Abstract
Several lines of evidence suggest that acetylcholinesterase inhibitors (AChE) inhibitors have their cognitive enhancing effects by stimulating cholinergic receptors within the medial septum. However, intraseptal administration of cholinergic enhancing drugs produce mixed results that appear to depend on both the integrity of the medial septum as well as task demands. Three experiments were conducted to determine the relationship between increased cholinergic activity within the medial septum and hippocampus and behavioral recovery in a model of diencephalic amnesia produced by pyrithiamine-induced thiamine deficiency (PTD). In Experiment 1, systemic tacrine (0.0, 0.75, 1.5 mg/kg) was administered to PTD and pair-fed (PF) rats prior to a spontaneous alternation task. Without tacrine, PF rats alternated at a higher rate than PTD rats. Both doses of tacrine increased alternation in PTD rats to within the range of PF rats. In Experiment 2, three doses of intraseptal tacrine (2.5, 5.0, 12.5 μg) were administered to PTD and PF rats and changes in hippocampal acetylcholine efflux were assessed. Both the 5.0 and 12.5 μg doses significantly increased hippocampal acetylcholine levels, but the change was greater in the PTD rats. In Experiment 3, despite the fact that both intraseptal doses of tacrine (5.0, 12.5 μg) increased hippocampal acetylcholine levels, only 5.0 μg significantly improved alternation scores in PTD rats. Thus, when there is basal forebrain cholinergic cell loss in conjunction with diencephalic pathology, the therapeutic range of AChE-I in the medial septum and the effective doses do not directly map onto changes in acetylcholine efflux in the hippocampus.
Index words: Diencephalic amnesia, Septohippocampal pathway, acetylcholine, Tacrine, Rat
1. Introduction
Wernicke-Korsakoff syndrome, a diencephalic amnesia produced by thiamine deficiency, can be modeled in rodents using pyrithiamine-induced thiamine deficiency (PTD). PTD-treated animals display damage in the anterior and midline thalamus, mammillary bodies, (Langlais and Savage, 1995; Langlais et al., 1996; Mair, 1994), and selective regional and phenotypic loss of choline acetyltransferase (ChAT)-positive neurons in the medial septum/diagonal band (Savage et al., 2007). This neuropathology likely contributes to the loss of functional acetylcholine efflux in the hippocampus in PTD rats that parallels their poor spatial performance (Roland and Savage, 2007; Savage et al., 2007; Vetreno et al., 2008).
Previous work has shown that intrahippocampal physostigmine can increase spontaneous alternation in rats that have a loss of cholinergic neurons in the medial septum (Chang and Gold, 2004). Similarly, both systemic and intrahippocampal physostigmine administration increases hippocampal acetylcholine release and spontaneous alternation performance in PTD animals (Roland et al., 2008). However, it has been suggested that acetylcholinesterase (AChE) inhibitors are likely exerting their cognitive enhancing effects via activation of cholinergic receptors in the medial septum/diagonal band (Mulder et al., 2005; Wu et al., 2003). The medial septum has abundant cholinergic receptors on both cholinergic and GABAergic neurons (Alreja et al., 2000b; Gao et al., 1995) and modulation of these receptors affect hippocampal neurochemistry and physiology (Freund and Buzsaki, 1996; Frotscher and Leranth, 1985; Wu et al., 2000).
Behavioral studies that have examined intraseptal infusions of AChE inhibitors and other cholinomimetic drugs have produced mixed results (Bunce et al., 2003; 2004; Frick et al., 1996; Givens and Olton, 1990; 1995; Markowska et al., 1995; Pang and Nocera, 1999). For example, intraseptal infusion of tacrine, at doses of 2.5 and 12.5 μg, has been shown to increase memory performance in cognitively impaired young rats (Sabolek et al., 2004a), but impair performance in aged (Sabolek et al., 2004b) and normal adult rats (Sabolek et al., 2005). Given past research with tacrine and related drugs, it appears that cholinergic manipulations of the medial septum/diagonal band may depend on the interaction of several factors, including brain integrity, cognitive status and the task used for behavioral assessment.
The current series of studies combine behavioral and neurochemical approaches to directly relate increased cholinergic receptor activity in the medial septum to changes in hippocampal acetylcholine levels and behavior in control- and PTD-treated rats. This series of experiments is the first to assess how systemic administration and direct infusion of tacrine into the medial septum/diagonal band alter behavioral outcomes in normal and PTD-treated rats with a loss of cholinergic neurons and diencephalic neuropathology. Furthermore, changes in acetylcholine levels in the hippocampus were assessed after intraseptal infusion of tacrine and during behavioral testing. Our laboratory has shown that increasing hippocampal acetylcholine levels in PTD animals improved cognitive performance (Roland et al., 2008; Roland and Savage, 2009b). Consequently, these experiments are aimed at determining how increasing cholinergic activity in the medial septum alters both behavioral and hippocampal functioning in normal and amnestic rodents.
2. Materials and methods
2.1 Animals
Male Sprague-Dawley rats, initially 3–4 months old, were obtained from Harlan Corporation (Harlan; Indianapolis, IN). The animals were pair-housed in clear plastic cages with free access to water in a controlled vivarium [temperature: 22 ± 1 °C; humidity: 50 ± 10%; 12-h light/dark cycle (lights on at 07:00 AM)]. Naive groups of animals were used in each experiment (Exp 1–3). After cannulae implantation (Exp 2, 3), animals were individually housed. Every effort was made to minimize animal suffering and the number of animals used. All experiments were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1996). The experimental procedures were approved by the Binghamton University Committee on Animal Care and Use (IACUC).
2.2 Pyrithiamine-induced thiamine deficiency (PTD) and pair-fed (PF) treatment
Animals for all three experiments were randomly assigned to one of the following treatments: (i) pair-fed control (PF), or (ii) pyrithiamine-induced thiamine deficiency (PTD). Subjects in the PTD group were free-fed a thiamine deficient chow (Teklad Diets, Madison, WI) and given daily injections (0.25 mg/kg, i.p.) of pyrithiamine hydrobromide (Sigma, St. Louis, MO). On days 14–16 of treatment, animals displayed signs of local tonic-clonic movement of the front and hind limbs, and generalized convulsions (seizures). Within 4 h of the onset of seizure, PTD-treated animals were given an injection of thiamine hydrochloride (Sigma, 100 mg/kg, i.p.) every 8 h until the seizure activity disappeared and the rats regained upright posture. The PF animals were fed an amount of thiamine-deficient chow equivalent to the average amount consumed by the PTD groups on the previous day of treatment, and were given daily injections of thiamine hydrochloride (0.4 mg/kg, i.p.). After treatment, all subjects were placed on regular chow and allowed to regain the weight lost.
2.3 Apparatus and non-rewarded spontaneous alternation testing
The testing apparatus used for all non-rewarded spontaneous alternation testing was a plus maze with clear Plexiglas sidewalls 12 cm high and a painted black wood floor. Each of the four arms was 55 cm from the center of the maze, and the maze was elevated 80 cm from the floor. The maze was located in a moderately lit room that contained several extraneous cues such as black geometric shapes, posters, chairs and a lamp.
During spontaneous alternation testing, animals were placed in the center of the maze and allowed to roam freely for 18 min. Entry into an arm was defined as all four paws over a line that was located at the entrance of the arm. The number and the sequence of arms entered was used to determined percent alternation. An alternation was defined as alternating left or right when the rat reached the center area. The percent alteration score was equal to the ratio of: (actual alternations ÷ possible alternations) × 100. The rate of perseveration behavior (defined as re-entry into an arm within 3 choices) was also recorded.
