Abstract
Nucleostemin (NS) is a nucleolar protein abundantly expressed in a variety of proliferating cells and undifferentiated cells. Its known functions include cell cycle regulation and the control of pre-rRNA processing. It also has been proposed that NS has an additional role in undifferentiated cells due to its downregulation during stem cell differentiation and its upregulation during tissue regeneration. Here, however, we demonstrate that skeletal muscle cell differentiation has a unique expression profile of NS in that it is continuously expressed during differentiation. NS was expressed at similar levels in non-proliferating muscle stem cells (satellite cells), rapidly proliferating precursor cells (myoblasts) and post-mitotic terminally differentiated cells (myotubes and myofibers). The sustained expression of NS during terminal differentiation is necessary to support increased protein synthesis during this process. Downregulation of NS inhibited differentiation of myoblasts to myotubes, accompanied by striking downregulation of key myogenic transcription factors, such as myogenin and MyoD. In contrast, upregulation of NS inhibited proliferation and promoted muscle differentiation in a p53-dependent manner. Our findings provide evidence that NS has an unexpected role in post-mitotic terminal differentiation. Importantly, these findings also indicate that, contrary to suggestions in the literature, the expression of NS cannot always be used as a reliable indicator for undifferentiated cells or proliferating cells.
Keywords: myogenesis, nucleostemin, p53, protein synthesis
Introduction
Nucleostemin (NS) is a nucleolar protein that regulates cell proliferation [1, 2]. Up- and downregulation of NS result in cell cycle arrest, primarily through interactions with the tumor suppressor protein p53. Several reports have also suggested that NS might serve as a marker for an undifferentiated or dedifferentiating state. NS is highly expressed in neural stem cells, embryonic stem cells and cardiac stem cells and downregulated during differentiation [2, 3]. The downregulated NS can be upregulated in the regions surrounding cardiac infarction sites. NS level is also downregulated during the differentiation of bone marrow stem cells during their differentiation into chondrocytes, adipocytes or osteocytes [4]. During regeneration of a newt lens, NS accumulates in the dedifferentiating pigmented epithelial cells two days before they reenter the cell cycle [5]. Additionally, NS is expressed in degenerating multinucleated muscle fibers during limb regeneration in a newt before formation of the blastema, a multipotent stem cell-like population [5]. However, we have recently found that NS has another function as a regulator of pre-rRNA processing and consequently ribosome synthesis [6]. This finding prompted us to hypothesize that NS might be also expressed in non-proliferating cells, including terminally differentiated cells, as long as they are actively synthesizing proteins. We tested this hypothesis using the differentiation process of skeletal muscle cells as an experimental model.
Myogenic stem cells, called satellite cells [7], are mitotically quiescent in adult muscle and their protein synthesis level is relatively low. However, they will initiate proliferation and enhance protein synthesis upon stimulation by weight bearing or through damage. The progeny of activated satellite cells, now called myoblasts, undergo multiple rounds of cell division prior to terminal differentiation and formation of multinucleated myotubes by cell fusion [7]. Nuclei in myotubes are generally post-mitotic. During maturation myotubes continuously enlarge through additional cell fusion as well as increased cytoplasmic volume per nucleus, resulting in functional myofibers with the capability of contraction. Myotube formation and myofiber maturation are characterized by a striking increase in protein synthesis. For instance, chicken embryonic myotubes increase their protein amount per nucleus more than tenfold in ten days [8]. Mouse myotubes derived from C2C12 cells increase their protein synthesis rate fourfold in two days [9]. Thus, skeletal myogenesis provides an experimental model in which a radical increase of protein synthesis can be separated from cell proliferation. Taking advantage of this feature in skeletal muscle cells, in the current work we uncovered a unique expression pattern of NS and its role during skeletal myogenesis.
