Abstract
Charcot-Marie-Tooth disease type 2C (CMT2C) is an autosomal dominant neuropathy characterized by limb, diaphragm, and laryngeal muscle weakness. Two unrelated families with CMT2C showed significant linkage to chromosome 12q24.11. All genes in this region were sequenced and heterozygous missense mutations were identified in the TRPV4 gene at positions c.805C>T and c.806G>A, causing the amino acid substitutions R269C and R269H. TRPV4 is a well known member of the TRP superfamily of cation channels. In TRPV4-transfected cells, the CMT2C mutations caused marked cellular toxicity and increased constitutive and activated channel currents. Mutations in TRPV4 were previously associated with skeletal dysplasias. Our findings indicate that TRPV4 mutations can also cause a degenerative disorder of peripheral nerves. The CMT2C mutations lie in a distinct region of the TRPV4 ankyrin repeats, suggesting that this striking phenotypic variability may be due to differential effects on regulatory protein-protein interactions.
Motor and sensory peripheral nerve cells are highly specialized, with long axons that connect the spinal cord with the periphery. Like all neurons, these cells are excitable and depend on ion channels for multiple functions including action potential propagation, synaptic transmission, plasticity, and cell survival. Charcot-Marie-Tooth (CMT) disease (or hereditary motor and sensory neuropathy) is a heterogeneous group of degenerative peripheral nerve disorders that together constitute the most common inherited neurological disease with an incidence of 1 in 2,5001. In CMT, progressive axonal degeneration and cell death result in disabling muscle weakness and sensory loss.
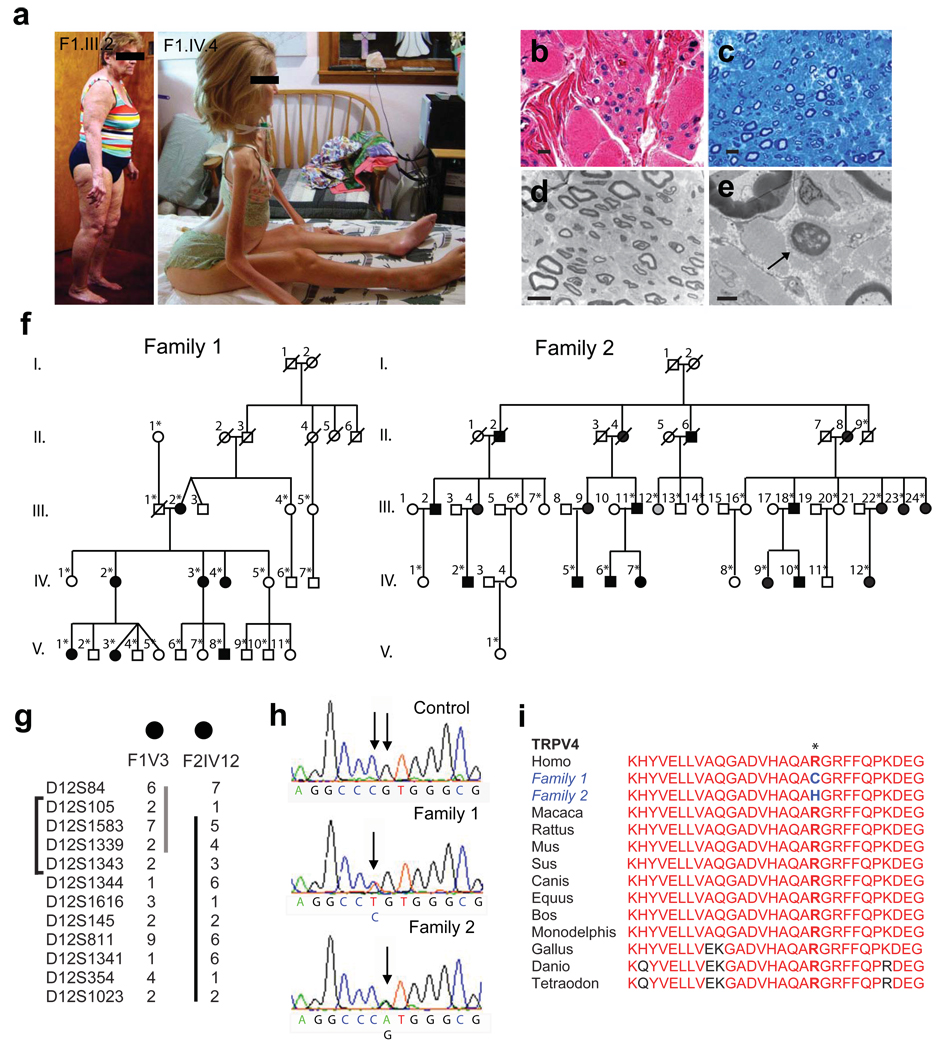
We examined subjects from two large families with CMT type 2C (Table and Fig. 1a–f), one of which was previously reported2. As described in prior studies2–4, affected individuals showed evidence of a motor greater than sensory axonal neuropathy causing progressive weakness of distal limb, diaphragm, and laryngeal muscles (Table). Few complained of sensory loss, but all had reduced or absent tendon reflexes. The age of onset and disease severity were highly variable (Table and Fig. 1a). Most affected patients reported worsening of hand weakness in the cold. Bilateral sensorineural hearing loss was documented in ten subjects, and nine subjects complained of bladder urgency and incontinence. Muscle and nerve biopsies in a severely affected individual showed marked neurogenic atrophy of the gastrocnemius muscle, indicating severe loss of motor nerve terminal innervation (Fig. 1b), and modest loss of sensory axons in the sural nerve with rare axons undergoing degeneration (Fig. 1c–e).
Table.
