Abstract
Objective
Extremely low body mass index (BMI) values are associated with increased risk for death and poor long-term prognosis in individuals with AN. The present study explores childhood personality characteristics that could be associated with the ability to attain an extremely low BMI.
Methods
Participants were 326 women from the Genetics of Anorexia Nervosa (GAN) Study who completed the Structured Interview for Anorexia Nervosa and Bulimic Syndromes and whose mother completed the Child Behavioral Check List and/or Revised Dimensions of Temperament Survey.
Results
Children who were described as having greater fear or anxiety by their mothers attained lower BMIs during AN (p <0.02). Path analysis in the GAN and a validation sample, Price Foundation Anorexia Nervosa Trios Study, confirmed the relation between early childhood anxiety, caloric restriction, qualitative food item restriction, excessive exercise, and low BMI. Path analysis also confirmed a relation between childhood anxiety and caloric restriction, which mediated the relation between childhood anxiety and low BMI in the GAN sample only.
Conclusion
Fearful or anxious behavior as a child was associated with the attainment of low BMI in AN and childhood anxiety was associated with caloric restriction. Measures of anxiety and factors associated with anxiety-proneness in childhood may index children at risk for restrictive behaviors and extremely low BMIs in AN.
Keywords: Anorexia Nervosa, Anxiety, Body Mass Index
INTRODUCTION
Anorexia nervosa (AN) is a debilitating and potentially lethal disorder (Berkman, Lohr, & Bulik, 2007; Fichter, Quadflieg, & Hedlund, 2006; Papadopoulos, Ekbom, Brandt, & Ekselius, 2009; Sullivan, 1995; Sullivan, Bulik, Fear, & Pickering, 1998) in which compulsive and unrelenting food avoidance results in dangerously low body mass index (BMI). Low BMI is associated with elevated mortality in the general population (Engeland, Bjorge, Selmer, & Tverdal, 2003; Kivimaki et al., 2008; Reis et al., 2009; Troiano, Frongillo, Sobal, & Levitsky, 1996; Whitlock et al., 2009) and individuals with AN are at significantly increased risk of sudden cardiac death (Lesinskiene, Barkus, Ranceva, & Dembinskas, 2008). Low BMI also has therapeutic and prognostic implications, has been associated with longer time to remission (Clausen, 2008), persistence of lower BMI following initiation of treatment (Pinter, Probst, Vandereycken, Pieters, & Goris, 2004), overall poorer prognosis (Steinhausen, Grigoroiu-Serbanescu, Boyadjieva, Neumarker, & Metzke, 2009), increased risk of relapse in the year following hospital discharge (Walsh et al., 2006), and increased likelihood for re-hospitalization (Steinhausen, Grigoroiu-Serbanescu, Boyadjieva, Neumarker, & Winkler Metzke, 2008).
Food intake in the weeks prior to treatment and shortly following treatment termination is also related to long-term prognosis. Caloric restriction and qualitative food item restriction, avoidance of certain food items or macronutrients (i.e., fat), are associated with a less favorable treatment outcome in those with eating disorders. Individuals with greater caloric restriction prior to treatment have higher rates of relapse following treatment (McFarlane, Olmsted, & Trottier, 2008). Following hospitalization for AN, women with limited dietary variety have a poorer prognosis than women who eat a wider range of food items (Schebendach et al., 2008).
As it is well known that anxiety is present in a substantial majority of individuals with AN prior to any signs of abnormal eating or distortions of body image (Godart, Flament, Lecrubier, & Jeammet, 2000; Raney et al., 2008; Salbach-Andrae et al., 2008), the identification of early developmental factors that confer risk of restrictive eating behaviors and attaining low BMI among those with AN has theoretical and clinical importance. One factor of potential significance is anxiety, which has been linked in cross-sectional studies of AN to elevations in resting energy expenditure (Van Wymelbeke, Brondel, Marcel Brun, & Rigaud, 2004), more extreme exercise (Penas-Lledo, Vaz Leal, & Waller, 2002; Shroff et al., 2006), and generally higher physical activity (Brewerton, Stellefson, Hibbs, Hodges, & Cochrane, 1995).
Given how often anxiety phenotypes are present in women with AN (Godart et al., 2000; Raney et al., 2008; Salbach-Andrae et al., 2008), and that anxiety disorders tend to predate the onset of AN (Godart et al., 2000; Kaye, Bulik, Thornton, Barbarich, & Masters, 2004; Raney et al., 2008), and considering that persisting, morbid fear of weight gain and its avoidance is central to its descriptive psychopathology, an intuitive hypothesis is that caloric restriction is anxiolytic. This hypothesis is partially supported by neurobiological processes. Increased extracellular levels of serotonin in AN may lead to increased anxiety and decreased appetite; serotonin levels are reduced during starvation and could thereby reduce anxiety. Refeeding is associated with increased serotonin levels and increased anxiety (Kaye, Fudge, & Paulus, 2009). Reductions in dietary tryptophan, the dietary precursor to serotonin, has been shown to reduce anxiety in individuals with and recovered from AN (Kaye et al., 2003). This effect could account, at least in part, for the reinforcing nature of starvation in the ill state (Kaye, 2008; Kaye et al., 2003), accentuated further by the inherent, general anxiolytic properties of physical activity (Norris, Carroll, & Cochrane, 1992; Sexton, Maere, & Dahl, 1989). Thus, a biologically and psychologically plausible speculation is that more extreme anxiety indexes greater disease liability to AN and is associated with greater caloric restriction, consequent lower BMI, and perhaps a poorer long-term prognosis.
Other related personality and temperamental factors that may be associated with attainment of low BMI are timidity (Wilbur & Colligan, 1981) and low self-esteem (Halvorsen & Heyerdahl, 2006; Wilksch & Wade, 2004). Personality and temperamental characteristics, such as timidity and low self-esteem are related to anxiety-proneness and captured by constructs such as harm avoidance (Cloninger, 1986; Cloninger & Svrakic, 1992; Joyce et al., 2003), often exhibited in individuals with AN (Fassino, Abbate-Daga et al., 2002; Fassino, Svrakic et al., 2002), and associated with chronic AN (Bulik, Sullivan, Fear, & Pickering, 2000). One study has identified neurotrophic tyrosine kinase receptor type 2 that may be associated with eating disorders, harm avoidance, and low BMI in those with eating disorders (Ribases et al., 2005).