2.4. Stereotaxic surgery
Three weeks after PTD/PF treatment and one week prior to microdialysis, subjects for Experiments 2 and 3 were anesthetized with a Ketamine (8.25 ml)/Xylazine (1.75 ml) mixture (50 mg/kg i.p.) in preparation for stereotaxic surgery. All animals in Experiments 2 and 3 were implanted with a microdialysis guide cannula (CMA/11) aimed at the hippocampus and a drug cannula aimed at the MS. Within each group, subjects were matched in terms of hippocampal microdialysis cannula placement (right vs. left hemisphere). Stereotaxic coordinates were obtained from the atlas of Paxinos and Watson (1986) and were based on previous studies (see Chang and Gold, 2003a). The coordinates for the hippocampus microdialysis cannula were: Anterior-Posterior (AP): −5.1 mm; Medial-Lateral (ML): ±5.0 mm; Dorsal-Ventral (DV): −4.2 mm. The coordinates for the MS drug cannula (28 gauge; Plastics One, Roanoke, VA) were: AP: +0.30 mm; ML: 0; DV: −5.0 mm. Acrylic cement and 2 skull screws were used to hold the cannulae in place. Immediately after surgery, subjects were placed in a warm incubator until they regained an upright posture. All subjects received the analgesic buprenorphine (0.3 ml, s.c.) directly after surgery and another injection 24 h later. Subjects were allowed to recover for 1 week with ad libitum access to food and water.
2.5. Experimental Design
2.5.1 Experiment 1: Systemic injection of tacrine and spontaneous alternation testing
In Experiment 1, animals (PF: n = 8; PTD: n = 8) were handled for 4 consecutive days prior to behavioral testing for 10 min each day to reduce maze anxiety and were fasted overnight to increase exploration behavior. Prior to i.p. injections, rats were habituated in the maze room in a 30 cm × 40 cm, depth 35 cm cage that contained wood shavings at the bottom for 60 min. Following habituation rats were given i.p. injections of saline, 0.75, or 1.5 mg/kg of tacrine. Drug order was counterbalanced over three consecutive days of behavioral testing and all animals received all treatment doses. After receiving an injection, rats were placed back in the holding cage for 18 min to allow the drug to be absorbed. Animals were placed in the center of the plus maze for 18 min of spontaneous alternation testing. Upon completion of spontaneous alternation testing, rats were transferred back to the holding cage
2.5.2 Experiment 2: Pharmacological challenge during awake in vivo microdialysis and high performance liquid chromatography analysis for acetylcholine
In Experiment 2, animals (PF: n = 7; PTD: n = 7) received intraseptal infusions of saline (1 μl) and tacrine (2.5, 5.0, or 12.5 μg/μl) in conjunction with in vivo microdialysis collection of hippocampal acetylcholine. Microdialysis occurred across three sessions; each session included saline and one infusion of tacrine. Subjects were transported to the testing room and placed in a microdialysis holding cage [acrylic cage (30 cm × 40 cm, depth 35 cm) with wood shavings at the bottom]. The microdialysis probe (CMA/11, 3 mm) was connected to a microinfusion system (CMA 100) and perfused continuously at a rate of 2 μl/min with artificial cerebrospinal fluid [aCSF (127.6 mM NaCl, 4.0 mM KCl, 1.3 mM CaCl2 dihydrate, 1.0 mM glucose, 0.9 mM MgCl2, 0.9 mM NaH2PO4, and 2.0 mM Na2HPO4, pH 7.0)], which contained the AChE inhibitor neostigmine (500 nM). Following an initial period of habituation to the cage (60 min), dialysate samples (volume ~30 μl) were collected every 15 min for a period of 30 min to determine basal levels of acetylcholine in awake rats. After baseline samples were collected the saline infusion was performed; a drug infusion needle (Plastics One, 28 gauge) was inserted into the septal cannula and 1 μl of saline was infused over 1 min. The drug needle was always left in place for an additional 2 min to allow the entire compound to diffuse into the tissue. After the saline infusion, microdialysis continued for two 15 min time samples. Thirty min after the saline infusion, the infusion needle was inserted into the septal cannula and 1 μl of tacrine (either 2.5, 5.0, or 12.5 μg) was infused over 1 min. Tacrine administration was counterbalanced across subjects over 3 consecutive days of microdialysis testing so that during each day of testing all animals received a saline infusion followed by an infusion of one of the three doses of tacrine. The drug phase consisted of six 15 min samples for a total of 90 min. A study that administered tacrine (1 mg/kg; i.v. into the jugular) and measured hippocampal dialysate for rate of elimination found that the half-life of tacrine was 99.1 ± 17.7 min (Telting-Diaz and Lunte, 1993). Following the drug phase, two 15 min post-baseline samples were collected. To test the reliability of the microdialysis probe, the probe was placed into a standard solution (100 nM concentration of acetylcholine and choline). After collection, brain dialysis samples were frozen at −30°C for later analysis using high performance liquid chromatography (HPLC).
Acetylcholine output was assayed by HPLC (Epison, BAS, West Lafayette, IN) along with an enzyme reactor. The assay system included an ion-exchange microbore analytical column (BAS, MR-8904), a microbore acetylcholine/choline immobilized enzyme reactor containing AChE and choline oxidase (BAS MF-8903), an auxiliary electrode with a radical flow electrochemical thin-layer cell and 13 mm thin layer gasket, a wired enzyme electrode kit (a redox polymer film containing horseradish peroxidase coated in the surface of a 3 mm glassy carbon working electrode), and a low dispersion injected value with a 10 μl polytheretheketone loop. The mobile phase was a 50 mM dibasic potassium phosphate buffer (pH = 8.5) containing Kathon (BAS). The mobile phase was delivered at a rate of 140 μl/min by a PM-91 pump (BAS) and the detection level was about 10 fentomoles. Acetylcholine standards (5 μl of 20 and 100 nM acetylcholine + Ch) were injected before and after samples to verify detection stability.
2.5.3 Experiment 3: Pharmacological treatment in combination with in vivo microdialysis and HPLC analysis of acetylcholine before, during and after behavioral testing
In Experiment 3, animals (PF: n = 10; PTD: n = 10) received intraseptal administration of vehicle and two doses of tacrine in conjunction with in vivo microdialysis during behavioral testing. All animals received three sessions of microdialysis with counterbalancing of drug infusion (Saline, 5.0 and 12.5 μg tacrine) across three sessions. Each session started with the rats being transported to the testing room and placed in a microdialysis holding cage [acrylic cage (30 cm × 40 cm, depth 35 cm) with wood shavings at the bottom]. The microdialysis probe (CMA/11, 3 mm) was connected to a microinfusion system and perfused continuously at a rate of 2 μl/min with aCSF. Following an initial period of habituation to the cage (60 min), dialysate samples (volume ~12 μl) were collected every 6 min for a period of 18 min to determine basal levels of acetylcholine in awake rats. After baseline, a drug infusion needle (Plastics One, 28 gauge, extended 0.5 mm beyond the cannula) was inserted into the septal drug cannula and 1 μl of saline or tacrine (5.0 or 12.5 μg) was infused over 1 min. The infusion needle was left in place for an additional 2 min to allow for the entire compound to diffuse into the tissue. Three 6 min samples were collected during this drug infusion phase. Following the 18 min infusion phase, the animal was placed on the maze to perform spontaneous alternation. All animals performed the non-rewarded spontaneous alternation plus-maze procedure for 18 min. Microdialysis samples continued to be collected every 6 min during the maze and post-baseline phases, which were both 18 min. The probe was removed after the post-baseline period. To test the reliability of the microdialysis probe, the probe was placed into a standard solution (100 nM concentration of acetylcholine and choline). After collection, brain dialysis samples were frozen for later analysis using HPLC as described in Experiment 2.
