Abstract
A growing body of evidence implicates small G-proteins [e.g., Cdc42 and Rac1] in glucose-stimulated insulin secretion [GSIS] in the islet β-cell. These signaling proteins undergo post-translational modifications [e.g., prenylation] at their C-terminal cysteine residue and appear to be essential for the transport and fusion of insulin-containing secretory granules with the plasma membrane and the exocytotic secretion of insulin. However, potential regulation of the prenylating enzymes by physiological insulin secretogues [e.g., glucose] has not been investigated thus far. Herein, we report immunological localization, subcellular distribution and regulation of farnesyltransferases [FTases] and geranylgeranyltransferase [GGTase] by glucose in insulin-secreting INS 832/13 β-cells and normal rat islets. Our findings suggest that an insulinotropic concentration of glucose [20 mM] markedly stimulated the expression of the α-subunits of FTase/GGTase-1, but not the β-subunits of FTase or GGTase-1 without significantly affecting the predominantly cytosolic distribution of these holoenzymes in INS 832/13 cells and rodent islets. Under these conditions, glucose significantly stimulated [2.5–4.0 fold over basal] the activities of both FTase and GGTase-1 in both cell types. Together, these findings provide the first evidence to suggest that GSIS involves activation of the endogenous islet prenyltransferases by glucose, culminating in the activation of their respective G-protein substrates, which is necessary for cytoskeletal rearrangement, vesicular transport, fusion and secretion of insulin.
Keywords: Prenylation, farnesylation, geranylgeranylation, islet β-cell, insulin secretion and G-proteins
Introduction
Glucose-stimulated insulin secretion [GSIS] involves a series of metabolic events resulting in significant increase in intracellular ATP concentration which, in turn, leads to the closure of membrane-associated ATP-sensitive potassium channels, resulting in membrane depolarization and influx of extracellular calcium through voltage-sensitive calcium channels [1–3]. A net increase in intracellular calcium that occurs via the influx of extracellular calcium into the stimulated β cell, in addition to the mobilization of intracellular calcium from the storage pools, has been shown to be critical for the transport and fusion of insulin-laden secretory granules to the plasma membrane and release of insulin [1–3].
In addition to ATP, many studies have examined possible contributory roles for guanosine triphosphate [GTP] in GSIS. Although the precise cellular mechanisms underlying the roles of GTP in GSIS remain to be defined, available evidence indicates that it might involve activation of one [or more] G-proteins endogenous to the islet β cell. In support of such postulation, published evidence from multiple laboratories demonstrated critical involvement of small G-proteins [e.g., Rac1, Cdc42 and Rap1] in GSIS from normal rat islets, human islets, and clonal β-cell preparations [4, 5 for recent reviews]. Such conclusions were drawn primarily based on data from multiple experimental approaches including use of Clostridial toxins, which monoglucosylate and inactivate specific G-proteins, expression of dominant negative mutants and/or selective knockdown [i.e., siRNA methodology] of candidate G-proteins, and use of pharmacological inhibitors of requisite post-translational modifications of G-proteins, all of which inhibited GSIS [4,5].
The majority of small G-proteins and the γ-subunits of trimeric G-proteins undergo posttranslational prenylation at their C-terminal cysteine residues. Farnesyl transferase [FTase] and the geranylgeranyl transferases [GGTase] catalyze the incorporation of either a 15-carbon [farnesyl moiety] or a 20-carbon [geranylgeranyl moiety] derivative of mevalonic acid into the C-terminal cysteine residues of their respective substrate proteins [6,7]. Collectively, the FTases and GGTases are referred to as prenyl transferases. Small G-proteins such as H-Ras undergo farnesylation and Cdc42, Rac and Rho are modified by geranylgeranylation. In addition to prenylation, the C-terminal cysteine residues of the prenylated proteins have been shown to undergo further modifications, including cleavage of the three carboxyl-terminus amino acid residues and carboxyl methylation. Data derived from studies with islet β-cells suggest that carboxyl methylation of specific G-proteins [e.g., Cdc42] increases their hydrophobicity resulting in their targeting to the relevant membranous sites. Thus, prenylation might represent the first committed step for glucose-induced activation of specific G-proteins in the pancreatic islet [5, 7].