An additional trait of interest in attainment of low BMI is early display of a rigid and unvarying schedule. Elevated rhythmicity could be associated with more severe eating disorder symptomatology. Teens with AN have less variation (greater rhythmicity) in their daily eating and sleep routines than teens with bulimia nervosa (BN) and adolescents with AN had less daily variation overall than adolescents with BN or depression (Shaw & Steiner, 1997). Childhood rhythmicity in those who develop AN needs to be explored to determine if early rhythmicity could serve as an early indication that a child may be prone to develop a low BMI. In children, high sleep activity (more restlessness during sleep) is associated with later development of anxiety disorders (Gregory et al., 2005). Childhood rhythmicity could be an early harbinger of later obessionality and sleep activity could index underlying anxious temperament.
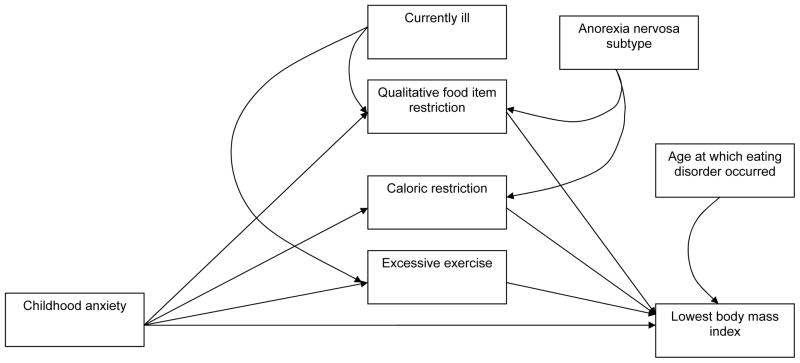
Early identification of at risk individuals could allow for tailored, targeted prevention and early intervention for AN. In this study, we examined childhood measures of temperament that index anxiety proneness to determine their association with lifetime lowest BMI (lowest BMI) in a cohort of individuals with AN. We further hypothesized that the relation between childhood anxiety and low BMI is mediated by illness related behavioral factors, specifically caloric restriction (Figure 1).
Figure 1.
Path Diagram
METHODS
Participants
Participants were from the Genetics of Anorexia Nervosa (GAN) Collaborative Study; a full description of the study is available in a separate publication (Kaye et al., 2008). The probands were age 16 or older, met criteria for a lifetime diagnosis of DSM-IV AN, with or without amenorrhea, at least 3 years prior to study entry and at or before age 45. All probands had at least one first, second, or third degree relative with AN (excluding parents and monozygotic twin) who was willing to participate in the study.
Exclusion criteria for probands included: (1) binge eating at least twice a week for at least three months [this criterion was implemented as the primary goal of the study was to attempt to replicate our previous linkage findings (Grice et al., 2002) so we ascertained a sample of probands who were predominantly of the restricting type, see (Kaye et al., 2008) for a more detailed discussion]; (2) a history of severe central nervous system trauma; (3) psychotic disorders or developmental disability; (4) medical, neurological, or substance use disorder that could confound the diagnosis of AN or interfere with the ability to complete assessments; (5) a maximum lifetime BMI exceeding 30 kg/m2 (those with a maximum lifetime BMI of greater than 30 were excluded to insure sample homogeneity for genetic studies of AN, theoretically minimizing individuals with potentially obscuring obesity-related genotypes); or (6) did not speak either English or German. Affected relatives were required to meet the same inclusion criteria as probands, but could have regular binge eating and AN diagnostic criteria could have been met less than three years prior to the study. Relatives were required to have had a minimum BMI at or below 18 kg/m 2 for females and 19.6 kg/m2 for males [which corresponds to the 5th percentile BMI values of the National Health and Nutrition Examination Survey (NHANES) epidemiological sample of females and males, respectively] for a minimum duration of 3 months, in order to be certain of an AN diagnosis, and could have an additional diagnosis of BN. Additional affected relatives with the diagnosis of AN, BN or Eating Disorder Not Otherwise Specified were included if the proband and the initial affected relative met the specified criteria.
The total number of probands and affected relatives included in this study was 904. Twenty-nine were excluded from the current analysis because they did not have a lifetime history of AN. Males (n=34) were removed because there were not enough affected with AN for meaningful analysis. From this pool of participants, only those who had data for the Childhood Behavioral Check List (CBCL)(Achenbach, 1991) or DOTS-R (Windle & Lerner, 1985) were included. Many participants’ mothers did not complete the CBCL or DOTS-R; thus the sample for this study was 326 women.
Each participating site had approval from their local Institutional Review Board and all participants signed informed consent.
Validation Sample
Validation of the path model, used to examine the main study hypotheses, was performed in a second sample of women who had participated in the Price Foundation Anorexia Nervosa Trios Study (AN Trios). Inclusion criteria for that study have previously been described (Reba et al., 2005). All participants met diagnostic criteria for AN prior to age 26, were currently between ages 13 and 65, had met AN diagnostic criteria at least three year prior to entry into the study, and did not engage in regular binge eating (Reba et al., 2005). Participants from this study were included if they had complete information for the caloric restriction, qualitative food item restriction, and exercise questions on the Structured Interview for Anorexia Nervosa and Bulimic Syndromes (SIAB) (Fichter, Herpertz, Quadflieg, & Herpertz-Dahlmann, 1998) and their mother had completed the CBCL. This yielded a sample of 383 women.
Assessments
Eating disorder pathology
Eating disorder diagnosis was established using several interviews. First, the Structured Clinical Interview for DSM-IV Axis I Disorder (SCID-I) (First, Spitzer, Gibbon, & Williams, 1997) was used to assess inclusion and exclusion criteria. An expanded modified version of the SCID Module H was used to establish the diagnoses of eating disorders. Individuals were grouped based on the subtype of AN: 1) restricting AN and 2) not restricting AN (which includes individuals with binge/purge AN and those with a lifetime history of both AN and BN).