Materials and methods
Myoblast culture and preparation of muscle sections
p53+/− mice, homozygous dystrophin gene mutant (DMDmdx-5Cv) mice and wild-type BALB/c and C57BL/6 mice were purchased from Jackson Laboratory. p53−/− mice were generated by crossing p53+/− parent mice. Satellite cell-derived myoblasts were isolated from the hind limbs of two-month-old wild-type BALB/c mice, p53−/− mice and DMDmdx-5Cv mice [10]. The myoblasts were maintained in collagen-coated dishes in myoblast growth medium consisting of HAM’s F-10 medium supplemented with 20% fetal bovine serum (FBS) and 5 ng/ml basic fibroblast growth factor (FGF) (R&D Systems). To induce differentiation of myoblasts, the culture medium was replaced with differentiation medium that contained Dulbecco’s Modified Eagle Medium (DMEM) with 5% horse serum on Day 0. The cells were harvested on Day 0 (before switching to the differentiation medium), 1 and 3 for Western blotting and immunostaining. Frozen sections were prepared from the tibialis anterior muscle of fetal and two-month-old C57BL/6 mice and DMDmdx-5Cv mice. The protein amount in the Day 0, 1 and 3 cells was measured with a Quant-iT Protein Assay kit (Invitrogen). Single myofibers were isolated from the extensor digitorum longus muscle prepared from one to two-month-old wild-type BALB/c mice by digestion as previously described [11].
Fluorescent Activated Cell Sorting (FACS)
Satellite cells were isolated from the hind limb skeletal muscle of one to two-month-old mice [12]. The sources of the antibodies are listed in Supplementary Table 1. Sorting gates were strictly defined based on single antibody-stained control cells, as well as the forward scatter (FSC) and side scatter (SSC) patterns of satellite cells. After the FSC/SSC gating the triple-negative cells for CD45- phycoerythrin (PE), CD31-PE and Sca-1-PE were gated out. Lastly, double-positive cells for integrin 7-Alexa 488 and integrin 1-APC were sorted to enrich for satellite cells [13].
Knockdown of NS
Myoblasts were transfected with NS or control siRNA using Lipofectamine 2000 (Invitrogen) on Day -3, -2 and -1 while cultured in the growth medium. The culture medium was replaced with the differentiation medium on Day 0 and the cells were harvested on Day 0 (before exposed to the differentiation medium), 1 and 3 for immunostaining and Western blotting. The sequences of NS siRNA and control siRNA were described before [6].
Metabolic labeling of protein with 35S methionine
The abovementioned Day 0 myoblasts were washed and cultured in methionine-free DMEM containing 5% dialyzed FBS for 1 hr. After addition of L-[35S] methionine (GE Healthcare) to a final concentration of 15 μCi/ml, the cells were incubated for an additional 4 hrs. Whole cell extracts prepared from these cells were resolved by SDS-PAGE at 2×105 nuclei equivalent per well and analyzed by autoradiography of dried gels.
Overexpression of NS
NS cDNA with a 6xHis tag at the carboxy terminus was inserted into the retrovirus vector plasmid pMXs [14]. The empty pMXs plasmid or the pMXs-NS plasmid was transfected into the packaging cell line Plat-E [15] with Lipofectamine 2000 to prepare retrovirus. Wild-type or p53−/− myoblasts were infected with the empty virus or the NS virus for 3 hrs. For a proliferation assay, cell number was counted with trypan blue staining on Day 0 (the day after transfection), 1, 2 and 3 while maintained in the growth medium. For a differentiation assay, the culture medium was replaced with the differentiation medium the day after transfection as Day 0 and the cells were harvested on Day 0 (before exposed to the differentiation medium), 1 and 3 for Western blotting and immunostaining.
Antibodies
Antibodies used for immunostaining and Western blotting are listed in Supplementary Table 1.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was purified from muscle cells with a PureLink RNA Purification System (Invitrogen) and treated with DNase I as indicated by the manufacturer. Primer sequences are listed in Supplementary Table 2. The relative amount of each mRNA was calculated as follows. ΔCT (threshold cycle) of each mRNA = average CT value of each mRNA obtained from three PCRs − average CT value of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA obtained from three PCRs. ΔΔCT of each mRNA = ΔCT of the cells infected with the empty or the NS-expressing retrovirus on each day − ΔCT of the Day 0 cells infected with the empty retrovirus. Relative expression (fold) = 2−ΔΔCT. For all these analyses mean and standard error of the mean (SEM) were calculated from the values obtained from three independent experiments.