At least three distinct prenyltransferases have been described in the literature. FTase and GGTase-I are referred to as CAAX prenyltransferases because they share the CAAX-substrate motif of the C-terminal cysteine region of their substrate proteins. GGTase-II [referred to as Rab GGTase] prenylates the Rab subfamily of proteins at a different motif, and hence this group of prenyltransferases is often referred to as non-CAAX prenyltransferases. FTase and GGTase-I are heterodimeric [i.e., consisting of α- and β-subunits] in nature. Interestingly, both FTase and GGTase-I share a common α-subunit and different β-subunits. The α-subunit is the regulatory subunit, whereas the β-subunit confers substrate specificity [7–10]. In the light of recent evidence implicating post-translational prenylation of specific G-proteins [e.g., Cdc42 and Rac1] in GSIS [5], we undertook the current investigation to precisely determine the immunological localization, subcellular distribution and functional regulation of both FTase and GGTase-I by glucose in pancreatic β-cells. Our data provide the first evidence for novel regulation of these enzymes by glucose in insulin-secreting INS 832/13 cells and normal rat islets.
Materials and Methods
Materials
All general laboratory reagents were from Sigma-Aldrich [St. Louis, MO]. Tritiated farnesyl pyrophosphate ([3H]FPP, NET 1042, 50 μCi/0.1ml) and geranylgeranyl pyrophosphate ([3H]GGPP, NET 1052, 50 μCi/0.1 ml) were from PerkinElmer/NEN [Waltham, MA]. Ras-Cys-Val-Lys-Ser protein and Ras CVLL [Rho analog] proteins were from Calbiochem/EMD [Gibbstown, NJ]. Bicinchoninic Acid Assay [BCA] was from Pierce-Thermo-Fisher [Waltham, MA]. Primary antibodies directed against the FTase/GGTase I-α were from Santa Cruz Biotechnology, Inc. [Santa Cruz, CA] and secondary antibodies [anti-rabbit-HRP] were from the ECL kit of GE Healthcare (Piscataway, NJ). SDS-PAGE gels and polyvinylidene fluoride [PVDF] membranes were from Millipore/Thermo-Fisher and Pierce/Thermo-Fisher, respectively.
Insulin-secreting cells
INS 832/13 cells [provided by Dr. Chris Newgard, Duke University Medical Center, Durham, NC] were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1mM sodium pyruvate, 50μM 2-mercaptoethanol and 10mM HEPES [pH 7.4]. Pancreatic islets from male Sprague-Dawley rats [200–250 g body wt; Harlan Laboratories] were isolated by the collagenase digestion method as described earlier [11–13].
Isolation of total particulate and soluble fractions from INS 832/13 cell lysates
INS 832/13 cells homogenates were prepared in 250 mM sucrose, 1 mM EDTA, 50 mM Tris-HCl, 1 mM DTT, and protease inhibitor cocktail, pH 7.4 and total membrane and soluble fractions were further isolated from homogenates by differential centrifugation method as in [13].
Isolation of hydrophobic and hydrophilic compartments from INS 832/13 cells
These were isolated using the Triton X-114 phase separation method described by us recently [13]. Briefly, about 400 μg of protein prepared in 400 μl of homogenization buffer supplemented with 1% (w/v) Triton X-114 and overlaid on a 400 μl sucrose cushion (20 mM Tris-HC1, pH 7.4, 6% (w/v) sucrose, and 0.06% (w/v) Triton X-114). Following a brief incubation [3 min at 30 °C], the samples were centrifuged [3 min at 300g] and the aqueous phase was mixed with 0.5% [w/v] fresh Triton X-114. After dissolution in Triton X-114 at 4 °C, the mixture was again overlayed on the same sucrose cushion, incubated for 3 min at 30 °C and centrifuged for 3 min at 300g. The lipid phase was then diluted to a final volume of 400 μl with homogenization buffer. Further, the aqueous phase was transferred into a separate tube supplemented with 2% fresh Triton X-114 incubated for 3 min at 30 °C and centrifuged [3 min at 300g] without sucrose cushion. The supernatant thus collected from the top of the tube is referred to as aqueous phase [13].