The SIAB (Fichter et al., 1998) is a detailed structured interview that assesses information relevant to lifetime severity of psychopathological factors, eating disorder symptoms, and substance abuse. This instrument was administered to confirm the eating disorder diagnosis and to obtain additional information on core eating disorder behaviors. Cronbach’s alpha for internal consistency on the SIAB lifetime version has been shown to be moderate to high, ranging from .78 – .91 for five of the six components (Fichter et al., 1998). Interrater reliability has been shown to be excellent, ranging between .86 – .96 (Fichter et al., 1998).
Specific items from the SIAB were used in this study. Interviewers asked participants to report worst lifetime symptoms on the SIAB. Lowest BMI was calculated as the lowest weight since puberty or during eating disorder if onset of eating disorder was prior to puberty measured in kilograms divided by the squared value of height in meters at that same age measured. Lowest BMI was used as a continuous variable. Lowest caloric intake was determined by participant’s answer to the following question: “What was your lowest limit in calories per day at the time when you were restricting the most?” To establish qualitative restriction, each participant was asked “Have you tried to avoid high caloric foods that you enjoy eating? Did you intentionally eat less fat or carbohydrates in order to stay slim? Did you restrict the range of your food? What types of foods were avoided? What types of food did you allow yourself to eat?” Based on the responses to these questions, the interviewer reported qualitative restriction as one of five categories: no qualitative food restriction, slight, marked, severe, or very severe. The interviewers were instructed to endorse “very severe” qualitative food item restriction only if the participant indicated that the variety of food consumed was reduced such that only a few low-calorie foods were consumed (i.e., salad without dressing, steamed vegetables) and fat, sugar, and carbohydrates were not consumed. In addition, very restricted diets (i.e., one food-group diets such as eating only vegetables) were coded as “very severe.” Excessive exercise was assessed by the SIAB questions: “How much exercise did you engage in? If you couldn’t exercise did you feel uneasy or stressed?” Those who exercised more than once a day were considered excessive exercisers and those who exercised once a day or less were not considered excessive exercisers.
Childhood temperament characteristics
Participating mothers of affected individuals retrospectively completed several questionnaires on childhood behaviors and temperament of their affected children up to age 10. The CBCL ages 4–18 (Achenbach, 1991) is a widely used instrument that assesses childhood symptoms and behaviors including anxiety and stubbornness. Each item from the CBCL had 3 response choices and was used as a categorical variable in all analyses. The mothers of the affected individuals were asked if each of the following characteristics was “not true, somewhat or sometimes true, or very true or often true” of the affected child: too fearful or anxious; self-conscious or easily embarrassed; shy or timid; and stubborn, sullen, or irritable. The Child Behavior Checklist anxiety scale (CBCL-A) has been used as a measure of childhood anxiety and is able to discriminate between children with and without anxiety disorders (Kendall et al., 2007). This scale was used as a continuous measure in the path analysis with a higher score indicating greater anxiety. If an individual had one item from this scale missing, the value was imputed using mean item substitution; individuals missing more than one item were excluded from the analysis.
The DOTS-R (Windle & Lerner, 1985) measures ten temperament attributes of children, five of which (activity-general, activity-sleep, rhythmicity-sleep, rhythmicity-eating, and rhythmicity-habits) were assessed in this study. In order to assess how similar and how precisely the affected child followed a schedule for sleeping, eating and “other habits” from day to day, each mother was asked if each item was “usually false, more false than true, more true than false, or usually true.” Rhythmicity captured whether the child did various things at the same time each day and whether being in a different environment altered the child’s schedule. The same response choices were used to determine general and sleep activity. Activity questions asked the mother to access how much the child moved around while both awake and asleep. Responses to each question were used to create scales for sleep rhythmicity, eating rhythmicity, habit rhythmicity, general activity, and sleep activity. All scales are continuous and higher scores indicate higher rhythmicity or activity for each scale.
Statistical Analyses
All statistical analyses were performed using SAS/STAT® 9.1 software (SAS Institute Inc., 2004). All continuous variables were standardized prior to analysis. Using PROC GENMOD, lowest BMI, caloric restriction, and qualitative food item restriction were each predicted from individual items from CBCL and DOTS-R measures of temperament scales. Generalized Estimating Equations (GEE) corrections were used in the analyses for lowest BMI and caloric restriction to account for the non-independence of the data due to the inclusion of affected relatives in the analyses. These statistical analyses were conducted using the GENMOD procedure. Such correction could not be applied to the item on qualitative food item restriction as GEE models cannot be applied to ordinal data. Age at interview, age of onset of eating disorder, and eating disorder subtype were entered into all models as covariates. Given that some women developed AN as children or adolescents and others developed AN as adults, we were unable to use BMI percentiles, which are used to evaluate BMI in children and adolescents. It is possible that it would be easier for younger individuals to attain lower BMI values. We accounted for this by entering age of eating disorder onset in all models. All tests were two-tailed. Adjustments to p-values were completed using the method of false discovery rate (FDR) (Benjamini & Hochberg, 1995).
Path analysis was performed to further elucidate the relation between childhood anxiety as assessed by the CBCL-A scale and lowest BMI (Figure 1) using the PROC CALIS statement. In this model, age at which eating disorder occurred, AN subtype, and whether or not the individual exhibited any eating disorder symptom in the last year were entered in as covariates. The sample size for the path analysis was 262 women who had complete information for the caloric restriction, qualitative food item restriction, and exercise questions on the SAIB and whose mother had completed the CBCL. The qualitative restriction variable was dichotomized as follows: very severe qualitative food item restriction or less than very severe qualitative food item restriction.
Comparison of Samples
Using PROC GENMOD, the comparisons were made between the GAN and AN Trios sample. GEE corrections were used in the analyses to account for the non-independence of the data due to the inclusion of affected relatives in the analyses. It is possible that differences exist between the GAN and AN Trios sample and these differences may or may not have implications on the results of the path analysis. Therefore, FDR corrections were not performed for these comparisons so that all differences between the samples could be investigated.
RESULTS
Of all eligible participants in the GAN sample, 155 (47.6%) had AN restricting type and 171 (52.4%) had AN subtypes other than restricting. Mean (standard deviation) age of participants at the time of the interview was 25.6 (8.1) years, age of eating disorder onset was 16.4 (3.2) years and duration of eating disorder was 7.3 (6.2) years.