2.6 Histology
After completion of each experiment, animals were anesthetized with 0.5 mg/kg, i.p. injection of Sleep-Away (26% sodium pentobarbital in 7.8% isopropyl alcohol and 20.7% propylene glycol solution; Fort Dodge, Iowa). The brains were extracted, post-fixed in a 10% formalin solution for at least 72 h and then transferred to a 30% sucrose solution. Coronal sections from the brains were cut (60 μm thick) on a sliding microtome (Sm2000r; Lecia Instruments, Wetzlar, Germany) and later stained with cresyl violet for assessment of thalamic lesion status and probe locations. Only rats with the correct cannula and probe placements were included in the final analysis for Experiments 2 and 3.
3. Results
3.1 Histology
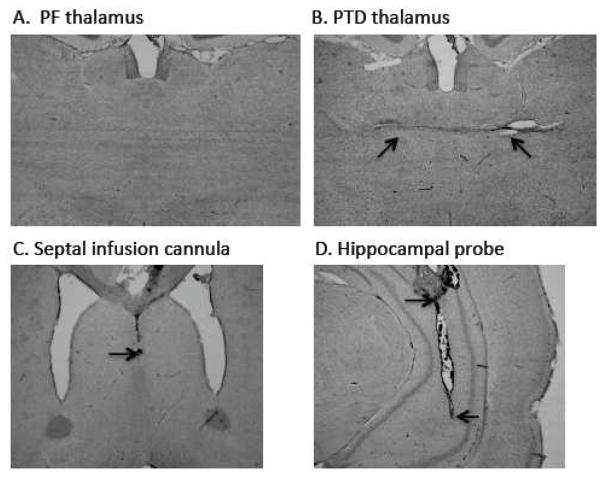
Figure 1 displays an intact PF brain (A) relative to PTD-induced thalamic neuropathology (B) with arrows pointed at the midline thalamic lesion. In the lower panels are representative cannula placements in the medial septum (C) and a microdialysis probe tract into the ventral hippocampus (D). In Experiments 2 and 3, some rats had missed cannulae locations in the medial septum and/or the ventral hippocampus. The final subjects counts were 12 for Experiment 2 (PF = 6; PTD = 6) and 16 for Experiment 3 (PF = 8; PTD = 8).
Figure 1. Histology.
Cresyl violet stained sections demonstrating the thalamus of a PF-rat (A) for comparison to a PTD-induced lesion of the thalamus (B). The arrows on panel B point to the midline thalamic lesion caused by thiamine deficiency. Panels CD shows acceptable placement of septal drug probe (C) and the ventral hippocampal microdialysis probe (D). The arrows in panel C point to the top and bottom of where the microdialysis probe was located (The infusion needle extended 0.5 mm beyond the drug cannula). The arrows in panel D points to the end of the cannula in the hippocampus and the beginning and end of the microdialysis probe tract.
3.2 Effects of systemic tacrine on spontaneous alternation
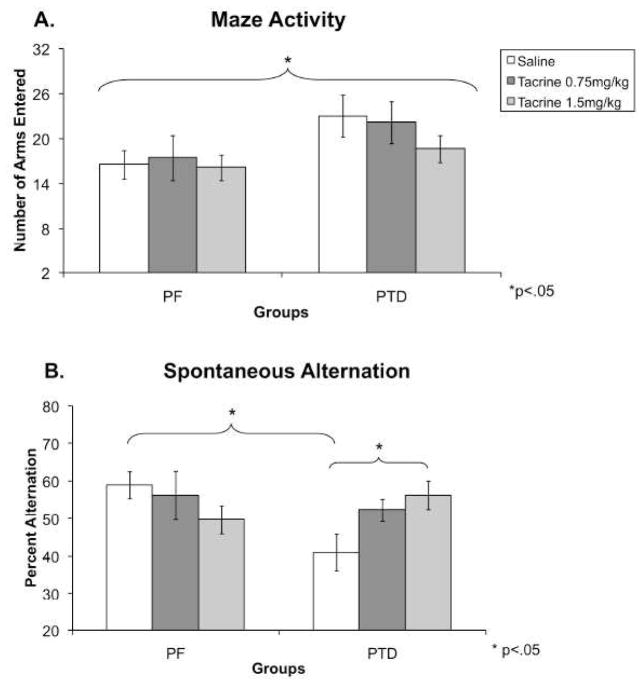
Counterbalancing the drug dose across sessions did not affect behavior (both F’s <1.6). Figure 2A displays that PTD rats had higher activity levels (number of arms entered) than PF rats during spontaneous alternation testing. A mixed model design of one between-subjects factor (Group: PF, PTD), one within-subjects factor (Dose: Saline, 0.75, 1.5 mg/kg) ANOVA (StatView 5, SAS Institute Inc., Cary, NC, USA) provided statistical confirmation that PTD animals were more active than PF rats (Group effect: F [1,14] = 4.77, P < 0.05). However, there was no effect of dose on the number of arms entered (F [2,28] = 1.26) nor a Group X Dose interaction (F<1).
Figure 2. Experiment 1: Spontaneous alternation performance during systemic saline and tacrine administration.
Behavioral data (Mean ± SEM) from spontaneous alternation testing for PF (n = 8) and PTD (n = 8) rats after systemic administration of saline, 0.75 and 1.50 mg/kg of tacrine. Panel A displays that across drug treatments; PTD rats were more active (entered more arms) than PF rats (P < 0.05). Panel B shows the differences between PF and PTD-rats in modified (corrected for number of arms entered) spontaneous alternation scores under the saline condition. In addition, tacrine dose-dependently improved alternation scores only in the PTD group (P < 0.01).
Given that the groups differed in activity, the data for alternation rates were corrected for only the first 17 arms entered. The same ANOVA model [one between-subjects factor (Group), one within-subjects factor (Dose)] on the modified alternation scores revealed the alternation performance of two groups (PF and PTD) was differentially affected by the drug conditions (Group X Dose interaction: F [2,28] = 5.53, P < 0.01; see Figure 2B; Note: The uncorrected data also had a significant Group X Dose interaction, P<0.01). Follow-up analyses of the interaction revealed that in the PTD rats, as the dose of tacrine increased, alternation scores improved (F [1,14] = 6.36, P < 0.01). In contrast, there was no significant effect of dose on PF animals. Pair-fed animals displayed a non-significant trend for a decrease in percent alternation (F [1,14] = 1.31).
Control animals alternated at a significantly higher level than PTD animals when administered saline (F [1,14] = 8.76, P < 0.01). When comparing the alternation rates between groups after administration of the 0.75 and 1.5 mg/kg of tacrine, no significant differences were found [both F’s (1,14) < 1.6]. PTD animals that received 1.5 mg/kg of tacrine performed at (56.2%) comparable levels to PF animals on saline (58.9%), demonstrating complete behavioral recovery.
Similarly to the alternation scores, systemic tacrine only affected the perseveration scores in the PTD group (Group X Dose interaction: F [2,28] = 3.44, P < 0.05). Under the saline control condition, the PTD group had more perseveration behavior than PF rats (PF = 24.27% ± 3.09; PTD = 43.45 ± 6.36; F [1,14] = 7.35, P < 0.02). However, after i.p. injections of tacrine there were no differences in perseveration behavior between the groups (0.75 mg/kg: PF = 38.64% ± 7.51; PTD = 30.48 ± 5.88; 1.50 mg/kg: PF = 34.51% ± 5.88; PTD = 36.37 ± 6.14; both F’s [1,14] < 1).