Western Blot Analysis
Aliquots of equal protein concentration from cleared lysates were loaded onto 8–16% graduated polyacrylamide HEPES-based gels and resolved by SDS-PAGE. Proteins were transferred to PVDF membranes and incubated in 5% BSA for 2 h. Membranes were washed two times with 1% Tween in Tris-buffered saline [1% TBST] and then immunoblotted with primary antibody to the respective protein in question for 24 hours at 4°C. The membranes were washed two times with 1% TBST and then immunoblotted with anti-rabbit-HRP conjugated secondary antibody for 2 h at room temperature. Membranes were washed two times with 1% TBST and then once with TBS. The chemiluminescent solution was added to the membrane and allowed to react for 1 minute. Membranes were placed on film for 5 minutes. The films were developed and band profiles quantified by densitometry.
FTase and GGTase Assays
These assays were performed using a modified methods of Moore et al [14] and as previously described [15]. Briefly, INS 832/13 cells or isolated rat islets were treated with low [5 mM] or high [25 mM] glucose for indicated times. Subsequently, cells were lysed in 500 μl of buffer [150 mM NaCl. 5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 1 mM sodium vanadate, 1 mM sodium phosphate, 1% Triton X-100, 0.05% SDS, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 50 mM HEPES, pH 7.5]. Crude lysates were sonicated and centrifuged 10,000 x g. Protein concentration was determined in cleared lysates using the bicinchoninic acid assay. The in vitro filtration assay was initiated by adding a 5-μl aliquot of diluted and normalized extract to 45 μl of reaction assay solution [5 mM MgCl2, 5 mM dithiothreitol, 100 nM Ras or Rho protein, 100 nM [3H]-farnesyl- or [3H]-geranylgeranyl pyrophosphate [20 mCi/mmol, 50 mM HEPES, pH 7.5] and incubated for 30 minutes. The reaction was stopped using 1 ml of ice-cold 1 M HCl in ethanol and samples were placed on ice for 15 min. The reaction mixtures were individually filtered through Whatman GF/C glass-fiber filters, air dried and placed in scintillation fluid and quantified by scintillation spectrometry.
Results
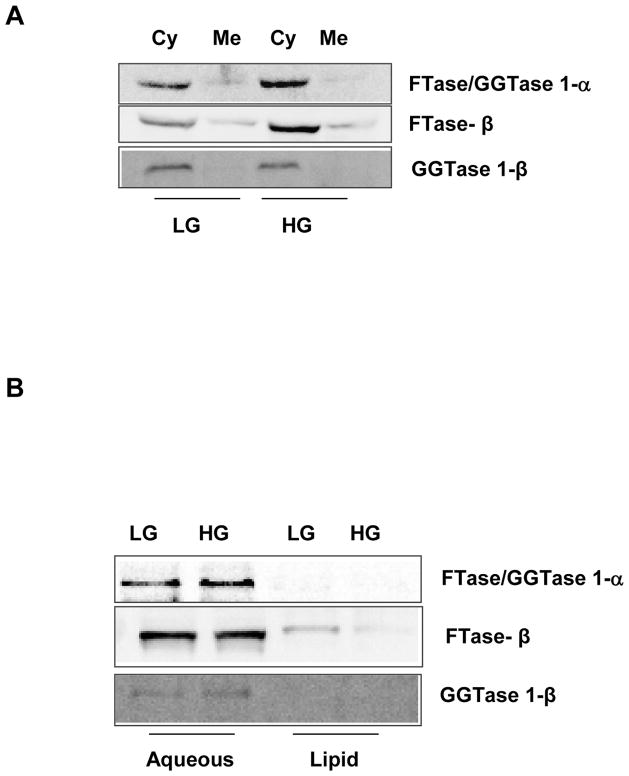
At the outset, we determined the effect of stimulatory glucose concentrations on the subcellular distribution of the common FTase/GGTase1-α subunit and the corresponding β-subunits of FTase and GGTase-1 in insulin-secreting INS832/13 cells. To address this, total membrane and soluble fractions from cell lysates derived from either low [2.5 mM] or high glucose [20 mM]-treated cells were subjected to a single-step centrifugation method and the relative abundance of the FTase and GGTase subunits was determined in those fraction by Western blotting method. Data in Figure 1 [Panel A] suggested that the common α-subunit of FTase/ GGTase-I is predominantly localized in the cytosolic compartment. Exposure of these cells to a stimulatory glucose concentration [20 mM] had minimal effects on the subcellular distribution of this protein. Likewise, the corresponding β-subunits of FTase and GGTase-I were also predominantly cytosolic in their distribution and did not undergo redistribution between the cytosolic and membrane compartments following exposure to stimulatory glucose [Figure 1; Panel A].