Results and discussion
NS is expressed in terminally differentiated skeletal muscle cells
To understand the expression level of NS during skeletal muscle differentiation, sections of the tibialis anterior muscle of wild-type fetal mice were co-immunostained with anti-NS antibody and anti-dystrophin antibody. Dystrophin, a protein localized under the plasma membrane [16], was used to identify NS signals located within the cytoplasm of myofibers. NS signals were clearly detectable in the centrally located nuclei of a mouse embryo 20 days post coitum (Fig. 1A, 20 dpc). Centrally located nuclei are characteristic for newly formed and terminally differentiated myofibers of fetal mice and regenerating muscle in adult [7, 17]. During maturation these nuclei migrate to the periphery of the cytoplasm and majority of myofibers in adult mice contain peripherally located nuclei. The peripherally located nuclei expressed NS as demonstrated by NS-positive nuclei underneath the dystrophin-positive plasma membrane in the wild-type adult tibialis anterior muscle sections (Fig. 1A, 1B). We also examined the expression of NS in adult dystrophin mutant mice (DMDmdx-5Cv), an animal model for Duchenne and Becker type muscular dystrophy. In these mice myofibers are constantly degenerating and regenerating, and display a large number of regenerating myofibers with centrally located nuclei [7]. These centrally located nuclei also expressed NS as shown by co-immunostaining of integrin α7, which was stained to locate the plasma membrane instead of the absent dystrophin epitopes (Fig. 1A). These results indicated that NS is expressed at significant levels in terminally differentiated skeletal muscle cells in embryonic, normal adult and regenerating adult muscle tissues.
Figure 1. Expression of NS during differentiation of skeletal muscle cells.
(A) and (B) Immunofluorescence staining of sections prepared from the tibialis anterior muscle of 20 dpc and adult wild-type mice and an adult DMDmdx-5Cv mouse. The sections were double immunostained with a combination of anti-dystrophin antibody and anti-NS antibody or with a combination of anti-integrin α7 antibody and anti-NS antibody. DNA was counterstained with DAPI. Arrowheads indicate NS signals in centrally located nuclei in myofibers. The areas denoted by arrows in the adult wild-type mouse section were enlarged threefold in (B) to demonstrate the presence of NS-positive nuclei inside the plasma membrane of myofibers. Bar, 30 μm in (A) and 10 μm in (B).
(C) Expression pattern of NS during differentiation of mouse myoblasts in vitro. Bar, 40 μm.
(D) Myoblasts (Day 0) and myotubes on Day 1 and Day 3 were applied for Western blotting with anti-NS antibody. Histone H2B was monitored as a loading control. Whole cell extract corresponding to 2×105 nuclei was loaded in each well.
(E) The total amount of cellular protein was compared among the muscle cells harvested on Day 0, 1 and 3. Protein amount in 1×106 muscle cells was divided by the number of DAPI-stained nuclei to determine the normalized protein amount per nucleus. The relative protein amount per nucleus on each day was calculated by using the normalized protein amount in the Day 0 cells as 100%. Results obtained from three sets of experiments are presented as mean + SEM.