Phenotypic characteristics of subjects with CMT2C
| Patient | Age (yr) |
Sex | Age of onset (yr) |
First symptom |
Vocal fold paresis |
Limb weakness | Sensory loss |
Hearing loss |
|
|---|---|---|---|---|---|---|---|---|---|
| Proximal | Distal | ||||||||
| F1 III2 | 71 | F | 44 | Foot weakness | Mild | None | Moderate | Minimal | Yes |
| F1 IV2 | 48 | F | 5 | Foot weakness | Moderate | Mild | Severe | Mild | No |
| F1 IV3 | 46 | F | 5 | Stridor | Moderate | Mild | Moderate | Mild | No |
| F1 IV4 | 44 | F | 3 | Foot weakness | Severe | Severe | Severe | Moderate | Yes |
| F1 V1 | 24 | F | 7 | Foot weakness | ND | None | Mild | Mild | No |
| F1 V3 | 20 | F | 5 | Stridor | ND | None | Mild | Mild | No |
| F1 V8 | 18 | M | 2 | Stridor | Severe | None | Mild | Minimal | No |
| F2 III11 | 65 | M | 57 | Hearing loss | Mild | None | Mild | Minimal | Yes |
| F2 III18 | 61 | M | 34 | Foot weakness | Mild | Mild | Moderate | Minimal | Yes |
| F2 III22 | 64 | F | 35 | Foot weakness | Mild | Mild | Severe | Minimal | Yes |
| F2 III23 | 57 | F | 22 | Foot weakness | Moderate | None | Mild | Minimal | Yes |
| F2 III24 | 49 | F | <2 | Stridor | Moderate | Mild | Moderate | Mild | Yes |
| F2 IV2 | 50 | M | 16 | Hoarseness | Mild | Mild | Moderate | Mild | Yes |
| F2 IV5 | 53 | M | 28 | Hoarseness | Moderate | None | Mild | Minimal | Yes |
| F2 IV9 | 31 | F | 5 | Stridor | Moderate | None | Mild | None | No |
| F2 IV10 | 29 | M | 5 | Hoarseness | Moderate | None | Mild | Minimal | Yes |
| F2 IV12 | 29 | F | 2 | Stridor | Severe | Moderate | Severe | Mild | No |
ND: not done
Figure 1.
Phenotypic and genetic characteristics of CMT2C. a) Marked variability of disease severity is demonstrated by mild, late onset weakness in subject F1.III.2, but severe quadriparesis and respiratory failure in her daughter, subject F1.IV.4. Written consent was obtained to publish these photographs. b) Light microscope images of hematoxylin and eosin-stained sections of gastrocnemius muscle biopsy from subject F1.IV.4 demonstrating profound denervation atrophy of muscle. Scale bar=10 µm. c) Toluidine blue-stained section of sural nerve shows mild loss of large, sensory myelinated axons. Scale bar=10 µm. d) Electron microscopy of sural nerve confirms mild loss of large and small myelinated axons and unmyelinated axons. Scale bar=10µm. e) Arrow demonstrates a rare axon undergoing Wallerian-like axonal degeneration. Scale bar=2µm. f) Pedigrees of Families 1 and 2 demonstrating affected subjects in each of 3 generations. White=unaffected, black=affected, and grey=unknown disease status. *Sample collected. g) The haplotypes for subjects F1.V.3 and F2.IV.12 are demonstrated. Family 1 defines the lower border (marker D12S1343) and Family 2 defines the upper border (marker D12S105) of the region of interest. The bracket indicates the linked region. h) Sequencing shows a heterozygous C>T change at position 805 in Family 1 and a G>A change at position 806 in Family 2 in the TRPV4 gene. i) Protein homology of TRPV4 in various species. The mutations result in amino acid substitutions at R269, a highly conserved residue.
Fine mapping analysis in both families showed significant linkage to chromosome 12q24.11-12q24.21 (lod scores 3.1, 6.9, respectively), confirming the previous studies4–5. Haplotype reconstruction matched the disease status in all clinically affected subjects. Two individuals in family 1 carried the disease allele but were not found to be affected during limited examination in their homes. Combined data from families 1 and 2 narrowed the region of interest to a 2.6 Mb interval between the markers D12S105 and D12S1343 (Fig. 1g). Single nucleotide polymorphism (SNP) array analysis showed no duplications or deletions in this region (data not shown).
Sequencing of all 38 predicted protein-coding genes in the region of interest revealed heterozygous nucleotide variants c.805C>T and c.806G>A in exon 5 of the transient receptor potential vanilloid 4 (TRPV4) gene in affected family members of families 1 and 2, respectively (Fig. 1h). These sequence variants were not present in 209 ethnically matched controls. The identified mutations predict substitutions at the same amino acid (R269C, R269H) in the TRPV4 protein. This residue is conserved from fish and birds to humans (Fig. 1i). Sequencing of TRPV4 in two other families with a CMT2C-like phenotype failed to identify mutations, and in one linkage to chromosome 12q24.11 was excluded, suggesting that CMT2C may be genetically heterogeneous (Supplementary Fig. 1).
Mutations in TRPV4 have been previously associated with skeletal dysplasias6–7. TRPV4 was identified as the causative gene in these disorders in part due to high expression levels in cartilage compared to other tissue types6. TRPV4 has been reported to be expressed in motor neurons8, ventral root8, and dorsal root ganglion (DRG) neurons9. In situ hybridization studies show TRPV4 mRNA expression in ventral and dorsal horn neurons of adult mouse spinal cord (Allen Institute for Brain Science Spinal Cord Atlas: http://mousespinal.brain-map.org/imageseries/show.html?id=100017703). We investigated TRPV4 protein expression in spinal cord by immunohistochemistry (IHC) in TRPV4 null mice10 and wild type littermates (Supplementary Fig. 2). These studies showed low levels of expression in motor neurons. We further quantified TRPV4 expression levels in adult human tissues by quantitative reverse transcription PCR (qRT-PCR). Compared to tracheal cartilage, TRPV4 was expressed at approximately 20 fold lower levels in dorsal and ventral human spinal cord (Fig. 2a). Although it has been suggested that there may be alternatively spliced isoforms of TRPV4 lacking exon 5 and/or exon 711, we detected no differential expression of such isoforms in spinal cord tissue (Fig. 2a).