Figure 1.
Figure 1A. Sub-cellular distribution of FTase and GGTase subunits in INS-832/13 following exposure to basal or stimulatory glucose concentrations
INS-832/13 cells were cultured overnight in low glucose-low serum media. Cells were further incubated in presence of either basal [2.5 mM; LG] or high glucose [20 mM; HG] for 45 min. Homogenates of these cells were centrifuged at 100,000g for 90 min and the total cytosolic [Cy] and membrane [Me] fractions were separated by SDS-PAGE, transferred to a membrane and probed with corresponding antibodies for detection of FTase/GGTase 1- α, FTase β and GGTase 1-β subunits. Data are representative of two separate experiments yielding identical results.
Figure 1B. Phase partition of prenyltransferase subunits in INS-832/13 cells following exposure to basal or stimulatory glucose concentrations
INS-832/13 cells were cultured overnight in low glucose-low serum media. Cells were further incubated in presence of either basal glucose [2.5 mM; LG) or stimulatory glucose [20 mM; HG] for 45 min. Homogenate proteins were partitioned into hydrophobic and hydrophilic compartment using Triton X-114 partition method as described in Methods section. Fractions were separated by SDS-PAGE, transferred to a membrane and probed with corresponding antibodies for detection of FTase/GGTase 1- α, FTase β and GGTase 1-β subunits. Data are representative of two separate experiments yielding identical results.
We next isolated total hydrophilic and hydrophobic fractions from unstimulated [2.5 mM glucose] or stimulated [20 mM] pancreatic β-cells by Triton X-114 phase separation method to determine potential effects of glucose on the distribution [or association] of FTase and GGTase subunits with either the aqueous or lipid phase. Data depicted in Figure 1 [Panel B] suggested that the common FTase/GGTase-1α subunit and the β-subunits of FTase and GGTase-1 are associated predominantly with the aqueous compartment. Furthermore, under these conditions glucose exerted minimal effects on distribution of these proteins between the hydrophilic and hydrophobic compartments. Together, these data [Figure 1; Panels A and B] suggested that the FTase and GGTase-1 holoenzymes are soluble in their cellular distribution and that stimulatory glucose concentrations elicit minimal effects on the subcellular distribution of these proteins.