3.3 Effects of intraseptal tacrine infusion on hippocampal acetylcholine efflux
Basal amounts of acetylcholine in fentomoles in the hippocampus were assessed with a one between-subjects factor (Group: PF vs. PTD) ANOVA. The analyses revealed that there were no differences as a function of Group for any condition (Mean PF = 138.25 ± 13.04 [SEM]; PTD = 106.90 ± 19.45; all F’s [1,10] < 1). Given that there was a 20% rise in acetylcholine efflux after the saline injection (relative to baseline), the data under each dose was transformed to reflect change from saline. In other words, instead of calculating drug rise as a percent of baseline it was calculated as a percent rise relative to saline to account for the increase in acetylcholine efflux caused by the injection procedure. There was no overall order effect of counterbalancing drug dose (F [1,10]< 1). Thus, a mixed model ANOVA with one between-subjects (Group) and two within-subjects factors (Dose [2.5, 5.0, 12.5 μg]; Sample Time (15, 30, 45, 60, 75, 90 min) was used to assess changes in acetylcholine efflux across time.
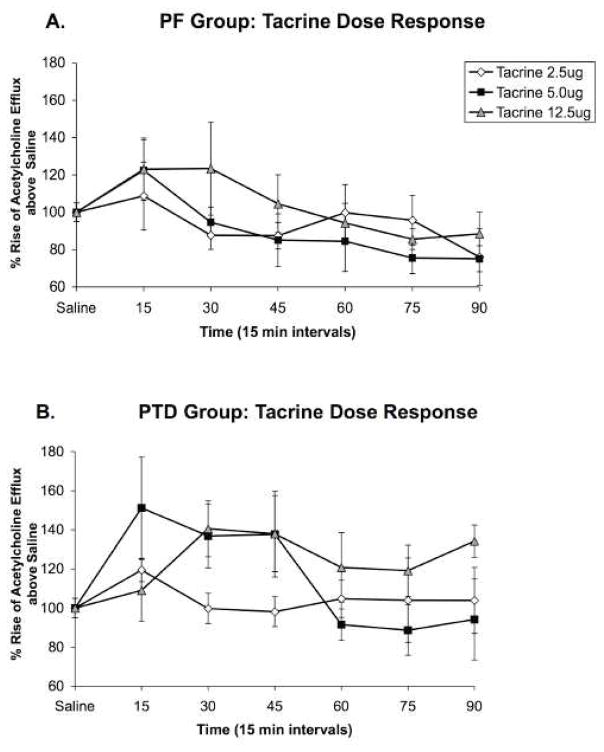
As shown in Figure 3, intraseptal tacrine infusions increased hippocampal acetylcholine levels in both groups, but the effect of the drug was greater in the PTD rats. There were main effects for Group (F [1, 10] = 5.63; P < 0.05) and Sample Time (F [5,50] = 4.85; P < 0.01) and a significant interaction of Dose X Sample Time (F [10,100] = 1.95; P < 0.05; see Figures 3AB). No other main effects or interactions were significant.
Figure 3. Experiment 2: Hippocampal acetylcholine release after intraseptal saline or tacrine administration.
Profiles of hippocampal acetylcholine release (Mean percent rise above saline ± SEM) after intraseptal tacrine infusions in PF (n = 6; Panel A) and PTD (n = 6; Panel B) animals shown in 15-minute sample bins for 90 min after infusion. The lowest dose of tacrine (2.5 ug) did not alter hippocampal acetylcholine effux in PF or PTD rats. Although the medium (5.0 ug) and high (12.5 ug) doses of tacrine infused into the medial septum increased hippocampal acetylcholine efflux in both PF and PTD rats, the percent change in acetylcholine efflux was greater in PTD rats (all P’s < 0.05).
Follow-up analyses were conducted to investigate the main effect of Group and the Dose X Sample Time interaction. One between-subjects factor (Group), one within-subjects factor (Time: early, late) ANOVAs were conducted to contrast group differences as a function of dose and time. The main effect of Group was caused by the PTD rats having an overall greater change in hippocampal acetylcholine efflux, relative to PF rats, at the early time points (15–45 min) at both 5.0 and 12.5 μg (both F’s [1,10] > 5.49; P < 0.05). Furthermore, the change in acetylcholine efflux persisted longer (from 60-90 min) in the PTD group, relative to the PF group, at 12.5 μg (F [1,10] = 8.3; P < 0.05). The Dose X Sample Time interaction was driven by 12.5 μg across both groups, creating a greater rise in hippocampal acetylcholine efflux than 2.5 μg shortly after infusion (F [1,10] = 9.05, P < 0.05) and for a longer duration than 5.0 μg (F [1,10] = 7.74, P < 0.05).
3.4 Effects of intraseptal tacrine infusion on behavioral performance and hippocampal acetylcholine efflux
3.4.1 Effects of intraseptal tacrine infusion on behavioral performance
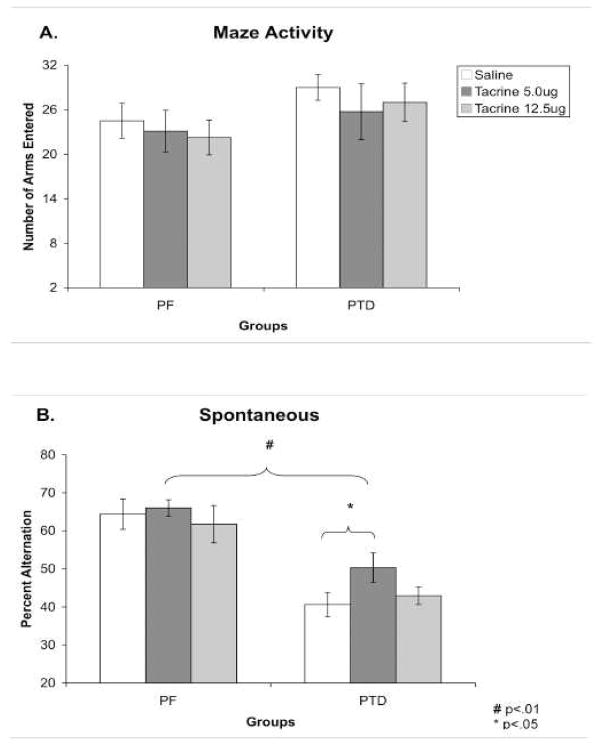
An analysis of order effects revealed that counterbalancing the drug condition across sessions did not affect alternation rates, number of arms entered or acetylcholine efflux in either group (all P’s > 0.17). A mixed model ANOVA with one between-subjects (Group) factor and one within-subjects (Dose) factor revealed that there were no differences in activity scores as a function of group (F [1,14] = 1.56), drug dose (F [1,14] = 1.02) or the interaction of those variables (F [1,14] < 1; Figure 4A).
Figure 4. Experiment 3: Spontaneous alternation performance after intraseptal saline or tacrine administration.
Behavioral data (Mean ± SEM) from spontaneous alternation testing for PF (n = 8) and PTD (n = 8) rats after septal infusions of saline or tacrine (5.0, 12.5 ug). Panel A shows that activity on the maze was equal across groups and did not change as a function of drug dose. Panel B shows the overall significant difference between PTD and PF rats in alternation behavior: Regardless of drug condition PF alternated at higher rates than PTD rats (saline: P < 0.001; 5.0 ug: P < 0.01; 12.5 ug: P < 0.01). However, intraseptal infusion of 5.0 ug tacrine did improve alternation rates in the PTD group (P < 0.05).