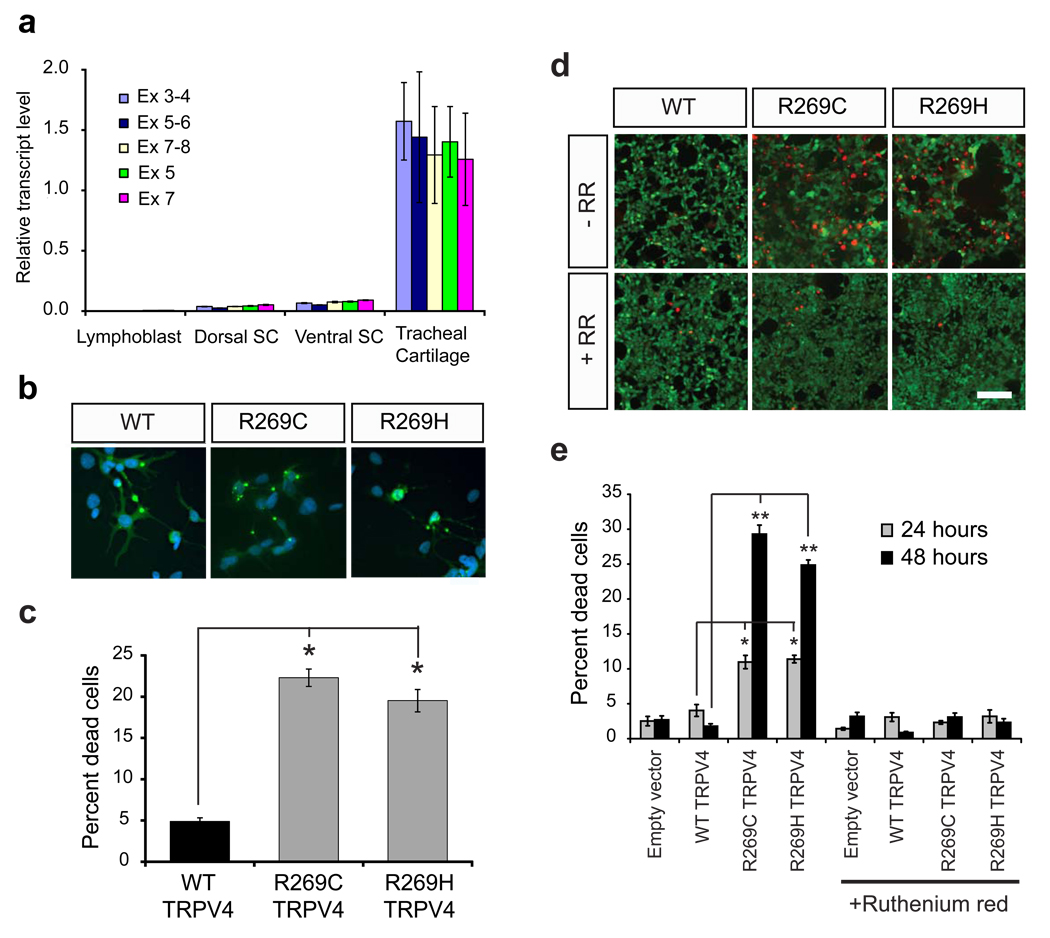
Figure 2.
Mutant TRPV4 causes neuronal toxicity. a) Quantification of TRPV4 transcript by qRT-PCR in control human lymphoblast lines (n=4), human dorsal spinal cord (n=3), ventral spinal cord (n=3), and tracheal cartilage (n=3) using qRT-PCR primers specific for following exon regions: exons 3–4, exons 5–6, exons 7–8, exon 5, and exon 7. Values are normalized to the first tracheal cartilage sample. Data is averaged; error bars, s.e.m. b) DRG neurons were transfected with WT and mutant forms of TRPV4 (green). At 16 hours, some cells expressing mutant forms of TRPV4 show evidence of early cellular toxicity with a collapsed cytoplasm. The nuclear DAPI stain is blue. Scale bar=40 µm. c) Quantification of propidium iodide uptake in DRG neurons expressing WT and mutant TRPV4 reveals a marked increase of cell toxicity in mutant expressing cells at 48 hours. *p<0.0001. d) HEK293 cells expressing R269C and R269H TRPV4 show an increase in the number of dead cells (red channel is EthD-1 stain) at 48 hours that is prevented by the TRP channel blocker ruthenium red (RR). Green channel is calcein-AM stain for live cells. e) Quantification of cell death in HEK293 cells indicates a time dependent increase in cell death that is blocked by the TRP channel blocker ruthenium red (RR). *p<0.01, **p<0.001. Data in d, f are averaged from three independent experiments; error bars, s.e.m.
Although TRPV4 was expressed at rather low levels in spinal neurons, mutant forms of TRPV4 were highly toxic to cultured neuronal cells. The CMT2C mutations caused a four-fold increase in cell death in DRG neurons compared to wild type (WT) TRPV4 at 48 hours (Fig. 2b,c). We further evaluated toxicity in human embryonic kidney (HEK) 293 cells (Fig. 2d,e). R269C and R269H TRPV4 caused a time-dependent increase in cell death, which was two-fold increased at 24 hours and approximately six-fold increased at 48 hours. This was associated with increased intracellular calcium levels at 24 hours (Supplementary Fig. 3). Importantly, increased calcium levels and cell death were blocked by the TRP channel inhibitor, ruthenium red (Fig, 2d,e, Supplementary Fig. 3), by the specific TRPV4 inhibitor RN173412 (Supplementary Fig. 4), and by the TRPV4 pore-inactivating mutation M680K13 (Supplementary Fig. 4). Together, these data indicate that the cell death is due to aberrant activity of the TRPV4 channel.