Lowest BMI values were used on a continuum and ranged from 8.8 to 18.0 kg/m2 with a mean (std) value of 14.3 (2.1) kg/m2. At the time of greatest caloric restriction for those who endorsed restricting calories, participants endorsed consuming between 0 and 1600 kcal/day with an average value of 532 (385) kcal/day. When asked about qualitative food restriction on the SIAB responses were as follows: 143 (45.5%) of participants were classified as having “very severe qualitative food restriction;” 102 (32.3%) had “severe qualitative food restriction;” 56 (17.7%) had “marked qualitative food restriction;” and 15 (4.8%) had little or no qualitative food restriction.
Lowest BMI was significantly associated with being described by mothers as too fearful or anxious prior to age 10 (p < 0.02) on the CBCL. Those who were anxious had a more extreme lowest BMI. Other variables from the CBCL and DOTS-R analyzed were not predictive of lowest BMI. Those who scored higher on sleep activity on the DOTS-R reported a significantly lower limit for daily caloric consumption (p < 0.02). The participants whose mothers rated them higher (indicating the item was more likely true) on the stubborn, sullen, or irritable CBCL item or on the self-conscious or easily embarrassed CBCL item endorsed significantly greater qualitative food item restriction (Table 1).
Table 1.
Results from analysis of variance and regression models predicting lowest BMI, caloric limit and qualitative restriction from items from the CBCL and DOTS-R. GEE were applied to all models and all models had age of onset, age at interview, and AN subtype as covariates. Chi-square (FDR corrected p-values) are presented.
| Childhood Measure | Lowest BMI Χ2 (p-value) | Caloric Limit Χ2 (p-value) | Qualitative Restriction Χ2 (p-value) |
|---|---|---|---|
| Childhood Behavior Checklist (CBCL) | |||
| Too Fearful or Anxious | 15.81 (0.02) | 5.42 (0.30) | 2.76 (0.46) |
| Stubborn, Sullen or Irritable | 0.72 ( 0.85) | 2.78 (0.46) | 10.22 (0.05) |
| Shy or Timid | 4.40 (0.30) | 3.59 (0.38) | 1.77 (0.59) |
| Self-Conscious or Easily Embarrassed | 5.11 (0.30) | 5.80 (0.30) | 10.20 (0.05) |
| Revised Dimensions of Temperament Survey- Child (DOTS-R) | |||
| General Activity | 0.89 (0.55) | 2.62 (0.30) | 0.73 (0.59) |
| Sleep Activity | 2.87 (0.30) | 16.44 (0.02) | 1.20 (0.47) |
| Sleep Rhythmicity | 0.09 (0.87) | 0.01 (0.97) | 0.22 (0.83) |
| Eating Rhythmicity | 0.03 (0.95) | 1.93 (0.38) | 0.13 (0.85) |
| Habit Rhythmicity | 1.33 (0.46) | 0.26 (0.83) | 0.00 (0.99) |
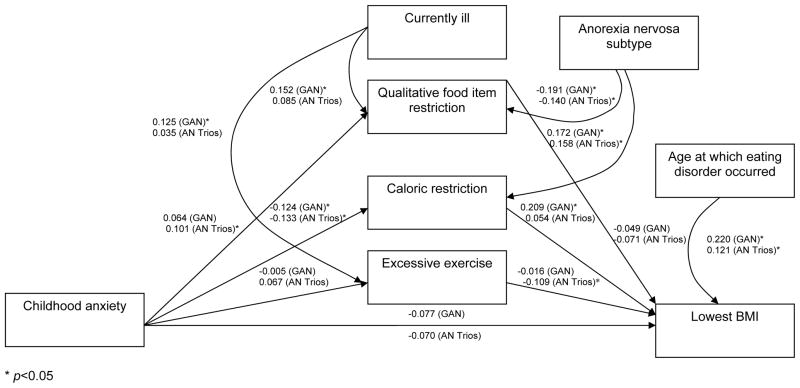
Characteristics of women in the GAN and AN Trios sample are listed in Table 2. Women in the GAN sample were significantly older at the time of AN onset (p <0.001), had a higher lowest BMI (p<0.002), and consumed more calories at the time of greatest caloric restriction (p< 0.02). Fewer women in the GAN sample were excessive exercisers compared with women in the AN Trios sample (41.2% vs. 52.2%, respectively p<0.006). Path analysis showed that the overall model predicted lowest BMI and this was validated using the AN Trios sample. In the GAN sample, higher childhood anxiety, as assessed by the CBCL-A scale, was associated with consuming a lower number of calories, and caloric restriction predicted lowest BMI. In the AN Trios sample, excessive exercise predicted lowest BMI, but neither caloric restriction nor food item restriction was significantly associated with lowest BMI. However, the relation between childhood anxiety and caloric restriction was confirmed in this sample. Standardized coefficients are presented in Figure 2.
Table 2.
| GAN (N=262) | AN Trios (N=383) | Results | |||
|---|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Χ2 (p-value) | ||
| Age (years) | 25.3 (8.1) | 25.0 (6.7) | 0.09 (0.77) | ||
| Age at Eating Disorder Onset (years) | 16.2 (3.1) | 14.5 (3.2) | 38.64 (<0.001) | ||
| Lowest BMI (kg/m2) | 14.2 (2.0) | 13.7 (1.9) | 10.43 (0.002) | ||
| Lowest Calorie Intake (kcal/day) | 533.3 (390.7) | 458.1 (320.9) | 6.17 (0.02) | ||
| Childhood Anxiety Scale | 6.7 (6.0) | 8.3 (5.7) | 0.15 (0.70) | ||
| No | Yes | No | Yes | Χ2 (p-value) | |
| N (%) | N (%) | N (%) | N (%) | ||
| Qualitative Food Restriction | 139 (53.0) | 123 (47.0) | 210 (54.8) | 173 (45.2) | 0.07 (0.80) |
| Excessive Exercise | 154 (58.8) | 108 (41.2) | 183 (47.8) | 200 (52.2) | 7.62 (0.006) |
| Currently Ill | 68 (26.0) | 194 (74.0) | 87 (22.7) | 296 (77.3) | 0.87 (0.36) |
| Restricting Anorexia Nervosa | 152 (58.0) | 110 (42.0) | 193 (50.4) | 190 (49.6) | 3.64 (0.06) |
Genetics of Anorexia Nervosa (GAN)
Price Foundation Anorexia Nervosa Trios Study (AN Trios)
p-values are not FDR corrected
Figure 2.