We next compared the protein levels of NS between undifferentiated proliferating muscle cells (myoblasts) and terminally differentiated non-proliferating muscle cells (myotubes) in vitro. Primary myoblasts were prepared from adult muscle satellite cells and initially maintained in the growth medium to prevent differentiation (Day 0). The medium was then replaced with the differentiation medium and the cells were fixed for immunostaining on Day 1 and 3. The expression of sarcomeric myosin heavy chain (MHC) and cell fusion (appearance of multinuclei in a single cell) were monitored as definitive indicators for myotubes [10]. As shown in Fig. 1C, NS was detected at similar levels in the nucleoli on Day 0, 1 and 3. The expression levels of NS in these cells were also compared by Western blotting. To normalize the decrease in cell number due to cell fusion, muscle cell extracts corresponding to the same number of nuclei were loaded into each well of an SDS-PAGE gel. The amount of NS per nucleus did not visibly change after the induction of differentiation (Fig. 1D) while the total amount of cellular protein per nucleus increased 1.4 ± 0.07 times by Day 3 (Fig. 1E). These results indicated that muscle cells maintain a stable expression level of NS during in vitro differentiation.
NS is expressed in quiescent and activated satellite cells
The above expression pattern of NS led us to investigate if NS is also expressed in the earlier stage of myogenesis, satellite cells. We co-immunostained tibialis anterior muscle sections of adult mice with anti-NS antibody and anti-Pax7 antibody. The transcription factor Pax7, which is expressed in satellite cells and myoblasts but not in myotubes, is essential for satellite cell specification and survival [18]. NS signal was clearly detectable in 71 ± 8% of the Pax7-positive satellite cell nuclei in the muscle sections (Supplementary Fig. 1A and 1C). To verify this observation we co-immunostained single myofibers freshly isolated from the extensor digitorum longus muscle of adult mice (Supplementary Fig. 1B). NS was also clearly detected in 82 ± 5% of the Pax7-positive satellite cell nuclei in these myofibers (Supplementary Fig. 1C). Thus, NS is also expressed in satellite cells which are mitotically quiescent under physiological conditions in adult mice.
Previous works demonstrated that quiescent satellite cells can be prospectively isolated by FACS as a CD45− CD31− Sca-1− integrin α7+ integrin β1+ fraction [19]. We purified these cells with a FACS (Supplementary Fig. 1D) and confirmed the purification quality of the cells using the expression of the satellite cell markers, Pax7, c-Met and CD34 (data not shown). Immunostaining showed that the purified satellite cells also expressed NS in their nuclei (Supplementary Fig. 1E, 1F, Day 0). The cells were cultured to obtain activated satellite cells which initiated cell division. Immunostaining showed NS signals in most of the activated satellite cells on Day 2 and 4 (Supplementary Fig. 1E, 1F). Collectively, NS is expressed during muscle development and differentiation regardless of their cell proliferation status, including both quiescent and activated stem cells (satellite cells), proliferating precursor cells (myoblasts) and terminally differentiated post-mitotic cells (myotubes and myofibers).
Knockdown of NS inhibits muscle cell differentiation
To understand if NS is necessary for the differentiation of muscle cells, we knocked down NS in myoblasts and induced differentiation of the cells. The efficiency of siRNA transfection, monitored with fluorescence-labeled siRNA, was higher than 90% in preliminary experiments (data not shown). Western blotting and immunostaining of the Day 0 cells confirmed highly efficient knockdown of NS in the myoblasts (Fig. 2A, 2B). A minor population of myoblasts (generally lower than 10%) tended to spontaneously differentiate in the growth medium and expressed MHC; however, this tendency was inhibited by NS siRNA (Fig. 2B, 2C, Day 0). This inhibition of differentiation became more obvious when the cells were induced to differentiate. Only 19 ± 7% of the cells transfected with NS siRNA expressed a detectable level of MHC on Day 1, compared with 43 ± 5% of the cells transfected with control siRNA (Fig. 2B, 2C, Day 1). This delay in differentiation, however, was almost completely overcome by Day 3, accompanied by a significant increase in NS levels in the NS-depleted cells, which likely reflects rapid turnover of siRNA.
Figure 2. Delayed muscle cell differentiation by NS depletion.
(A) Western blotting of Day 0 myoblast extract after transfection of siRNAs.
(B) Differentiation pattern of muscle cells after siRNA transfection. Cells were double immunostained with anti-NS antibody and anti-MHC antibody. Bar, 40 μm. (C) Mean + SEM of the ratio between the number of DAPI-positive nuclei in MHC-positive cells and the total number of DAPI-positive nuclei shown in (B) obtained from three independent experiments. Two hundred nuclei were counted in each experiment. Asterisks indicate pairs where differences were statistically significant (t-test, P < 0.05).