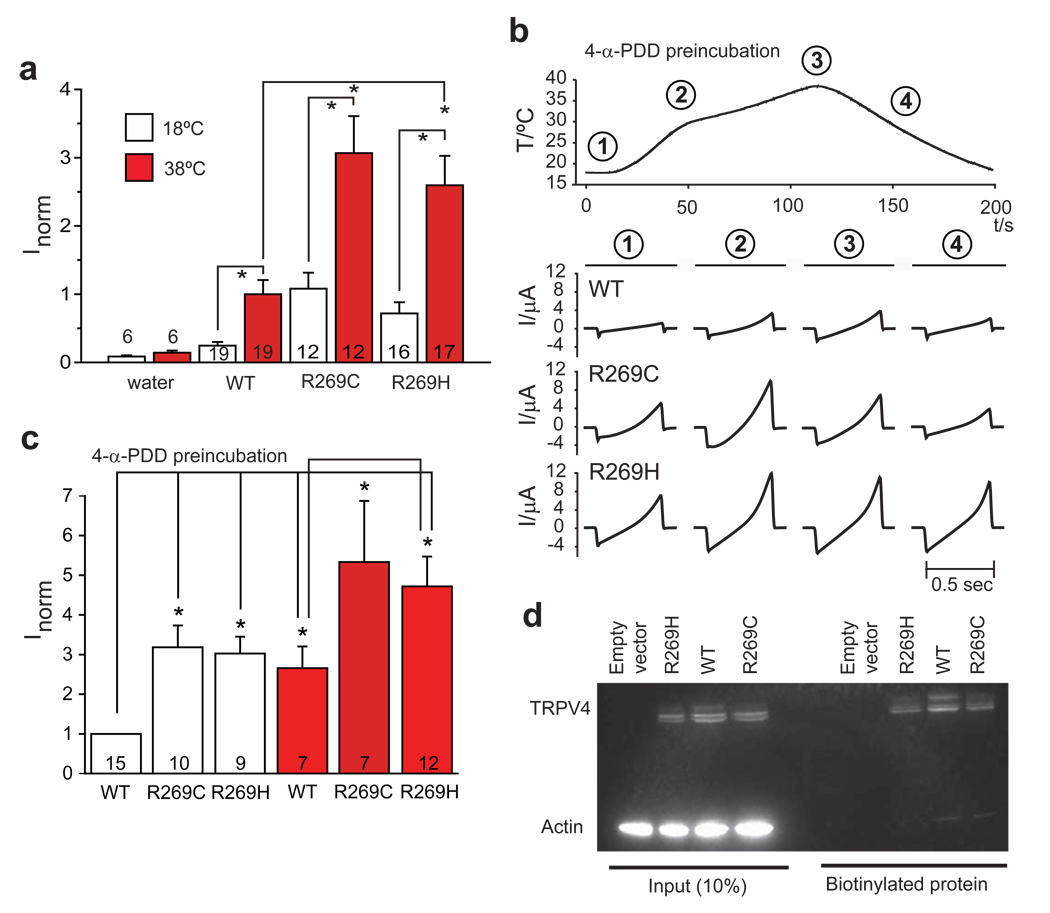
TRPV4 is a non-selective cation channel that responds to environmental stimuli such as temperature, hypotonicity, and chemicals including phorbol derivatives and arachidonic acid14–20. To investigate the effects of CMT2C mutations on TRPV4 channel activity, we expressed WT and mutant TRPV4 in Xenopus oocytes incubated in Ca2+-free frog’s Ringer solution. Oocytes expressing R269C and R269H TRPV4 showed a 2.5 fold increase in channel currents compared to WT under resting conditions (18°C) (Fig. 3a). Ruthenium red at 10 µM reduced WT, R269H, and R269C currents by 53%, 52%, 48%, respectively (p<0.05). When temperature was increased to 38°C, both WT and mutant channel currents increased 2–3 fold above their constitutive levels, with mutant channel current remaining 2–3 fold higher than WT (Fig. 3a). Similar results were obtained when the TRPV4 channels were activated by 4α-phorbol didecanoate (4αPDD) (Fig. 3b & c). Increasing temperature in the oocytes pre-incubated with 4αPDD resulted in a further increase in channel activity (Fig. 3b & c). Higher basal currents and enhanced responses to both 4αPDD and hypotonic stimulation were also observed in HEK293 cells transfected with R269C or R269H TRPV4 compared to WT (Supplementary Fig. 5). Increased channel activity could not be accounted for by changes in TRPV4 channel subcellular localization. Analysis of surface incorporation of TRPV4 by cell surface biotinylation assays showed approximately equal amounts of TRPV4 incorporated into the cell membrane (Fig. 3d). Furthermore, WT and mutant TRPV4 expressed in HeLa and HEK293 cells showed similar spatial distributions (Supplementary Fig. 6).
Figure 3.
The R269C and R269H mutations cause increased TRPV4 currents without a change in membrane localization. a) WT and mutant TRPV4 were expressed in Xenopus oocytes and currents were measured at 18°C and after heating to 38°C. Currents obtained at −100 mV were normalized to currents in WT at 38°C. Basal and stimulated channel activities of both mutants were significantly increased compared to WT, p<0.006. Data are averaged from the number of experiments per condition shown in the columns; error bars, s.e.m. *p<0.006. b) Representative current ramps obtained after pre-incubation in 5 µM 4-α-PDD (obtained from clamping cells from −100 to +100 mV in 500 ms). c) Summary of the data obtained in b). Currents obtained at −100 mV were normalized to WT at 18°C. *p<0.05. d) Immunodetection of TRPV4 proteins shows appropriately sized bands for TRPV4 in 10% of whole cell extract inputs and the biotinylated fraction. There is no difference in the amount of WT compared to mutant TRPV4 in the membrane fraction (biotinylated fraction). Shown is one representative blot of four independent experiments.