Relation between Childhood Anxiety and Low Body Mass Index (BMI) in the Genetics of Anorexia Nervosa (GAN) and Price Foundation Anorexia Nervosa Trios (AN Trios) Studies
* p<0.05
DISCUSSION
Anxiety disorders often presage the development of AN (Godart et al., 2000; Kaye et al., 2004; Raney et al., 2008) and multiple anxiety phenotypes have also been shown to aggregate strongly in families of probands with AN, suggesting familial transmission of anxiety proneness (Strober, Freeman, Lampert, & Diamond, 2007). Findings from this study extend previous research implicating anxiety in developmental liability to AN suggesting that it may also portend the attainment of an extremely low BMI during AN. Using childhood personality and temperamental features from 326 women affected with AN, we found a positive association between childhood anxiety and lowest BMI, and between anxiety-related childhood features and caloric and qualitative food item restriction, which could contribute to low BMI. Path analysis in the GAN sample revealed a relation between childhood anxiety and lowest BMI mediated through caloric restriction. Results from our validation sample confirmed the relation between childhood anxiety and caloric restriction in the context of AN.
AN is associated with numerous health risks (Berkman et al., 2007; Fichter et al., 2006; Papadopoulos et al., 2009; Sullivan, 1995; Sullivan et al., 1998), which increase with decreasing BMI (Lesinskiene et al., 2008; Swenne & Larsson, 1999). Preventing a slide to an extremely low BMI could be an important intervention goal facilitated by being able to identify those at risk. We hypothesized that women with AN who have greater inherent developmental difficulty in regulating anxious states have greater motivation to experience the anxiolytic effects of severe caloric restriction and higher physical activity, culminating in lower BMI in the context of AN.
In this retrospective study, children who were described by their mothers as “too fearful or anxious” reported a more extreme lowest BMI relative to their less anxious counterparts illustrating that early display of anxiety may be a harbinger of low BMI if AN develops. When applying the full CBCL-A scale, the relation between childhood anxiety and caloric restriction was present in both the GAN and AN Trios samples. Even though a direct relation between childhood anxiety measured by the CBCL-A scale and lowest BMI was not observed in the path analysis, findings of the relation between childhood anxiety and caloric restriction are in accordance with hypothesized anxiolytic properties of caloric restriction. Although exercise may also have anxiolytic properties, we did not observe a relation between childhood anxiety and excessive exercise in women with AN. It is possible that another factor is influencing the relation between childhood anxiety and lowest BMI. Perfectionism is also common in individuals with AN (Bulik et al., 2003; Fairburn, Cooper, Doll, & Welch, 1999; Wade et al., 2008) and is associated with being underweight for a longer period of time in the context of AN (Anderluh, Tchanturia, Rabe-Hesketh, Collier, & Treasure, 2008). Latent profile analyses in females with AN revealed that the severity of traits indicative of anxiety and perfectionism clustered together in mother daughter dyads (Jacobs et al., 2009). It is possible that perfectionism influenced the relation among childhood anxiety, caloric restriction, qualitative food item restriction, excessive exercise, and low BMI.
Children whose mothers reported them as being more stubborn, sullen, or irritable or more self-conscious or easily embarrassed were more likely to engage in qualitative food item restriction, but not more likely to have a lower BMI compared with the other individuals in the sample. In healthy men and women, higher food variety is associated with increased caloric intake and lower food variety with lower caloric intake (McCrory et al., 1999), suggesting that qualitative food item restriction may be another contributory mechanism to the attainment of a low BMI in at least some populations. It is possible that our measure of qualitative food item restriction was not sufficiently precise to indicate differences in BMI between individuals who engaged in high levels of qualitative food item restriction.
Individuals who were described by their mothers as being more active and restless during sleep (i.e., higher sleep activity) as a child, reported greater caloric restriction. Supporting this observation are reported associations between poor sleep quality and both anxiety disorders and eating disorders (Gregory et al., 2005; Ivanenko, Crabtree, Obrien, & Gozal, 2006; Lacey, Crisp, Kalucy, Hartmann, & Chien, 1975; Lauer & Krieg, 2004; Nobili et al., 2004), although this observation is not universal (Ong, Wickramaratne, Tang, & Weissman, 2006; Shaw & Steiner, 1997). On a related note, difficulty sleeping and sleep problems in children predict the onset of anxiety disorders in adulthood (Gregory et al., 2005). The observed high sleep activity could denote underlying anxiety which also indexes more severe restrictive behaviors during AN ultimately resulting in the attainment of low BMI, consistent with the relation between childhood anxiety and low BMI being mediated through caloric restriction.
The nature of the relation among rhythmicity, anxiety, and AN is not entirely clear. AN is associated with high eating and high sleep rhythmicity (Shaw & Steiner, 1997), but anxiety is associated with low eating and low sleep rhythmicity (Ong et al., 2006; Windle & Windle, 2006). In this sample we found no relation between any of the childhood rhythmicity variables and lowest BMI. It is plausible that the disconnect in rhythmicity between those with anxiety and those with AN could negate the possible influence of rhythmicity on BMI or rhythmicity may not be present until the actual development of AN and is not captured by early childhood measurements.
Limitations
There are several limitations to this study. First, the proposed overall model was validated by the AN Trios sample and the relation between childhood anxiety and caloric restriction was validated by the AN Trios sample, but caloric restriction did not mediate a relation between childhood anxiety and lowest BMI in the AN Trios sample. It is possible that differences in inclusion criteria, differences in our two samples, or the non-independence of the GAN sample (i.e., selected on the basis of affected relative pairs) contributed to differences in our findings. Families who have more than one affected individual may be different than families with only one affected individual; the GAN study required that each proband have a relative affected with AN while AN Trios study did not require participants to have another affected relative.