(D) Autoradiograph of an SDS-PAGE gel loaded with Day 0 myoblast extracts after siRNA transfection. These myoblasts were metabolically labeled with 35S methionine for 4 hrs while cultured in the growth medium.
(E) Relative intensity of the total radioactivity in each lane shown in (D). Each lane in the dried gel was excised and the radioactivity was measured with a scintillation counter. Radioactivity of the control siRNA lane was defined as 100%.
Knockdown of NS decreases the overall protein synthesis rate (uptake of 35S methionine) by approximately 25% in HeLa cells, probably by disrupting ribosome synthesis [6]. The observed delay in muscle differentiation by NS knockdown could be due to the downregulation of some key transcription factors for muscle differentiation, such as MyoD and myogenin. MyoD is abundantly expressed in myoblasts and myogenin is upregulated in myocytes (post-mitotic mononuclear muscle cells) and myotubes, which both spontaneously appear in myoblast culture [20]. As we expected, the levels of these two proteins were drastically decreased in the NS-depleted Day 0 myoblasts (Fig. 2A) while overall protein synthesis rate remained 62 ± 9% of that in the control cells (Fig. 2D, 2E). The extremely short half-lives of MyoD and myogenin (30–60 min and 20–30 min for each protein) could be a major reason for their disproportionately severe depletion compared with the decrease of the total protein synthesis rate [21, 22]. The significant loss of MyoD, myogenin and potentially other key transcription factors almost certainly contributed to the delay in muscle differentiation observed in these experiments.
Overexpression of NS promotes muscle differentiation
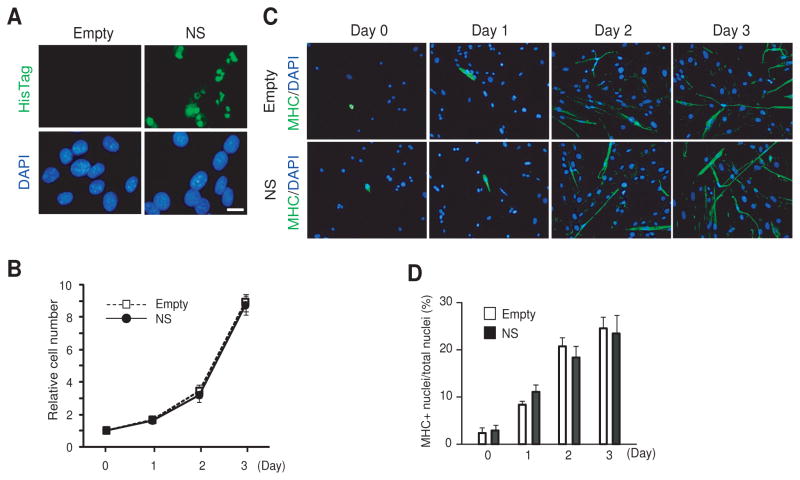
As a complementary study we investigated its effects of overexpressed NS on myoblast differentiation. Infection with NS retrovirus resulted in nucleolar expression of the 6xHis-tagged NS in more than 90% of myoblasts (Fig. 3A). Overexpression of NS in myoblasts reduced cell proliferation compared with control myoblasts infected with the empty retrovirus while cultured in the growth medium (Fig. 3B). In addition, NS overexpression facilitated muscle differentiation in the differentiation medium (Day 1–3) when compared with the control myoblasts (Fig. 3C, 3D). This facilitation of muscle differentiation was accompanied by clear upregulation of myogenin, as well as a more subtle increase in myotube-specific proteins, such as -sarcomeric actin, muscle creatine kinase (MCK) and MHC, compared with control cells (Fig. 3E). The facilitation of differentiation could potentially be explained by the increased level of myogenin [23]. It is known that upregulation of NS does not increase the global protein synthesis rate [6]. Indeed, levels of desmin and MyoD, expressed in both myoblasts and myotubes, did not show any apparent increases during this process. The increase in the levels of myogenin, sacromeric actin and MCK following NS overexpression appears to be regulated at the post-transcriptional level because the mRNA levels of these proteins were not significantly altered by the overexpressed NS (Fig. 3F). Details of this regulatory mechanism remain unknown.