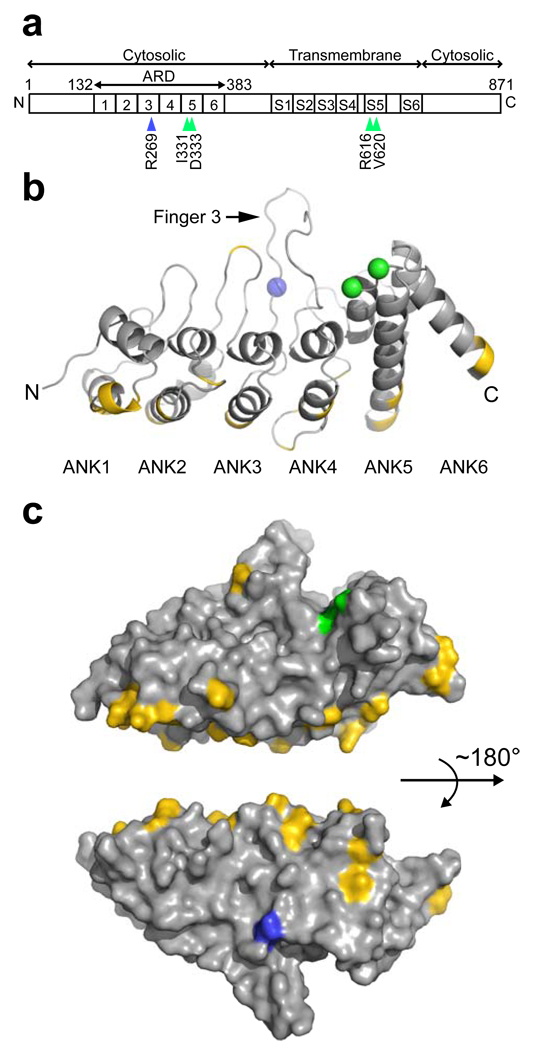
The TRPV4 protein has distinct domains including six transmembrane segments with a putative ion pore region between S5 and S6, an intracellular N-terminal domain containing six ankyrin repeats, and a C-terminal intracellular domain (Fig. 4a). Ankyrin repeats mediate protein-protein interactions, some of which have been shown to modulate TRPV4 activity21. The 2.3 Å crystal structure of the chicken TRPV4 ankyrin repeat domain (ARD) (Supplementary Table 1; Fig. 4b) shows a typical hand-shaped structure with a concave palm surface formed by inner helices and fingers, and a convex surface representing the back of the hand. R269 is located at the base of Finger 3 on the convex surface, opposite the palm surface harboring previously described skeletal dysplasia mutations in the ARD7 (Fig. 4b,c). Comparison with the TRPV1, TRPV2, and TRPV6 structures22–24 shows that the backbone structure is conserved at this position, although the arginine residue is not conserved in other TRPV channels (data not shown). The R269C and R269H substitutions are thus unlikely to disrupt the fold, but the R269 side chain is exposed and well-positioned to mediate protein-protein interactions that may be critical to TRPV4 function. Binding assays and co-immunoprecipitation experiments did not show changes in the interaction of mutant TRPV4 with two known protein partners, calmodulin21 and PACSIN325 (Supplementary Fig. 7), confirming that the CMT2C mutations do not grossly disrupt protein folding. Further experiments are needed to define whether specific intermolecular or intramolecular interactions are interrupted by the R269 mutations.
Figure 4.
The R269C and R269H mutations are located in the ankyrin repeat domain (ARD) of the TRPV4 protein. a) Primary structure of the TRPV4 protein, with the positions of the CMT2C (R269; blue arrowhead) and skeletal dysplasia6–7 (green arrowheads) mutations indicated below. b) Ribbon diagram of the chicken TRPV4 ARD, with the location of the R269 residue (R255 in chicken sequence) depicted as a blue sphere, and the I331 and D333 residues (I317 and D319 in chicken sequence, respectively) previously shown to be mutated in skeletal dysplasia as green spheres. c) Surface representation of the TRPV4 ARD with R269 in blue and I331 and D333 in green. Residues that differ between chicken and human TRPV4 are yellow, demonstrating that the palm and fingers regions are conserved.
CMT2C is an unusual peripheral neuropathy resulting in motor greater than sensory axonal degeneration. Here we have shown that despite low expression levels of TRPV4 in neurons, the TRPV4 CMT2C mutations are highly toxic due to increased channel activity causing calcium overload. Ca2+ is an essential intracellular signal in neurons regulating neurite outgrowth, synaptogenesis, synaptic transmission, and plasticity, but excessive influx of Ca2+ has also long been associated with neurodegeneration26. Interestingly, many of our severely affected patients had sensorineural hearing loss and bladder urgency (Table 1). TRPV4 knock-out mice have been shown to have sensorineural hearing loss, impairment of bladder voiding, and abnormalities of mechanosensation and nociception10,27–28 indicating that some of the clinical manifestations of CMT2C may be attributable to a loss of normal TRPV4 function, as well as to the toxic effects of abnormal channel opening and calcium influx.
Mutations of TRPV4 have been previously associated with autosomal dominant skeletal dysplasias. Brachyolmia is caused by R616Q and V620I mutations, which increase basal and stimulated channel activity, perhaps via direct alteration of the S5 pore domain6. More recently, the D333G and I331F mutations have been identified in the 5th ankyrin repeat in single patients with spondylometaphyseal dysplasia Kozlowski type and metatropic dysplasia, respectively7. TRP channels operate within macromolecular complexes29, and the ankyrin motifs are likely critical mediators of protein-protein interactions that regulate TRP channel localization and activity. The R269 residue localized to the convex face of the TRPV4-ARD may mediate distinct interactions with yet-to-be identified regulatory partners particularly important for TRPV4 function in peripheral neurons.