Second, mothers were interviewed about their affected child subsequent to the affected individual’s development of AN. It is possible that the questions about the child’s temperament and personality were shaped by factors mothers believed to have influenced the development of AN in their child. It is possible that the mother’s recollection of childhood personality and temperament features was influenced by the affected child’s position in family birth order, the personality and temperament of siblings, or time frame over which the parent was asked to recall childhood personality and temperament features. In addition, the children of the mothers who completed the CBCL may differ from those who did not complete the CBCL. In fact, there were no differences between the two groups in lowest BMI, age of eating disorder onset, caloric restriction, qualitative food item restriction, and excessive exercise. However, those affected individuals with CBCL data were younger, had a shorter duration of illness, and were more likely to have the restrictive AN subtype, but it is not known how these differences would influence the results of the present study. Third, the affected individuals’ recall of lowest BMI, caloric restriction, qualitative food item restriction, and excessive exercise could have been influenced by the woman’s point in the disorder (ill, recently recovered, long-term recovered) or the severity and cognitive impact of the lowest BMI when the participant was at her worst. We attempted to address this by controlling for current symptomatology in path analysis. Fourth, our measures may not have adequately captured AN behaviors. Measurements for qualitative food item restriction and excessive exercise may not have been sufficiently precise to detect relations among these items, childhood personality and temperamental features, and lowest BMI. In addition, determinations of the severity of qualitative food item restriction were made by the interviewers. However, all interviewers were trained in administration of the SIAB and used the standard criteria to make these determinations. To determine caloric restriction, participants were first asked “Did you try to set a limit to your caloric intake?” If the participant responded no, the interviewer did not ask “What was your lowest limit in calories per day at the time when you were restricting the most?”; therefore caloric limit was not available for all participants. Discrepancies between the number of calories a participant thought she was consuming and what she actually consumed may exist.
Conclusion
Anxiety may play a prominent role in the attainment of low BMI in individuals with AN and caloric restriction may have anxiolytic properties (Kaye et al., 2009). Certain behaviors associated with childhood anxiety may index individuals who may attain a low BMI if AN develops. The present study elucidates a relation between childhood anxiety and caloric restriction. Although the mechanisms underlying this association remain obscure, the association is intriguing in light of mounting developmental translational evidence linking anxiety early in life to behavioral and neurobiological sequelae which confer later risk to abnormal coping, alterations in hedonic motivation and locomotor activity, elevated stress reactivity, and elevated anxiety sensitivity. These factors may be mediated by abnormalities in stress related fronto-limbic structures now implicated in a broad range of neuropsychiatric phenotypes (see Pine, 2007). High levels of serotonin have been associated with increased anxiety, decreased appetite, and present in individuals with active AN and recovered from AN (Kaye et al., 2009). Although possibly a sequela of illness, elevated serotonin levels in recovered individuals may suggest that high premorbid serotonin levels presage illness (Kaye et al., 2009) and could index an underlying biological factor influencing the relation among childhood anxiety, caloric restriction, and lowest attained BMI. Of related potential significance is evidence of common neurotransmitter signaling and molecular variations in the central control of emotion arousal, exploratory behavior, and body weight regulation under conditions of stress (Domschke & Zwanzger, 2008; Smith, Lawrence, Sutton, & Gundlach, 2009). These works suggest the potential application of novel behavioral and neurodevelopmental strategies for exploring the mechanisms underlying clinically important phenotypic associations in AN and related eating disorders.
Acknowledgments
This research was supported by the National Institutes of Health Grant (MH66117). Dr. Dellava was supported by T32MH076694-03 (Bulik). Dr. Strober was supported in part by the Franklin Mint Endowed Chair in Eating Disorders. We express our gratitude to all families who participated in this research. The authors thank the Price Foundation for the support of the clinical collection of participants for the Price Foundation study. The authors thank the staff of the Price Foundation Collaborative Group for their efforts in participant screening and clinical assessments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T. Manual for Child Behavior Checklist/4–18 and 1991 Profile. Burlington VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Anderluh M, Tchanturia K, Rabe-Hesketh S, Collier D, Treasure J. Lifetime course of eating disorders: design and validity testing of a new strategy to define the eating disorders phenotype. Psychological Medicine. 2008:1–10. doi: 10.1017/S0033291708003292. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- Berkman ND, Lohr KN, Bulik CM. Outcomes of eating disorders: A systematic review of the literature. International Journal of Eating Disorders. 2007;40:293–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- Brewerton TD, Stellefson EJ, Hibbs N, Hodges EL, Cochrane CE. Comparison of eating disorder patients with and without compulsive exercising. International Journal of Eating Disorders. 1995;17:413–416. doi: 10.1002/1098-108x(199505)17:4<413::aid-eat2260170414>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Fear JL, Pickering A. Outcome of anorexia nervosa: eating attitudes, personality, and parental bonding. International Journal of Eating Disorders. 2000;28:139–147. doi: 10.1002/1098-108x(200009)28:2<139::aid-eat2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Tozzi F, Anderson C, Mazzeo SE, Aggen S, Sullivan PF. The relation between eating disorders and components of perfectionism. American Journal of Psychiatry. 2003;160:366–368. doi: 10.1176/appi.ajp.160.2.366. [DOI] [PubMed] [Google Scholar]