Figure 3. Facilitation of muscle cell differentiation by overexpressed NS.
(A) Myoblasts infected with the empty or the NS-expressing retrovirus were immunostained with anti-6xHis tag antibody to detect NS. Bar, 10 μm.
(B) Comparison of cell proliferation rates between myoblasts infected with the empty retrovirus and the NS-expressing retrovirus. The cells were cultured in the growth medium.
(C) Immunostaining of muscle cells with anti-MHC antibody after infection with the empty or the NS-expressing retrovirus. The cells were cultured in the differentiation medium. Bar, 20 μm.
(D) Mean + SEM of the ratio between the number of DAPI-positive nuclei in MHC-positive cells and the total number of DAPI-positive nuclei shown in (C) obtained from three independent experiments. One hundred nuclei were counted in each experiment.
(E) Western blotting of extracts prepared from muscle cells infected with the empty or the NS-expressing retrovirus. β actin was monitored as a loading control.
(F) Relative expression levels of three mRNAs measured by qRT-PCR after overexpression of NS. The value with the empty virus on Day 0 was defined as 1 for each mRNA.
NS-induced promotion of muscle differentiation is p53-dependent
Oerexpressed NS binds to the MDM2 protein and inhibits MDM2-mediated ubiquitylation and subsequent degradation of p53 [24]. This leads to the increased p53 and consequently G1 arrest of the cell cycle. Therefore, we next investigated whether NS-induced promotion of cell cycle arrest and differentiation in myoblasts is also dependent on p53. We isolated primary myoblasts from adult p53−/− mice and infected them with empty or NS-expressing retrovirus (Fig. 4A) to study their proliferation and differentiation profiles. p53−/− myoblasts are known to display an increased cell proliferation rate in the maintenance medium and continue proliferation even in the differentiation medium at the expense of differentiation [25]. As expected, our p53−/− myoblasts proliferated faster than wild-type counterparts as shown in the numbers of Day 3 cells infected with the empty virus (6.1 ± 0.6 in Fig. 3B vs 9.2 ± 0.3 in 4B). This increased proliferation rate was not brought down by overexpressed NS (Fig. 4B). In addition, emergence of MHC-positive cells was not facilitated by overexpressed NS in p53−/− myoblasts (Fig. 4C, 4D). These results demonstrated that NS-induced promotion of muscle differentiation is dependent on p53. The aggressive proliferation of p53−/− myoblasts could be a reason for the delayed differentiation because myoblasts need to exit from the cell cycle prior to differentiation [26].
Figure 4. Promotion of muscle differentiation by overexpressed NS is dependent on p53.
(A) Immunostaining of p53−/− myoblasts infected with the empty or the NS-expressing retrovirus. Anti-6xHis tag antibody was used to detect NS. Bar, 10 μm.
(B) Cell proliferation profiles of p53−/− myoblasts infected with the empty or the NS-expressing retrovirus.
(C) Immunostaining of p53−/− myoblasts during differentiation after infection with the empty or the NS-expressing retrovirus. Bar, 40 μm.
(D) Mean + SEM of the ratios between the number of DAPI-positive nuclei in MHC-positive cells and the total number of DAPI-positive nuclei shown in (C). One hundred nuclei were counted in each experiment.
In summary, our findings demonstrated that NS is continuously expressed throughout skeletal muscle cell differentiation regardless of cell proliferation and plays a vital role in the differentiation. This is evidence that the expression of NS is not necessarily a reliable indictor for proliferating cells or undifferentiated cells. Instead, the expression level of NS may depend on the level of protein synthesis or potentially other cellular activities which often, but not always, correlate with cellular proliferation or differentiation. This last conclusion is important when interpreting the expression patterns of NS in stem cell biology.