Figure 4 displays the significant difference in percent alternation between PF and PTD-treated rats under saline as well as after intraseptal infusion of tacrine. Given that tacrine produced an inverted-U function in PTD rats, the non-monotonic relationship was assessed with three separate one between-subjects factor (Group), one within-subject factor (Dose) ANOVAs to assess saline vs. 5.0 μg tacrine and saline vs. 12.5 μg tacrine, 5.0 μg vs. 12 μg (similar to the recommendation to use Dunnet’s test when non-monotonic curves exist (see Henck, 2002).
When performance between the groups was compared after saline infusion, PTD rats had significantly lower alternation performance than PF rats (F [1,14] = 21.46; P < 0.001). After 5.0 μg of tacrine there was a significant (10%) increase in the percent alternation of PTD animals (F [1,7] = 6.81; P < 0.05), but not the PF rats (F [1,7] < 1). However, when comparing the alternation rates between Groups after the infusion of 5.0 μg of tacrine, PF rats still had a significantly higher (16%) rate of percent alternation than the PTD rats (F [1,14] = 12.48; P < 0.01). Thus, although 5.0 μg of tacrine increased performance in the PTD animals, it did not result in alternation rates comparable to PF animals.
When the high dose of tacrine was contrasted to saline there was no change in behavior in either group (no effect of Drug [F < 1]). Within each group, 12.5 μg of tacrine did not increase alternation levels above saline (both F’s [1,14] < 1). Thus, the PF rats alternated at significantly higher levels than the PTD-treated rats (F [1,14] = 12.13; P < 0.01).
Furthermore, when the performance of all rats was contrasted between when they were given 5 μg to when they were administered 12.5 μg, PF rats had higher alternation scores than PTD rats regardless of drug condition (main effect of Group: F [1,14] = 23.34; P < 0.01). These data suggest an inverted-U type of function.
A mixed model design of one between-subjects factor (Group), one within-subjects (Dose) ANOVA revealed that rates of perseveration were greater in the PTD rats (39.6% ± 1.79) relative to the PF rats (28% ± 2.21; main effect of Group: F [1,14] = 26.5, P < 0.01). In addition, the main effect of Dose was significant (F [2,28] = 4.9, P < 0.05): Intraseptal administration of tacrine produced an overall reduction in perseveration (Saline: 39.8% ± 2.8; 5.0 μg: 29.7% ± 2.8; 12.5 μg: 32% ± 2.5) in both groups.
3.4.2 The effects of intraseptal tacrine infusion on hippocampal acetylcholine efflux prior to, during and after behavior testing
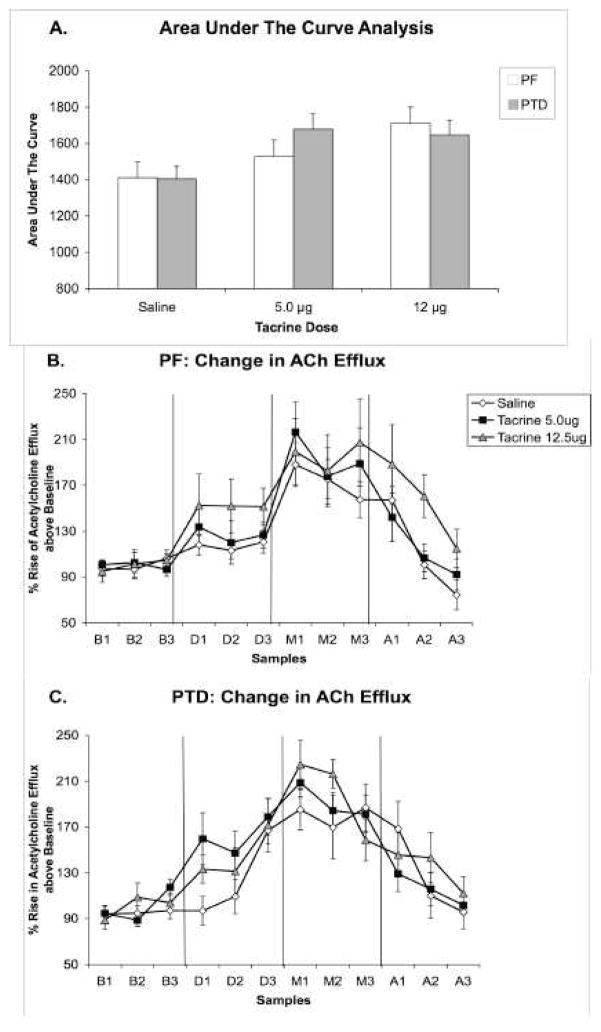
A one between-subjects factor (Group), one within-subjects factor (Dose) ANOVA verified that basal amounts of acetylcholine (fentomoles) in the hippocampus were not different as a function of Group or Dose condition (PF = 139.96 ± 13.04; PTD = 112.11 ± 15.87; all F’s [1,10] < 1). An area under the curve analysis, which collapsed acetylcholine values across phase and sample time for each dose (saline, 5.0, 12.5 μg) revealed that the two doses of tacrine infused into the medial septum increased acetylcholine levels beyond the saline infusion in both groups (main effect of Dose (F [2,28] = 3.31, P = 0.05; Group, Group X Dose, F’s<1). This composite of changes in acetylcholine levels as a function of dose is shown in Figure 5-A.
Figure 5. Experiment 3: Hippocampal acetylcholine release after intraseptal saline or tacrine administration.
Panel A represents the overall change in hippocampal acetylcholine efflux of PF and PTD rats collapsed across phase and time. In contrast, Panels B and C show the complete profiles of acetylcholine efflux (Mean percent rise above baseline ± SEM) in PF (n = 8; Panel B) and PTD (n = 8; Panel C) rats as a function of intraseptal drug dose (Saline, 5.0, 12.5 μg) during the phases of baseline (B1–B3), drug infusion (D1–D3), behavioral testing (M1–M3), and post-baseline (A1–A3). The acetylcholine efflux form the samples collected at 6-min intervals is expressed as percent change from baseline. There was a significant Dose X Phase X Sample interaction in the PTD group (P < 0.05).
However, given that the two groups responded differently to tacrine as a function of time in Experiment 2, two separate three within-subjects (Dose: saline, 5.0, 12.5 μg), Phase (baseline, infusion, maze, after), Sample time (1–3) ANOVAs were conducted for each Group (PF, PTD). In the PF group, acetylcholine efflux increased during the infusion phase and behavioral testing phase as a function of time (Phase [F (3,21) = 21.64, P < 0.01), Sample Time (F [2,14] = 7.33, P < 0.01), Phase X Sample Time interaction (F [6,42] = 6.05, P < 0.01). The Phase X Sample interaction was driven by an increase in acetylcholine efflux during all sample times during the infusion of 12.5 μg (all t’s [24] > 2.25, P’s < 0.05) and during maze testing (all t’s [24] > 4.35, P’s < 0.01), but a gradual decrease in acetylcholine efflux as a function of sample time in the post-baseline phase (S1 and S2: t’s [24] > 3.36, P’s < 0.01; S3: t [24] < 1). Performing on the maze increased acetylcholine level beyond the infusion phase by 52.82%, 67.5% and 46.94% in PF rats in the saline, 5.0 μg and 12.5 μg conditions, respectively. This effect is seen in Figure 5B.