TRPV4 is a cation channel known to play a role in fundamental cellular processes such as osmotic-, temperature-, and mechano-sensation. In this study, we have shown that CMT2C is associated with mutations at the R269 residue in the ARD of the TRPV4 protein. This work uncovers a previously unrecognized role for TRPV4 in neurons and highlights the importance of this ion channel in normal peripheral nerve function. Together with the observations that disruption of TRPML1 causes the central nervous system lysosomal storage disease, mucolipidosis type IV30, and mutation of TRPC3 causes degeneration of cerebellar neurons in mice31, this study emphasizes the importance of TRP channels in the pathogenesis of neurodegenerative diseases. Most surprisingly, our data indicate that distinct mutations in this important ion channel result in strikingly different phenotypes that involve separate organ systems.
MATERIALS AND METHODS
Subjects
Subjects were enrolled in NINDS Institutional Review Board or University College London Research Ethics Committee -approved protocols. Informed consent was obtained from all subjects. Family 2 was part of a kindred previously reported2.
Genetic Analysis
Samples were genotyped at deCODE Genetics (Reykjavik, Iceland). Twelve polymorphic markers at 0.04 to 1.6 cM intervals covering the previously reported CMT2C region5 were used for fine mapping. Multipoint parametric and nonparametric analyses and haplotype reconstruction was done using Genehunter 2.032 and Simwalk2 v2.9133. Mendelian inconsistencies were checked using PedCheck (version 1.1); genotyping data was formatted for Simwalk2 using mega2 (v4.0); and haplotypes were visualized using HaploPainter (v.029.5). SNP array analysis (Illumina Infinium II HumanHap550K SNP chips BeadStation v2.3.25, Illumina Inc., San Diego, CA, USA) was performed using DNA samples from one affected individual from families 1 and 2.
qRT-PCR
RNA was isolated from human lymphoblast cells, spinal cord, and tracheal cartilage (Maryland Brain and Tissue Bank) using Trizol (Invitrogen) and converted to cDNA using High Capacity cDNA RT kit (Applied Biosystems (ABI)). PCR reactions were run using TRPV4 and glucoronidase (hGUS) primers (ABI) and the ABI Prism 7900 Sequence Detector System as described34.
Expression of TRPV4 in Mammalian Cells and Xenopus Oocytes
Constructs (pcDNA3.1, pcDNA3.1-FLAG, and pcDNA3.1-YFP) expressing human TRPV4 cDNA were provided by Miguel Angel Valverde11. R269C, R269H, and M680K mutants were generated using QuickChange (Stratagene). For expression in Xenopus oocytes, TRPV4 cDNA constructs were obtained by PCR of mouse kidney cDNA and inserted into pTLB. These cDNAs were subcloned into a modified pIRES-CD8 for mammalian cell transfection. HEK293, HeLa, and DRG cells were transfected using Lipofectamine 2000 (Invitrogen) or polyethyleneimine in Optimem (Invitrogen). Cell surface expression of TRPV4 was determined by incubating transfected cells with SulfoLink NHS Biotin (Pierce) for 1 h at 4°C. Post-nuclear supernatant was incubated with Streptavidin agarose for 60 min at room temperature. Beads were pelleted, washed, and protein recovered by heating with reducing loading buffer. Five per cent input and half of the biotinylated protein were separated on 4–12 % SDS gels using MOPS buffer (Invitrogen) and visualized by Western blot using anti-TRPV4 (Sigma #T9075) and mouse anti-actin (Sigma) antibodies.
Immunohistochemistry
Cells were transfected and fixed with 4 % paraformaldehyde (PFA)/0.05 % NP-40. TRPV4 null and wild-type littermate mice were transcardially perfused with 4 % PFA. Spinal cords were cut into 50 µm cryostat sections. Antibodies used were: rabbit anti-TRPV4 (Sigma), rabbit anti-TRPV415, rabbit anti-TRPV4 (gift from Stefan Heller), monoclonal anti-TRPV4 (Alomone), rabbit anti-FLAG (Abcam), monoclonal anti-calnexin (Becton Dickinson), acetylated tubulin (Sigma), goat anti-choline acetyltransferase (Chemicon), and Alexa Fluor conjugated 488, 546, or 594 secondary antibodies (Molecular Probes). Images were obtained using Zeiss LSM-510 META confocal microscope.
Cell Death Assays
DRGs35 were transfected with TRPV4 pcDNA3.1-YFP constructs, harvested in PBS-10% serum containing 25 µg/ml propidium iodide (PI; Sigma), and sorted using FACS Calibur Flow Cytometer and CellQuest Pro software (BD Biosciences) recording ≥50,000 events. Percent cell death was the percentage of YFP/PI double positive cells.
HEK293 cells were transfected with TRPV4 pcDNA3.1-FLAG constructs using 0.1 µg of TRPV4 plasmid and 0.4 µg empty vector per well of a 24 well plate. Ruthenium red (RR) 10µM (Sigma) or RN1734 10µM was added 4 hours post-transfection. At 24 or 48 hours, cells were incubated with 2 µM calcein AM and 4 µM EthD-1 (Molecular Probes) and imaged using Zeiss AxioImager Z1 microscope. Dead cells (red) and live cells (green) were counted using Zeiss Axiovision 4.6 software. Equal expression of TRPV4 was confirmed by Western blot of cells transfected under identical conditions.
Calcium Imaging
HEK293 cells were transfected with human TRPV4 and the fluorescent marker mCherry. Cells were replated onto polyornithine-coated coverslips 4.5 hours after transfection and maintained at 37 °C/5% CO2. RR 10 µM was added at the time of replating. Calcium imaging was done at 22–24 hours15.