Supplementary Material
Acknowledgments
We thank Dr. Toshio Kitamura for providing the pMXs vector and Plat-E cells and Nobuko Katoku-Kikyo for technical assistance. This work was supported by grants from the Muscular Dystrophy Association, Korea Institute of Science and Technology and the Nash Foundation to A.A. and the NIH grant R01 GM068027 to N.K.
Abbreviations
- APC
allophycocyanin
- CT
threshold cycle
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s Modified Eagle Medium
- dpc
days post coitum
- FACS
fluorescent activated cell sorting
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- FSC
forward scatter
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- MCK
muscle creatine kinase
- MHC
sarcomeric myosin heavy chain
- NS
nucleostemin
- PBS
phosphate buffered saline
- PE
phycoerythrin
- qRT-PCR
quantitative reverse transcription PCR
- SEM
standard error of the mean
- SSC
side scatter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma H, Pederson T. Nucleostemin: a multiplex regulator of cell-cycle progression. Trends Cell Biol. 2008;18:575–579. doi: 10.1016/j.tcb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002;16:2991–3003. doi: 10.1101/gad.55671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi S, Gude N, Hosoda T, Muraski J, Rubio M, Emmanuel G, Fransioli J, Vitale S, Parolin C, D’Amario D, Schaefer E, Kajstura J, Leri A, Anversa P, Sussman MA. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24:1113–1120. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- 5.Maki N, Takechi K, Sano S, Tarui H, Sasai Y, Agata K. Rapid accumulation of nucleostemin in nucleolus during newt regeneration. Dev Dyn. 2007;236:941–950. doi: 10.1002/dvdy.21027. [DOI] [PubMed] [Google Scholar]
- 6.Romanova L, Grand A, Zhang L, Rayner S, Katoku-Kikyo N, Kellner S, Kikyo N. Critical Role of Nucleostemin in Pre-rRNA Processing. J Biol Chem. 2009;284:4968–4977. doi: 10.1074/jbc.M804594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 8.Orcutt MW, Young RB. Cell differentiation, protein synthesis rate and protein accumulation in muscle cell cultures isolated from embryos of layer and broiler chickens. J Anim Sci. 1982;54:769–776. doi: 10.2527/jas1982.544769x. [DOI] [PubMed] [Google Scholar]
- 9.Plaisance I, Morandi C, Murigande C, Brink M. TNF-alpha increases protein content in C2C12 and primary myotubes by enhancing protein translation via the TNF-R1, PI3K, and MEK. Am J Physiol Endocrinol Metab. 2008;294:E241–250. doi: 10.1152/ajpendo.00129.2007. [DOI] [PubMed] [Google Scholar]
- 10.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 11.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, Kumagai H. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- 15.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 16.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 17.Rosser BW, Dean MS, Bandman E. Myonuclear domain size varies along the lengths of maturing skeletal muscle fibers. Int J Dev Biol. 2002;46:747–754. [PubMed] [Google Scholar]
- 18.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 19.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson EN, Klein WH. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;8:1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Thayer MJ, Tapscott SJ, Davis RL, Wright WE, Lassar AB, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58:241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- 22.Edmondson DG, Brennan TJ, Olson EN. Mitogenic repression of myogenin autoregulation. J Biol Chem. 1991;266:21343–21346. [PubMed] [Google Scholar]
- 23.Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 24.Dai MS, Sun XX, Lu H. Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol Cell Biol. 2008;28:4365–4376. doi: 10.1128/MCB.01662-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarzkopf M, Coletti D, Sassoon D, Marazzi G. Muscle cachexia is regulated by a p53-PW1/Peg3-dependent pathway. Genes Dev. 2006;20:3440–3452. doi: 10.1101/gad.412606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitzmann M, Fernandez A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol Life Sci. 2001;58:571–579. doi: 10.1007/PL00000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.