In the PTD group, acetylcholine levels also changed during the infusion phase and testing phase, but the different doses effected peak acetylcholine levels at different time points (Phase [F (3,21) = 51.96, P < 0.01), Phase X Sample Time interaction (F [6,42] = 8.69, P < 0.01), Dose X Phase X Sample Time interaction (F [12,84] = 1.89, P < 0.05). Dunnett’s test was used as a method for comparing means to the saline reference given the significance of the 3-way interaction from the F-test. The Dose X Phase X Sample interaction was due to the fact that during the infusion phase, 5.0 μg of tacrine created a greater rise in acetylcholine efflux, relative to saline, during sample periods 1 and 2 (both t’s [14] > 3.1, P’s < 0.01). In contrast, during the maze-testing phase 12.5 μg of tacrine, relative to saline, produced a greater change in acetylcholine efflux during sample period 1 and 2 (both t’s [14] > 2.56, P’s < 0.05). Performance on the maze increased acetylcholine level beyond the infusion condition by 57.29%, 29.3,% and 57.57% in PTD rats in the saline, 5.0 μg and 12.5 μg conditions, respectively. Figure 5C displays the changes in the PTD rats as a function of drug dose and phase.
3.5 Comparison of behavior across systemic and intraseptal infusion experiments
Across the behavioral experiments, rats with intraseptal cannulae, regardless of treatment condition (PF, PTD), were more active on the maze (arms entered: 26.75± 1.54 vs. 20.31± 1.85; F[1,28]=8.52, P < .01). However, there was not a significant difference in alternation scores between the two experimental conditions (F[1,18]<1). No other differences in behaviors across the studies were observed.
4. Discussion
There has been some debate as to the putative neural targets for AChE inhibitors to exert their therapeutic pro-cognitive effects (Wu et al., 2004; Wu et al., 2003). In subjects with compromised cholinergic function, there is often improvement with systemic (Beninger et al., 1995; Chang and Gold, 2008; Matsuoka et al., 1991; Roland et al., 2008), intrahippocampal (Chang and Gold, 2004; Roland et al., 2008), and intraseptal (Frick et al., 1996; Sabolek et al., 2004a) infusions of AChE inhibitors. Currently, systemic tacrine dose-dependently increased spontaneous alternation behavior in PTD-treated rats to within the range of PF rats. However, intraseptal infusion of 5.0 μg of tacrine, but not 12.5 μg, led to partial recovery of spontaneous alternation behavior in PTD rats. Nevertheless, neither systemic nor intraseptal administration of tacrine significantly altered the behavior of intact PF rats.
Rats with a loss of cholinergic neurons in the medial septum/diagonal band have suboptimal levels of hippocampal acetylcholine (Chang and Gold, 2004) and intraseptal infusions of drugs like tacrine may enhance the signaling capability of the septohippocampal circuit to within a functional range. However, in normal rats these drugs may increase signal capacity within the septohippocampal pathway transforming hippocampal theta activity into an epileptiform that adversely affects behavior (Sabolek et al., 2004b; Wallenstein and Hasselmo, 1997). Evidence from a number of preparations suggests that there is a restricted range of acetylcholine levels in the medial septum and hippocampus that optimizes cognitive functions (Hasselmo, 2006; Roland et al., 2008; Sabolek et al., 2004a).
A caveat of microdialysis studies is that handling (Nilsson et al., 1990) and restraint (Imperato et al., 1991) increase hippocampal acetylcholine levels. Thus, the physiological stimulation created by the infusion procedure increased hippocampal acetylcholine levels greater than 20% in both PF and PTD rats. This procedure-induced change in acetylcholine efflux masked the behavioral differences in acetylcholine levels seen between PTD-treated and PF rats reported in our previous non-drug studies (Roland et al., 2008; Roland and Savage, 2007; Savage et al., 2003; Savage et al., 2007; Vetreno et al., 2008). However, as shown in Figures 4 and 5, intraseptal tacrine significantly increased hippocampal acetylcholine levels beyond saline infusion for the 12.5 μg dose in PF rats and both doses in PTD rats.
The behavioral differences seen after septal administration of tacrine between PF and PTD rats can be partially explained by differential reactions to the drugs effect of increasing hippocampal acetylcholine levels. PTD-treated rats had an overall greater change in hippocampal acetylcholine levels, relative to PF rats, at the early time points at both 5.0 and 12.5 μg of tacrine. Behaviorally, intraseptal administration of tacrine produced an inverted U curve in PTD rats: 5.0 μg improved performance, but the higher 12.5 μg dose had no effect. Neurochemically, there was also a trend for 5.0 μg to produce an initial accelerated rise in hippocampal acetylcholine levels in PTD rats in Experiment 2 and in Experiment 3 during the pre-testing infusion phase. However, 12.5 μg of tacrine also increased acetylcholine levels during maze testing. Thus, there is no direct linear relationship between the modulation of medial septal acetylcholine receptor activation, hippocampal acetylcholine levels and behavioral success. This is not a new finding as there are instances in which the effects of septal pharmacological manipulations on memory do not parallel their effects on hippocampal acetylcholine levels (see Parent and Baxter, 2004). Given that the septal region is rich in muscarinic receptors that reside upon cholinergic, GABAergic and glutamatergic neurons (Levey et al., 1995; Manseau et al., 2005; Rowntree and Bland, 1986; Van der Zee and Luiten, 1994), intraseptal drug infusion of AChE inhibitors have widespread effects on septal, hippocampal and cortical physiology across a range of neurotransmitter systems.
The integrity of GABA neurons in the medial septum is critical for hippocampal functioning (Givens and Olton, 1990; Smythe et al., 1992). In PTD rats, recovery of performance produced by intraseptal infusions of the GABA antagonist bicuculline (Roland and Savage, 2009b) was more complete than that observed in the present intraseptal infusions of tacrine. These results suggest a significant role for drug therapies that modulate the synergistic interactions between the forebrain cholinergic and GABAergic systems for the treatment of cognition decline.
Our previous work has demonstrated that ChAT-immunopositive medial septum/diagonal band neurons, but not those in the nucleus basalis magnocellularis, are reduced after thiamine deficiency (Savage et al., 2007). Furthermore, two populations of GABAergic medial septum/diagonal band neurons (those immunopositive for parvalbumin or calbindin) are spared after thiamine deficiency (Roland and Savage, 2009a). The cholinergic neurons of the medial septum/diagonal band may be particularly vulnerable to thiamine deficiency for three reasons: (1) Thiamine deficiency induces the inhibition of acetylcholine synthesis due to a reduction of the thiamine-dependent acetyl coenzyme A; (2) Thiamine deficiency leads to mitochondrial dysfunction, glutamate-related excitotoxicity, and increased oxidative stress reactions (Todd and Butterworth, 1999; 2001) and cholinergic cells have a higher demand for energy production, are more sensitive to glucose deprivation (Szutowicz et al., 2007) and oxidative stress (McKinney, 2005) relative to other neuronal populations; (3) The cholinergic neurons of the medial septum/diagonal band, but not nucleus basalis magnocellularis, have the neurotrophin p75 receptor that appear to pro-regulate cell death under neurotoxic conditions (Kaplan and Miller, 2000).
It should be noted that significant brain pathology beyond the loss of cholinergic neurons in the medial septum/diagonal band contributes to the amnestic syndrome in the PTD model. Previous studies have shown that learning and memory impairments in the PTD rat are closely related to damage in particular two key nuclei within the limbic thalamus: internal medullary lamina and anteroventral thalamic nuclei (Langlais and Savage, 1995; Mair et al., 1991).