- Clausen L. Time to remission for eating disorder patients: a 2(1/2)-year follow-up study of outcome and predictors. Nordic Journal Psychiatry. 2008;62:151–159. doi: 10.1080/08039480801984875. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric Developments. 1986;4:167–226. [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM. Personality dimensions as a conceptual framework for explaining variations in normal, neurotic, and personality disordered behavior. In: Burrows GD, Roth M, Noyes R, editors. Handbook of Anxiety. Vol. 5. Elsevier Science Publishers; 1992. [Google Scholar]
- Domschke K, Zwanzger P. GABAergic and endocannabinoid dysfunction in anxiety - future therapeutic targets? Current Pharmaceutical Design. 2008;14:3508–3517. doi: 10.2174/138161208786848784. [DOI] [PubMed] [Google Scholar]
- Engeland A, Bjorge T, Selmer RM, Tverdal A. Height and body mass index in relation to total mortality. Epidemiology. 2003;14:293–299. [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Doll HA, Welch SL. Risk factors for anorexia nervosa: three integrated case-control comparisons. Archives of General Psychiatry. 1999;56:468–476. doi: 10.1001/archpsyc.56.5.468. [DOI] [PubMed] [Google Scholar]
- Fassino S, Abbate-Daga G, Amianto F, Leombruni P, Boggio S, Rovera GG. Temperament and character profile of eating disorders: a controlled study with the Temperament and Character Inventory. International Journal of Eating Disorders. 2002;32:412–425. doi: 10.1002/eat.10099. [DOI] [PubMed] [Google Scholar]
- Fassino S, Svrakic D, Abbate-Daga G, Leombruni P, Amianto F, Stanic S, et al. Anorectic family dynamics: temperament and character data. Comprehensive Psychiatry. 2002;43:114–120. doi: 10.1053/comp.2002.30806. [DOI] [PubMed] [Google Scholar]
- Fichter MM, Herpertz S, Quadflieg N, Herpertz-Dahlmann B. Structured Interview for Anorexic and Bulimic disorders for DSM-IV and ICD-10: updated (third) revision. International Journal of Eating Disorders. 1998;24:227–249. doi: 10.1002/(sici)1098-108x(199811)24:3<227::aid-eat1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fichter MM, Quadflieg N, Hedlund S. Twelve-year course and outcome predictors of anorexia nervosa. International Journal of Eating Disorders. 2006;39:87–100. doi: 10.1002/eat.20215. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. European Psychiatry. 2000;15:38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, Oconnor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. Journal of Abnormal Child Psychology. 2005;33:157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- Grice DE, Halmi KA, Fichter MM, Strober M, Woodside DB, Treasure JT, et al. Evidence for a susceptibility gene for anorexia nervosa on chromosome 1. American Journal of Human Genetics. 2002;70:787–792. doi: 10.1086/339250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen I, Heyerdahl S. Girls with anorexia nervosa as young adults: personality, self-esteem, and life satisfaction. International Journal of Eating Disorders. 2006;39:285–293. doi: 10.1002/eat.20248. [DOI] [PubMed] [Google Scholar]
- Ivanenko A, Crabtree VM, Obrien LM, Gozal D. Sleep complaints and psychiatric symptoms in children evaluated at a pediatric mental health clinic. Journal of Clinical Sleep Medicine. 2006;2:42–48. [PubMed] [Google Scholar]
- Jacobs MJ, Roesch S, Wonderlich SA, Crosby R, Thornton L, Wilfley DE, et al. Anorexia nervosa trios: behavioral profiles of individuals with anorexia nervosa and their parents. Psychological Medicine. 2009;39:451–461. doi: 10.1017/S0033291708003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce PR, McKenzie JM, Luty SE, Mulder RT, Carter JD, Sullivan PF, et al. Temperament, childhood environment and psychopathology as risk factors for avoidant and borderline personality disorders. Australian and New Zealand Journal of Psychiatry. 2003;37:756–764. doi: 10.1080/j.1440-1614.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiology and Behavior. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, et al. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. International Journal of Eating Disorders. 2003;33:257–267. doi: 10.1002/eat.10135. discussion 268–270. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Plotnicov K, Thornton L, Devlin B, Fichter MM, et al. The genetics of anorexia nervosa collaborative study: methods and sample description. International Journal of Eating Disorders. 2008;41:289–300. doi: 10.1002/eat.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. American Journal of Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews of Neuroscience. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Puliafico AC, Barmish AJ, Choudhury MS, Henin A, Treadwell KS. Assessing anxiety with the Child Behavior Checklist and the Teacher Report Form. Journal of Anxiety Disorders. 2007;21:1004–1015. doi: 10.1016/j.janxdis.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Ferrie JE, Batty GD, Davey Smith G, Elovainio M, Marmot MG, et al. Optimal form of operationalizing BMI in relation to all-cause and cause-specific mortality: the original Whitehall study. Obesity (Silver Spring) 2008;16:1926–1932. doi: 10.1038/oby.2008.322. [DOI] [PubMed] [Google Scholar]
- Lacey JH, Crisp AH, Kalucy RS, Hartmann MK, Chien CN. Weight gain and the sleeping electroencephalogram: study of 10 patients with anorexia nervosa. British Medical Journal. 1975;4:556–558. doi: 10.1136/bmj.4.5996.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer CJ, Krieg JC. Sleep in eating disorders. Sleep Medicine Reviews. 2004;8:109–118. doi: 10.1016/S1087-0792(02)00122-3. [DOI] [PubMed] [Google Scholar]
- Lesinskiene S, Barkus A, Ranceva N, Dembinskas A. A meta-analysis of heart rate and QT interval alteration in anorexia nervosa. World Journal of Biological Psychiatry. 2008;9:86–91. doi: 10.1080/15622970701230963. [DOI] [PubMed] [Google Scholar]
- McCrory MA, Fuss PJ, McCallum JE, Yao M, Vinken AG, Hays NP, et al. Dietary variety within food groups: association with energy intake and body fatness in men and women. American Journal of Clinical Nutrition. 1999;69:440–447. doi: 10.1093/ajcn/69.3.440. [DOI] [PubMed] [Google Scholar]
- McFarlane T, Olmsted MP, Trottier K. Timing and prediction of relapse in a transdiagnostic eating disorder sample. International Journal of Eating Disorders. 2008;41:587–593. doi: 10.1002/eat.20550. [DOI] [PubMed] [Google Scholar]
- Nobili L, Baglietto MG, Beelke M, De Carli F, Di Comite R, Fiocchi I, et al. Impairment of the production of delta sleep in anorectic adolescents. Sleep. 2004;27:1553–1559. doi: 10.1093/sleep/27.8.1553. [DOI] [PubMed] [Google Scholar]
- Norris R, Carroll D, Cochrane R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. Journal of Psychosomatic Research. 1992;36:55–65. doi: 10.1016/0022-3999(92)90114-h. [DOI] [PubMed] [Google Scholar]