Plate-Based Intracellular Calcium Assay
Intracellular calcium levels were determined using a calcium assay kit according to manufacturer’s instructions (# R8041, Molecular Devices). HEK293 cells were transfected. At 4 hours, RN1734 was added at 10µM. At 24 hours, media was removed, 50 µl of Calcium Assay Reagent was added, and incubated at 37°C for 1 hour. Fluorescence was quantified using a fluorescent imaging plate reader (FLIPR, Molecular Devices).
Electrophysiology
Mouse TRPV4 cRNA was transcribed from pTLB constructs. 0.5–5 ng was injected into oocytes incubated in nominally Ca2+-free solution. Whole-cell membrane currents were measured using an npi Tec01 TEVC amplifier (npi, Tamm, Germany). Currents were activated by increasing temperature using peltier-based solution cooler/heater (CL-100, Harvard Apparatus, Kent, UK) or by preincubation with 5 µM 4αPDD (LC laboratories) for 30 minutes. Temperature-activated currents were measured initially and when the bath had reached 38°C (see Fig. 3b, points 1 and 3). Pre-incubation with 4αPDD rather than acute application was used due to the observed slow activation of TRPV4 currents in oocytes. A ramp or pulse protocol was used clamping oocytes from −100 to + 100 mV and currents compared at −100 mV. Each experimental condition was replicated in at least 3–4 different oocyte batches. Recordings were performed in a solution containing in mM: 100 Na-gluconate, 20 Ca-gluconate, 4 MgSO4, 5 HEPES, pH 7.5.
HEK293 cells were transfected as described with addition of pNEGFP for visual selection of transfected cells. Whole-cell currents were measured at 25°C with Axopatch 200B (Molecular Devices) using a repeated ramp protocol (−100 to +100 mV, 400 ms, every 2 s). The solutions were (in mM): standard extracellular, 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES, pH 7.4 with NaOH; hypotonic (200 mOsm), NaCl reduced to 80; isotonic (320 mOsm), 120 mannitol added to hypotonic solution; and pipette solution, 140 CsCl, 5 EGTA, 10 HEPES, pH 7.2 with CsOH.
Protein structure determination
TRPV4-ARD (132–383) was purified as described21. TRPV4-ARD crystals were grown in hanging drops at 4°C in 0.1 M sodium citrate pH 5.0, 10% MPD, 2% PEG8000, 4.3% trifluoroethanol and frozen in reservoir with 20% PEG4000 (crystal form I), or 0.025 M KH2PO4, 7% MPD, 7% PEG8000, and 5 mM DTT and frozen in reservoir with 25% PEG400 (crystal form II). Data were collected at 110 K and 1.54 Å on a Micromax007/R-AXIS IV++ (Rigaku/MSC Inc) and processed with HKL2000. Structures were determined by molecular replacement using TRPV2-ARD22 as search model in MOLREP, model building in COOT, and refinement using REFMAC. Data and refinement statistics are in Supplementary Table. Coordinates were deposited in the Protein Data Bank (3JXI and 2JXJ are crystal forms I and II, respectively).
Co-Immunoprecipation
HEK293 cells were co-transfected with pcDNA3 expressing human PACSIN1, PACSIN2, or PACSIN3 (gifts of Markus Plomann) and FLAG-TRPV4. At 24 hours, cells were harvested and cleared cell lysates were incubated with anti-FLAG M2 antibody (Sigma) for 6 hours followed by overnight incubation with Dynabeads Protein G (Dynal, Oslo). The beads were washed, and bound proteins were separated by SDS-PAGE and analyzed by immunoblotting with antibodies to PACSIN3, PACSIN2 and PACSIN1 (gifts of Marcus Plomann), respectively. TRPV4 imunoprecipitation was verified by immunoblotting with anti-FLAG M2 antibody.
Protein Binding Assays
WT and mutant human TRPV4-ARDs including the N-terminal proline-rich region (residues 136–397) were expressed and purified as the chicken TRPV4-ARD. Mouse PACSIN3-SH3 domain (residues 361–424; single difference between mouse and human PACSIN3-SH3 is V362A; cDNA provided by Stefan Heller) was cloned into NdeI and NotI sites of pET21-C6H, expressed in BL21 (DE3) induced with 0.4 mM isopropyl-β-D-thiogalactopyranoside at room temperature. Cells were lysed by sonication in lysis buffer (20 mM TrisHCl pH 8.0, 300 mM NaCl, 20 mM imidazole pH 7.0 and 1 mM phenylmethylsulfonyl fluoride) with 0.1% Triton X-100, 0.2 mg/ml lysozyme, 50 µg/ml RNAse A and 25 µg/ml DNAse I. The cleared lysate was loaded onto Ni-NTA (Qiagen) and eluted with steps of 25, 50, 100, 150 and 250 mM imidazole pH 7 in lysis buffer, adding 10 mM EDTA pH 8.0 and 1 mM dithiothreitol (DTT) after elution. Relevant fractions were pooled, dialyzed, purified on Superdex75 (GE Healthcare) in 10 mM TrisHCl pH 8.0, 50 mM NaCl, 1 mM DTT, and concentrated to >20 mg/ml in centrifugal filters. For PACSIN3-SH3 interaction assays, samples contained 25 nmol TRPV4-ARD with or without 37.5 nmol PACSIN3-SH3 in 125 µl pre-incubated for >30 min, and 100 µl were loaded onto a Superdex75 10/30 column (GE Healthcare) in 20 mM Tris pH 7.0, 150 mM NaCl, 1 mM DTT at 4°C. Calmodulin-agarose binding assays were performed essentially as described21.
Statistics
Data were analyzed using Excel (Microsoft) and the Strathclyde electrophysiology suite (University of Strathclyde). Statistical significance was determined using paired or unpaired Student's t tests where appropriate.