Furthermore, the loss of cholinergic neurons in the medial septum/diagonal band of PTD-treated rats correlated with both behavioral impairment and midline thalamic tissue loss (Roland and Savage, 2009a). Cortical abnormalities leading to decreased behavioral flexibility may also play a role in cognitive decline after thiamine deficiency (Carvalho et al., 2006). In contrast, simple cognitive and motor performance is relatively unaffected in the recovered thiamine deficiency rodent (Langlais and Savage, 1995; Nakagawasai et al., 2001). Thus, the severity of diencephalic amnesia is likely to be related to the extent of damage to multiple sites in the limbic system.
The current results suggest that AChE inhibitors are likely modulating the reactions of the depleted cholinergic neurons as well as the intact GABAergic neurons in the MS of PTD rats. This dual reaction of intraseptal tacrine administration is likely through both the excitatory M3 and the inhibitory M2 muscarinic receptor subtypes present in the medial septum/diagonal band region. The M3 receptors are located on the GABAergic neurons that project to the hippocampus and when activated, hippocampal GABAergic neurons are inhibited leading to an increase in the activity of hippocampal pyramidal neurons (Wu et al., 2003). However, intraseptal AChE inhibitor infusion has also been shown to decrease acetylcholine production through negative-feedback inhibition on the M2 receptor. Therefore, when acetylcholine levels increase after intraseptal AChE inhibitor administration, the activity of the medial septum/diagonal band cholinergic neurons is reduced—particularly in the intact subject (Levey et al., 1995; Wu et al., 2003). Acetylcholinesterase inhibitors are likely to produce differential behavioral-neurochemical reactions depending on the status of neuronal populations with in the medial septum/diagonal band.
Intraseptal AChE inhibitor infusion may be creating two opposing mechanisms; increased septohippocampal GABAergic activation and decreased acetylcholine production. Intrahippocampal infusion of AChE inhibitors has consistently resulted in increased spatial memory performance and hippocampal acetylcholine levels (Degroot and Parent, 2000; 2001; Roland et al., 2008), whereas intraseptal infusion of AChE inhibitors has produced mixed results. Nevertheless, manipulations in both of these regions have demonstrated inverted-U shape relationships in terms of memory improvement. Inverted-U shape functions have been exhibited by intraseptal tacrine (Sabolek et al., 2004a), systemic glucose (Ragozzino et al., 1996), intrahippocampal physostigmine (Roland et al., 2008) and intrahippocampal bicuculline (Zarrindast et al., 2002), where low and high doses impaired memory or where ineffective --but intermediate doses improved memory. High doses of memory-enhancing drugs may be increasing “noise” or attention to unimportant stimuli in the environment that is interfering with the relevant to-be remembered information (Gold, 2006). As discussed previously, the 5.0 μg dose of tacrine could be a dose that produces a physiological balance between muscarinic receptor activation across cholinergic and GABAergic neurons in the medial septum/diagonal band. Again, the results point towards a model of septohippocampal function in which there is an optimal range of cholinergic tone for optimal behavioral and hippocampal function (Hasselmo, 2006; Roland et al., 2008; Sabolek et al., 2004a).
In summary, systemic administration of the AChE inhibitor tacrine restored the deficit in spontaneous alternation in PTD-treated rats whereas intraseptal administration of tacrine produced an inverted-U curve in behavior and increased hippocampal acetylcholine levels in PTD rats. The effect of intraseptal tacrine on hippocampal acetylcholine levels was more pronounced in PTD rats, relative to control PF rats. This suggests that the memory deficits and the improvement after AChE inhibitors reflect the ability of dysfunctional forebrain cholinergic mechanisms to respond when appropriately stimulated. Understanding how the modulation of septohippocampal circuits in amnestic populations alters hippocampal function is critical to the development of therapeutics for cognitive dysfunction.
Acknowledgments
This work was supported by research grant NINDS RO1-054272 to LMS. Jessica Roland is now at University of Medicine and Dentistry of New Jersey, Newark New Jersey. We would like to thank Jessica Patel, Obeta Lavin and Jess Blackwolf for their assistance in behavioral testing, HPLC analyses, and histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alreja M, Wu M, Liu W, Atkins JB, Leranth C, Shanabrough M. Muscarinic Tone Sustains Impulse Flow in the Septohippocampal GABA But Not Cholinergic Pathway: Implications for Learning and Memory. J Neurosci. 2000;20:8103–8110. doi: 10.1523/JNEUROSCI.20-21-08103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Wirsching BA, Mallet PE, Jhamandas K, Boegman RJ. Physostigmine, but not 3,4-diaminopyridine, improves radial maze performance in memory-impaired rats. Pharmacol Biochem Behav. 1995;51:739–746. doi: 10.1016/0091-3057(95)00024-q. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, Chrobak JJ. Intraseptal infusion of oxotremorine impairs memory in a delayed-non-match-to-sample radial maze task. Neuroscience. 2003;121:259–267. doi: 10.1016/s0306-4522(03)00462-7. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, Chrobak JJ. Intraseptal Infusion of the Cholinergic Agonist Carbachol Impairs Delayed-Non-Match-to-Sample Radial Arm Maze Performance in the Rat. Hippocampus. 2004;14:450–459. doi: 10.1002/hipo.10200. [DOI] [PubMed] [Google Scholar]
- Carvalho FM, Pereira SRC, Pires RGW, Ferraz VP, Romano-Silva MA, Oliveria-Silva IF, Ribeiro AM. Thiamine deficiency decreases glutamate uptake in the prefrontal cortex and impairs spatial memory performance in a water maze test. Pharmacol Biochem Behav. 2006;83:481–489. doi: 10.1016/j.pbb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav Brain Res. 2003;144:19–24. doi: 10.1016/s0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Impaired and Spared Cholinergic Functions in the Hippocampus After Lesions of the Medial Septum/Vertical Limb of the Diagonal Band With 192 IgG-Saporin. Hippocampus. 2004;14:170–179. doi: 10.1002/hipo.10160. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Age-related changes in memory and in acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. Neurobiol Learn Mem. 2008;89:167–177. doi: 10.1016/j.nlm.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Parent MB. Increasing acetylcholine Levels in the Hippocampus or Entorhinal Cortex Reverses the Impairing Effects of Septal GABA Receptor Activation on Spontaneous Alternation Learn. Memory. 2000;7:293–302. doi: 10.1101/lm.32200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Parent MB. Infusions of physostigmine into the hippocampus or the entorhinal cortex attenuate avoidance retention deficits produced by intra-septal infusions of the GABA agonist muscimol. Brain Res. 2001;920:10–18. doi: 10.1016/s0006-8993(01)02798-6. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frick KM, Gorman LK, Markowska AL. Oxotremorine infusions into the medial septal area of middle-aged rats affect spatial reference memory and ChAT activity. Behav Brain Res. 1996;80:99–109. doi: 10.1016/0166-4328(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic Innervation of the Rat Hippocampus as Revealed by Choline Acetyltransferase Immunocytochemistry: A Combined Light and Electron Microscopic Study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Gao B, Hornung JP, Fritschy JM. Identification of distinct GABA-a receptor subtypes in cholinergic and parvalbumin-positive neurons of the rat and marmoset medial septum diagonal band complex. Neuroscience. 1995;65:101–117. doi: 10.1016/0306-4522(94)00480-s. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Cholinergic and GABAergic Modulation of Medial Septal Area: Effect on Working Memory. Behav Neurosci. 1990;104:849–855. doi: 10.1037//0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Bidirectional Modulation of Scopolamine-Induced Working Memory Impairments by Muscarinic Activation of the Medial Septal Area. Neurobiol Learn Mem. 1995;63:269–276. doi: 10.1006/nlme.1995.1031. [DOI] [PubMed] [Google Scholar]
