaminergic systems and smokers were not excluded, it is possible that tobacco use could have contributed to the present findings. To minimize this possibility, we required four hours of abstinence from nicotine products prior to testing procedures but cannot exclude very early withdrawal effects. These considerations aside, the current study provides new evidence that in schizophrenia, cerebral structures previously identified during task-dependent paradigms show COMT genotype effects even in the resting state and without the confound of psychotropic medications.
Supplementary Material
Acknowledgments
We would like to thank the staff of the NIH PET Center and the NIMH GCAP Inpatient Program for their assistance in data acquisition.
This research was supported by the Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, 20892.
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 2.Allen NC, Bagade S, McQueen MB, Ioannidis JPA, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 3.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, et al. Catechol-O-Methyltransferase val158met Genotype Affects Processing of Emotional Stimuli in the Amygdala and Prefrontal Cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic Dissection of the Role of Catechol-O-Methyltransferase in Cognition and Stress Reactivity in Mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, et al. An fMRI Study of Facial Emotion Processing in Patients With Schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 7.Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, et al. Physiological Dysfunction of the Dorsolateral Prefrontal Cortex in Schizophrenia Revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 8.Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological Psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, Jr, McMahon R. Correlations Between rCBF and Symptoms in Two Independent Cohorts of Drug-Free Patients with Schizophrenia. 2005;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 12.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting Activations in PET and fMRI: Levels of Inference and Power. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 13.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–6. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 14.Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 15.Berman K, Meyer-Lindenberg A. Functional brain imaging studies in schizophrenia. In: Charney D, Nestler E, editors. Neurobiology of Mental Illness. 2nd. New York: Oxford University Press; 2004. pp. 311–322. [Google Scholar]
- 16.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–86. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 17.Honea R, Crow TJ, Passingham D, Mackay CE. Regional Deficits in Brain Volume in Schizophrenia: A Meta-Analysis of Voxel-Based Morphometry Studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 18.Kircher TTJ, Bulimore ET, Brammer MJ, Williams SCR, Broome MR, Murray RM, et al. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophrenia Research. 2001;50:27–40. doi: 10.1016/s0920-9964(00)00042-6. [DOI] [PubMed] [Google Scholar]
- 19.Zinkstok J, Schmitz N, van Amelsvoort T, Moeton M, Baas F, Linszen D. Genetic variation in COMT and PRODH is associated with brain anatomy in patients with schizophrenia. Genes Brain Behav. 2008;7:61–9. doi: 10.1111/j.1601-183X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.