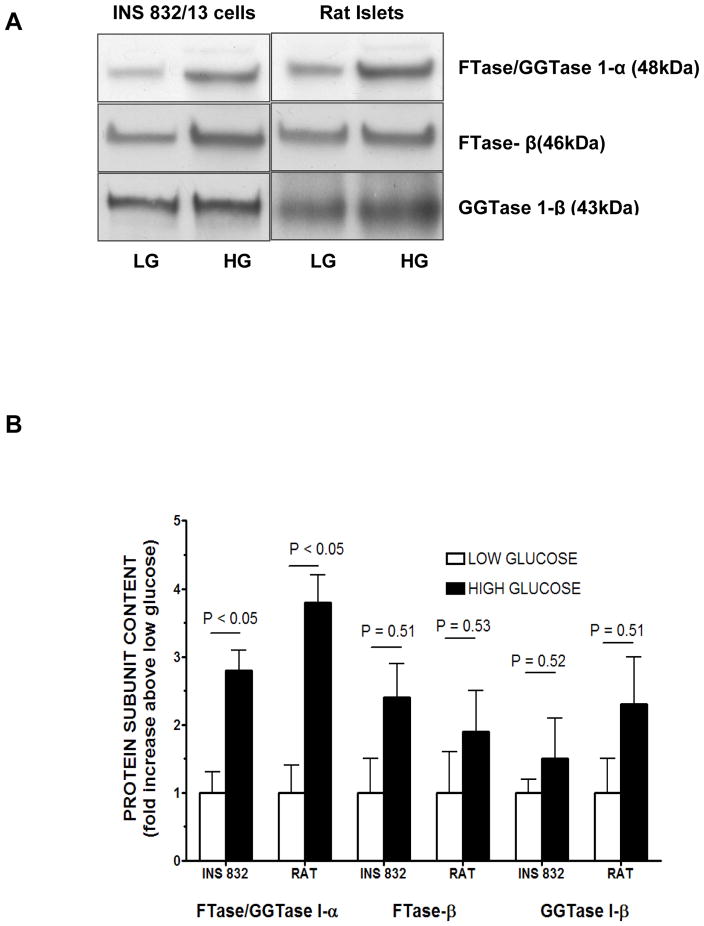
In the next series of studies, we determined the effects of glucose on the expression of these proteins in insulin-secreting INS 832/13 cells and normal rat islets. Data in Figure 2 [Panel A] suggested a significant (P < 0.05) increase in the expression of the common FTase/GGTase-1α subunits in cells exposed to high glucose [20 mM] in both cell types. However, no significant effects of glucose on the expression of either FTase β or GGTase-1β were seen in INS 832/13 cells or normal rat islets. Pooled data from multiple studies are depicted in Figure 2 [Panel B], which clearly suggested differential effects of glucose on the expression of these proteins, i.e., a significant increase in the expression of FTase/GGTase-1α, but not FTase β or GGTase-1β in INS 832/13 cells and normal rat islets following exposure to insulinotropic concentrations of glucose.
Figure 2. Changes in the protein contents of FTase and GGTase subunits in INS 832/13 cells and isolated rat pancreatic islets exposed to basal or stimulatory glucose.
Panel A: INS-832/13 cells and isolated rat pancreatic islets were cultured overnight in low glucose-low serum media. Cells were further incubated in presence of either low glucose [2.5 mM; LG] or high glucose [20 mM; HG] for 45 min. Homogenate proteins were separated by SDS-PAGE, transferred to a membrane and probed with corresponding antibodies for detection of FTase/GGTase 1- α, FTase β and GGTase 1-β subunits. A representative blot from two studies is shown here. Panel B: Relative intensities of protein bands from experiments described under Panel A are quanitiated by densitometry and plotted. Pooled data from two separate studies are given.
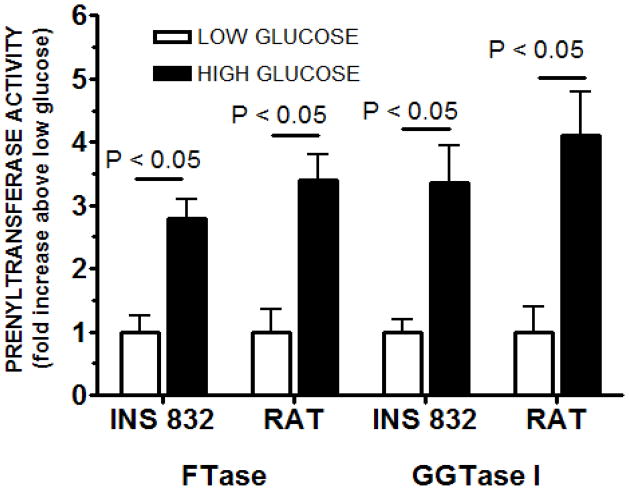
In the last set of experiments, we studied regulation by glucose of FTase and GGTase activities in INS 832/13 cells and normal rat islets. Data in Figure 3 demonstrated that an insulinotropic concentration of glucose [20 mM] markedly increased the FTase activity in INS 832/13 cells and rat islets by nearly 2.75 and 3.5 fold over the basal, respectively. Under these conditions, we also noticed a marked increase in the GGTase activity in INS 832/13 cells [nearly 3.5 fold] and rat islets [nearly 4-fold] following exposure to stimulatory glucose concentrations.