- Ong SH, Wickramaratne P, Tang M, Weissman MM. Early childhood sleep and eating problems as predictors of adolescent and adult mood and anxiety disorders. Journal of Affect Disorders. 2006;96:1–8. doi: 10.1016/j.jad.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Papadopoulos FC, Ekbom A, Brandt L, Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. British Journal of Psychiatry. 2009;194:10–17. doi: 10.1192/bjp.bp.108.054742. [DOI] [PubMed] [Google Scholar]
- Penas-Lledo E, Vaz Leal FJ, Waller G. Excessive exercise in anorexia nervosa and bulimia nervosa: relation to eating characteristics and general psychopathology. International Journal of Eating Disorders. 2002;31:370–375. doi: 10.1002/eat.10042. [DOI] [PubMed] [Google Scholar]
- Pine D. Developing developmental psychopathology. Journal of Child Psychology and Psychiatry. 2007;48:113–114. doi: 10.1111/j.1469-7610.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- Pinter O, Probst M, Vandereycken W, Pieters G, Goris M. The predictive value of body mass index for the weight evolution in anorexia nervosa. Eating and Weight Disorders. 2004;9:232–235. doi: 10.1007/BF03325073. [DOI] [PubMed] [Google Scholar]
- Raney TJ, Thornton LM, Berrettini W, Brandt H, Crawford S, Fichter MM, et al. Influence of overanxious disorder of childhood on the expression of anorexia nervosa. International Journal of Eating Disorders. 2008;41:326–332. doi: 10.1002/eat.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reba L, Thornton L, Tozzi F, Klump KL, Brandt H, Crawford S, et al. Relationships between features associated with vomiting in purging-type eating disorders. International Journal of Eating Disorders. 2005;38:287–294. doi: 10.1002/eat.20189. [DOI] [PubMed] [Google Scholar]
- Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of Overall Obesity and Body Fat Distribution in Predicting Risk of Mortality. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2008.664. [DOI] [PubMed] [Google Scholar]
- Ribases M, Gratacos M, Badia A, Jimenez L, Solano R, Vallejo J, et al. Contribution of NTRK2 to the genetic susceptibility to anorexia nervosa, harm avoidance and minimum body mass index. Molecular Psychiatry. 2005;10:851–860. doi: 10.1038/sj.mp.4001670. [DOI] [PubMed] [Google Scholar]
- Salbach-Andrae H, Lenz K, Simmendinger N, Klinkowski N, Lehmkuhl U, Pfeiffer E. Psychiatric comorbidities among female adolescents with anorexia nervosa. Child Psychiatry and Human Development. 2008;39:261–272. doi: 10.1007/s10578-007-0086-1. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® Software: Version 9. Cary, NC: SAS Institute, Inc; 2004. [Google Scholar]
- Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, et al. Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. American Journal of Clinical Nutrition. 2008;87:810–816. doi: 10.1093/ajcn/87.4.810. [DOI] [PubMed] [Google Scholar]
- Sexton H, Maere A, Dahl NH. Exercise intensity and reduction in neurotic symptoms. A controlled follow-up study. Acta Psychiatricia Scandinavica. 1989;80:231–235. doi: 10.1111/j.1600-0447.1989.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Steiner H. Temperament in juvenile eating disorders. Psychosomatics. 1997;38:126–131. doi: 10.1016/S0033-3182(97)71481-6. [DOI] [PubMed] [Google Scholar]
- Shroff H, Reba L, Thornton LM, Tozzi F, Klump KL, Berrettini WH, et al. Features associated with excessive exercise in women with eating disorders. International Journal of Eating Disorders. 2006;39:454–461. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- Smith CM, Lawrence AJ, Sutton SW, Gundlach AL. Behavioral phenotyping of mixed background (129S5:B6) relaxin-3 knockout mice. Annals of the New York Academy of Sciences. 2009;1160:236–241. doi: 10.1111/j.1749-6632.2009.03953.x. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Grigoroiu-Serbanescu M, Boyadjieva S, Neumarker KJ, Metzke CW. The relevance of body weight in the medium-term to long-term course of adolescent anorexia nervosa. Findings from a multisite study. International Journal of Eating Disorders. 2009;42:19–25. doi: 10.1002/eat.20577. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Grigoroiu-Serbanescu M, Boyadjieva S, Neumarker KJ, Winkler Metzke C. Course and predictors of rehospitalization in adolescent anorexia nervosa in a multisite study. International Journal of Eating Disorders. 2008;41:29–36. doi: 10.1002/eat.20414. [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Lampert C, Diamond J. The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: evidence from a family study with discussion of nosological and neurodevelopmental implications. International Journal of Eating Disorders. 2007;40(Suppl):S46–51. doi: 10.1002/eat.20429. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. Mortality in anorexia nervosa. American Journal of Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Bulik CM, Fear JL, Pickering A. Outcome of anorexia nervosa: a case-control study. American Journal of Psychiatry. 1998;155:939–946. doi: 10.1176/ajp.155.7.939. [DOI] [PubMed] [Google Scholar]
- Swenne I, Larsson PT. Heart risk associated with weight loss in anorexia nervosa and eating disorders: risk factors for QTc interval prolongation and dispersion. Acta Paediatrica. 1999;88:304–309. doi: 10.1080/08035259950170079. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Frongillo EA, Jr, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. International Journal of Obesity and Related Metabolic Disorders. 1996;20:63–75. [PubMed] [Google Scholar]
- Van Wymelbeke V, Brondel L, Marcel Brun J, Rigaud D. Factors associated with the increase in resting energy expenditure during refeeding in malnourished anorexia nervosa patients. American Journal of Clinical Nutrition. 2004;80:1469–1477. doi: 10.1093/ajcn/80.6.1469. [DOI] [PubMed] [Google Scholar]
- Wade TD, Tiggemann M, Bulik CM, Fairburn CG, Wray NR, Martin NG. Shared temperament risk factors for anorexia nervosa: a twin study. Psychosomatic Medicine. 2008;70:239–244. doi: 10.1097/PSY.0b013e31815c40f1. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, et al. Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. Journal of the American Medical Association. 2006;295:2605–2612. doi: 10.1001/jama.295.22.2605. [DOI] [PubMed] [Google Scholar]
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur CJ, Colligan RC. Psychologic and behavioral correlates of anorexia nervosa. Journal of Developmental and Behavioral Pediatrics. 1981;2:89–92. [PubMed] [Google Scholar]
- Wilksch S, Wade TD. Differences between women with anorexia nervosa and restrained eaters on shape and weight concerns, self-esteem, and depression. International Journal of Eating Disorders. 2004;35:571–578. doi: 10.1002/eat.10273. [DOI] [PubMed] [Google Scholar]
- Windle M, Lerner R. Revised dimensions of temperament survey. 1985 unpublished manuscript. [Google Scholar]
- Windle M, Windle RC. Adolescent temperament and lifetime psychiatric and substance abuse disorders assessed in young adulthood. Personality and Individual Differences. 2006;41:15–25. [Google Scholar]