Supplementary Material
Acknowledgements
We are grateful to the patients and their families for participating in this study. We thank John Kissel for discussions regarding clinical evaluation of family 1, Alison LaPean for aid in patient characterization, Mary Kay Floeter and Tanya Lehky for performing neurophysiological studies, Ruiqi R. Wang for crystallization and x-ray data collection, John Hardy for helpful discussion regarding genetic analysis, Andrew Singleton and Dena Hernandez for help with single nucleotide polymorphism array analysis, the NINDS DNA sequencing facility for help with sequencing, the Maryland Brain and Tissue Bank for providing human spinal cord and tracheal tissues, Makoto Suzuki for TRPV4 knockout mice, Roger Tsien for providing mCherry, Ahmet Hoke for providing DRG cells, John Griffin for aid in nerve pathology evaluation, Stefan Heller for providing TRPV4 antibody, Thomas Jentsch for cells and instruments, Camilo Rojas and Jesse Alt for help with FLIPR, Shane Minogue for assistance in confocal imaging, Miguel Angel Valverde for providing human TRPV4 expression constructs, and Marcus Plomann for providing PACSIN expression constructs and antibodies. This work was supported by intramural funds from the National Institute of Neurological Disorders and Stroke at NIH, funds from the Johns Hopkins Department of Neurology, the David and Elaine Potter Charitable Foundation, and NIH grant R01GM081340 and a McKnight Scholar Award to RG.
Footnotes
Author contributions
C.J.S., K.H.F., and R.K. directed the study and C.J.S wrote the paper. C.J.S., K.H.F., C.L.L., H.H. and G.L. evaluated patients. R.K., H.C.S., K.H.F. and G.L. did the genetic analysis and Y.S., A.A.T., R.P., R.K., K.H.F, and G.L. carried out the gene sequencing. B.G.B., T.L.M., L.K., and C.J.S. performed qRT-PCR, IHC, cell death assays, and co-IP experiments. A.A.Z. completed cell surface biotinylation and electrophysiology in Xenopus oocytes. H.I. and R.G. did electrophysiology in HEK cells and protein binding assays. Protein structure determination was done by R.G., S.S.C., and C.B.P. Cell calcium imaging was completed by C.H.M. and M.J.C.
The authors have no competing financial interests.
References
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 2.Dyck PJ, et al. Hereditary motor and sensory neuropathy with diaphragm and vocal cord paresis. Ann Neurol. 1994;35:608–615. doi: 10.1002/ana.410350515. [DOI] [PubMed] [Google Scholar]
- 3.Santoro L, et al. Charcot-Marie-Tooth disease type 2C: a distinct genetic entity. Clinical and molecular characterization of the first European family. Neuromuscul Disord. 2002;12:399–404. doi: 10.1016/s0960-8966(01)00305-4. [DOI] [PubMed] [Google Scholar]
- 4.McEntagart ME, et al. Confirmation of a hereditary motor and sensory neuropathy IIC locus at chromosome 12q23-q24. Ann Neurol. 2005;57:293–297. doi: 10.1002/ana.20375. [DOI] [PubMed] [Google Scholar]
- 5.Klein CJ, et al. The gene for HMSN2C maps to 12q23-24: a region of neuromuscular disorders. Neurology. 2003;60:1151–1156. doi: 10.1212/01.wnl.0000055900.30217.ea. [DOI] [PubMed] [Google Scholar]
- 6.Rock MJ, et al. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet. 2008;40:999–1003. doi: 10.1038/ng.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krakow D, et al. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet. 2009;84:307–315. doi: 10.1016/j.ajhg.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facer P, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VROAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 11.Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem. 2006;281:1580–1586. doi: 10.1074/jbc.M511456200. [DOI] [PubMed] [Google Scholar]
- 12.Vincent F, et al. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Voets T, et al. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 14.Alessandri-Haber N, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 15.Guler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe H, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 18.Vriens J, et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everaerts W, Nilius B, Owsianik G. The vallinoid transient receptor potential channel Trpv4: From structure to disease. Prog Biophys Mol Biol. 2009 doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Liedtke W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann N Y Acad Sci. 2008;1144:42–52. doi: 10.1196/annals.1418.012. [DOI] [PubMed] [Google Scholar]
- 21.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3 and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. Journal of Biological Chemistry. 2010 doi: 10.1074/jbc.M109.052548. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- 23.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R. Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry. 2008;47:2476–2484. doi: 10.1021/bi702109w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuajungco MP, et al. PACSINs bind to the TRPV4 cation channel. PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem. 2006;281:18753–18762. doi: 10.1074/jbc.M602452200. [DOI] [PubMed] [Google Scholar]
- 26.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 27.Tabuchi K, Suzuki M, Mizuno A, Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci Lett. 2005;382:304–308. doi: 10.1016/j.neulet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Gevaert T, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark K, Middelbeek J, van Leeuwen FN. Interplay between TRP channels and the cytoskeleton in health and disease. Eur J Cell Biol. 2008;87:631–640. doi: 10.1016/j.ejcb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Venkatachalam K, et al. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker EB, et al. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci U S A. 2009;106:6706–6711. doi: 10.1073/pnas.0810599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES. Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet. 1996;58:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 33.Sobel E, Sengul H, Weeks DE. Multipoint estimation of identity-by-descent probabilities at arbitrary positions among marker loci on general pedigrees. Hum Hered. 2001;52:121–131. doi: 10.1159/000053366. [DOI] [PubMed] [Google Scholar]
- 34.Avila AM, et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J Clin Invest. 2007;117:659–671. doi: 10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Mi R, Haughey N, Oz M, Hoke A. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J Peripher Nerv Syst. 2007;12:121–130. doi: 10.1111/j.1529-8027.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.