Figure 3. FTase and GGTase-I activities in INS 832/13 cells or isolated rat islets following exposure to basal [2.5 mM] or stimulatory [20 mM] glucose.
FTase and GGTase-I activities were determined using a modified filtration assay as previously described in Methods. Briefly, cells were grown to confluence and treated with low [2.5 mM] or stimulatory [20 mM] glucose for 45min. Subsequently, cells were lysed, sonicated and centrifuged 10,000 × g. Protein concentrations were determined from lysate supernatants. The in vitro filtration assay was initiated by adding a 5-μl aliquot of diluted and normalized extract to 45 μl of reaction assay solution, which contained tritiated farnesyl- or geranylgeranyl pyrophosphate and allowed to incubate for 30 minutes. The assay was stopped using ice-cold 1 M HCl in ethanol. Reactions solutions were individually filtered through Whatman GF/C glass-fiber filters, air dried, placed in scintillation fluid and quantified by scintillation spectrometry. Activities were first calculated in dpm/μg protein/unit time and then converted to relative amounts. FTase and GGTase-I activities are expressed as fold increase above low glucose level in the same treatment group and represent the mean ± SEM (n = 6 for each treatment).
Discussion
The overall objective of the current study was to determine potential regulation of prenyltransferase activity by glucose, the major physiologic regulator of insulin secretion in the pancreatic β-cell. This has not been studied thus far. Our findings provide the first evidence to suggest that insulinotropic concentrations of glucose markedly stimulate both FTase and GGTase activities in insulin-secreting clonal INS 832/13 cells and normal rat islets. Several recent studies, including our own, have demonstrated direct activation of small G-proteins [e.g., Rac1 and Cdc42] by glucose in a variety of β-cell preparations [4,5,11–13,16]. Previous data [reviewed in ref. 5] in insulin-secreting cells using inhibitors of FTases and GGTases implicated that G-protein prenylation is necessary for their association with relevant membranous sites [e.g., secretory granules], interaction with their regulatory proteins [e.g., GDP-dissociation inhibitors], and effector proteins [e.g., PAK-1].
Together, the above data suggested that GSIS involved direct activation of prenyltransferases by glucose. Thus, there appears to be a need to study this phenomenon further and in more depth. Our current studies demonstrated that glucose directly stimulated the activities of both GGTase-I and FTase in INS 832/13 cells and isolated rat islets. GGTases catalyze the geranylgeranylation of small G-proteins such as Cdc42 and Rac1, the activation of which has been shown to be critical for GSIS [4,5,7, 16]. Even though previously published evidence using inhibitors of farnesyltransferases [e.g., allyl or vinyl farnesols] implicated novel roles for protein farnesylation in GSIS, very little is known to date with regard to the potential identity of the farnesylated proteins whose activation is critical for GSIS [17]. Our current data provide additional support to our original hypothesis that GSIS involves activation of both farnesylated and geranylgeranylated proteins.
Our data also suggested that glucose differentially regulated the expression of the α and β-subunits of FTase and GGTase-I in the pancreatic β-cell. Specifically, it stimulated the expression of the common α-subunit of FTase/GGTase-I, without significantly affecting the expression of their cognate β-subunits. The significance of these findings remain unknown at this time, but it could represent additional regulatory mechanism underlying GSIS. Along these lines, previous studies have suggested functional regulation of prenyltransferase activity by phosphorylation-dephosphorylation [18]. They reported phosphorylation of the common FTase/GGTase-1α regulatory subunit by insulin in 3T3 cells leading to the activation of its catalytic function [18]. It should be noted that recent studies from our laboratory have demonstrated that overexpression of a dominant negative [i.e., nonphosphorylatable] FTase/GGTase 1-α resulted in a marked reduction in GSIS in INS 832/13 cells raising an interesting possibility that signaling steps underlying GSIS could involve multiple regulatory mechanisms involving the activation of FTase/GGTase activities, including phosphorylation of the common α-subunit [13]. Additional studies are needed to verify this in the islet β-cell. In conclusion, our current findings provide the first evidence to suggest that GSIS involves stimulation of endogenous islet prenyltransferases by glucose culminating in the activation of their respective G-proteins, to facilitate cytoskeletal rearrangement, vesicular transport, fusion and secretion of insulin.
Acknowledgments
These studies were supported by grants from the National Institutes of Health [DK94201 to AK] and Merit Review awards to AK and MG from the Department of VA Medical Research Service. AK is the recipient of Senior Research Career Scientist from the Department of VA. The authors thank Mr. Brandon Koch for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67:1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald MJ. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990;39:1461–1466. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- 3.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic β-cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis-roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowluru A. Small G-proteins in islet beta-cell function. Endocr Rev. 2009 doi: 10.1210/er.2009-0022. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 7.Kowluru A. Protein prenylation in glucose-induced insulin secretion from the pancreatic islet beta-cell: a perspective. J Cell Mol Med. 2008;12:164–173. doi: 10.1111/j.1582-4934.2007.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 9.Seabra MC, Reiss Y, Casey PJ, Brown MS, Goldstein JL. Protein farnesyltransferase and geranylgeranyltransferase share a common α subunit. Cell. 1991;65:429–434. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- 10.Fu HW, Casey PJ. Enzymology and biology of CaaX protein prenylation. Rec Prog Horm Res. 1999;54:315–342. [PubMed] [Google Scholar]
- 11.Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA. Glucose-and GTP-dependent stimulation of the carboxylmethylation of Cdc42 in rodent and human pancreatic islets and pure β cells: Evidence for an essential role for GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98:540–555. doi: 10.1172/JCI118822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A. Regulatory roles of Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol. 2009;77:101–113. doi: 10.1016/j.bcp.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veluthakal R, Kaur H, Goalstone M, Kowluru A. Dominant negative alpha-subunit of farnesyl-and geranylgeranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes. 2007;56:204–210. doi: 10.2337/db06-0668. [DOI] [PubMed] [Google Scholar]
- 14.Moores SL, Schaber MD, Mosser SD, Rands E, O’Hara MB, Garsky VM, Marshall MS, Pompliano DL, Gibbs JB. Sequence dependence of protein isoprenylation. J Biol Chem. 1991;266:4603–4610. [PubMed] [Google Scholar]
- 15.Goalstone ML, Draznin B. Effect of insulin on farnesyltransferase activity in 3T3-L1 adipocytes. J Biol Chem. 1996;271:27585–27589. doi: 10.1074/jbc.271.44.27585. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Luo R, Kowluru A, Li G. Novel regulation by Rac1 of glucose-and forskolin-induced insulin secretion in INS-1 beta cell. Am J Physiol Endocrinol Metab. 2004;286:818–827. doi: 10.1152/ajpendo.00307.2003. [DOI] [PubMed] [Google Scholar]
- 17.Amin R, Chen HQ, Tannous M, Gibbs R, Kowluru A. Inhibition of glucose- and calcium-induced insulin secretion from betaTC3 cells by novel inhibitors of protein isoprenylation. J Pharmacol Exp Ther. 2002;303:82–88. doi: 10.1124/jpet.102.036160. [DOI] [PubMed] [Google Scholar]
- 18.Goalstone M, Carel K, Leitner JW, Draznin B. Insulin stimulates the phosphorylation and activity of farnesyltransferase via the Ras-mitogen-activated protein kinase pathway. Endocrinology. 1997;138:5119–5124. doi: 10.1210/endo.138.12.5621. [DOI] [PubMed] [Google Scholar]