- Gold PE. The many faces of amnesia. Learn Memory. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:1–6. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henck J. Developmental Neurotoxicity: Testing and Interpretation. In: Massaro EJ, editor. Handbook of Neurotoxicology. Humana Press; New York, NY: 2002. pp. 3–56. [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Angelucci L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991;538:111–117. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transdunction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav Brain Res. 1995;68:75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Zhang SX, Savage LM. Neuropathology of thiamine deficiency: An update on the comparative analysis of human disorders and experimental models. Metab Brain Dis. 1996;11:19–37. doi: 10.1007/BF02080929. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of ml-m4 Muscarinic acetylcholine Receptor Proteins in Rat Hippocampus and Regulation by Cholinergic Innervation. J Neurosci. 1995;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG. On the role of thalamic pathology in diencephalic amnesia. Rev Neurosci. 1994;5:105–140. doi: 10.1515/revneuro.1994.5.2.105. [DOI] [PubMed] [Google Scholar]
- Mair RG, Otto TA, Knoth RL, Rabchenuk SA, Langlais PJ. Analysis of Aversively Conditioned Learning and Memory in Rats Recovered From Pyrithiamine-Induced Thiamine Deficiency. Behav Neurosci. 1991;105:351–359. [PubMed] [Google Scholar]
- Manseau F, Danik M, Williams S. A functional glutamatergic neurone network in the medial septum and diagonal band area. J Physiol. 2005;566:865–884. doi: 10.1113/jphysiol.2005.089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Givens BS. Cholinergic Manipulations in the Medial Septal Area: Age-Related Effects on Working Memory and Hippocampal Electrophysiology. J Neurosci. 1995;15:2063–2073. doi: 10.1523/JNEUROSCI.15-03-02063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka N, Maeda N, Ohkubo Y, Yamaguchi I. Differential effects of physostigmine and pilocarpine on the spatial memory deficits produced by two septo-hippocampal deafferentations in rats. Brain Res. 1991;559:233–240. doi: 10.1016/0006-8993(91)90007-i. [DOI] [PubMed] [Google Scholar]
- McKinney M. Brain cholinergic vulnerability: Relevance to behavior and disease Biochem. Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Mulder J, Harkany T, Czollner K, Cremers TIFH, Keijser JN, Nyakas C, Luiten PGM. Galantamine-induced behavioral recovery after sublethal excitotoxic lesions to the rat medial septum. Behav Brain Res. 2005;163:33–41. doi: 10.1016/j.bbr.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Nakagawasai O, Tadano T, Hozumi S, Taniguchi R, Yamadera F, Tan-No K, Niijima F, Arai H, Yasuhara H, Kinemuchi H, Kisara K. Involvement of Muscarinic Receptor on the Impairment of Avoidance Learning in Mice Fed a Thiamine-Deficient Diet. Biogenic Amines. 2001;16:199–210. [Google Scholar]
- Nilsson OG, Kalen P, Rosengren E, Bjorklund A. acetylcholine release in the rat hippocampus as studied by microdialysis is dependent on axonal impulse flow and increases during behavioural activation. Neuroscience. 1990;36:325–338. doi: 10.1016/0306-4522(90)90429-8. [DOI] [PubMed] [Google Scholar]
- Pang KCH, Nocera R. Interactions Between 192-IgG Saporin and Intraseptal Cholinergic and GABAergic Drugs: Role of Cholinergic Medial Septal Neurons in Spatial Working Memory. Behav Neurosci. 1999;113:265–275. doi: 10.1037//0735-7044.113.2.265. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: Involved in but not Necessary for Learning and Memory? Learn Memory. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1986. [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: Augmentation by glucose. Proc Natl Acad Sci U S A. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Mark K, Vetreno RP, Savage LM. Increasing Hippocampal acetylcholine Levels Enhances Behavioral Performance in an Animal Model of Diencephalic Amnesia. Brain Res. 2008;1234:116–127. doi: 10.1016/j.brainres.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. Blunted hippocampal, but not striatal, acetylcholine efflux parallels learning impairment in diencephalic-lesioned rats. Neurobiol Learn Mem. 2007;87:123–132. doi: 10.1016/j.nlm.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. The role of cholinergic and GABAergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia. Neuroscience. 2009a;160:32–41. doi: 10.1016/j.neuroscience.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. Blocking GABA-A receptors in the Medial Septum Enhances Hippocampal acetylcholine Release and Behavior in a Rat Model of Diencephalic Amnesia. Pharmacol Biochem Behav. 2009b;92:480–487. doi: 10.1016/j.pbb.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowntree CI, Bland BH. An analysis of cholinoceptive neurons in the hippocampal formation by direct microinfusion. Brain Res. 1986;362:98–113. doi: 10.1016/0006-8993(86)91403-4. [DOI] [PubMed] [Google Scholar]
- Sabolek HR, Bunce JG, Chrobak JJ. Intraseptal tacrine can enhance memory in cognitively impaired young rats. Neuroreport. 2004a;15:181–183. doi: 10.1097/00001756-200401190-00035. [DOI] [PubMed] [Google Scholar]
- Sabolek HR, Bunce JG, Giuliana D, Chrobak JJ. Within-subject memory decline in middle-aged rats: effects of intraseptal tacrine. Neurobiol Aging. 2004b;25:1221–1229. doi: 10.1016/j.neurobiolaging.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Sabolek HR, Bunce JG, Chrobak JJ. Intraseptal tacrine-induced disruptions of spatial memory performance. Behav Brain Res. 2005;158:1–7. doi: 10.1016/j.bbr.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Savage LM, Chang Q, Gold PE. Diencephalic Damage Decreases Hippocampal acetylcholine Release During Spontaneous Alternation Testing. Learn Memory. 2003;10:242–246. doi: 10.1101/lm.60003. [DOI] [PubMed] [Google Scholar]
- Savage LM, Roland JJ, Klintsova AY. Selective septohippocampal – but not forebrain amygdalar – cholinergic dysfunction in diencephalic amnesia. Brain Res. 2007;1139:210–219. doi: 10.1016/j.brainres.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe JW, Colom LV, Bland BH. The Extrinsic Modulation of Hippocampal Theta Depends on the Coactivation of Cholinergic and GABA-ergic Medial Septal Inputs. Neurosci Biobehav Rev. 1992;16:289–308. doi: 10.1016/s0149-7634(05)80203-9. [DOI] [PubMed] [Google Scholar]
- Szutowicz A, Bielarczyk H, Gul S, Ronowska A, Pawelczyk T, Jankowska-Kulawy A. Phenotype-dependent susceptibility of cholinergic neuroblastoma cells to neurotoxic inputs. Metab Brain Dis. 2007;21:149–161. doi: 10.1007/s11011-006-9007-4. [DOI] [PubMed] [Google Scholar]
- Telting-Diaz M, Lunte CE. Distribution of Tacrin Across the Blood-Brain Barrier in Awake, Freely Moving Rats Using in vivo Microdialysis Sampling. Pharm Res. 1993;10:44–48. doi: 10.1023/a:1018964727833. [DOI] [PubMed] [Google Scholar]
- Todd KG, Butterworth RF. Mechanisms of Selective Neuronal Cell Death due to Thiamine Deficiency. Ann N Y Acad Sci. 1999;893:404–411. doi: 10.1111/j.1749-6632.1999.tb07866.x. [DOI] [PubMed] [Google Scholar]
- Todd KG, Butterworth RF. In Vivo Microdialysis in an Animal Model of Neurological Disease: Thiamine Deficiency (Wernicke) Encephalopathy. Methods. 2001;23:55–61. doi: 10.1006/meth.2000.1105. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Luiten PGM. Cholinergic and GABAergic neurons in the rat medial septum express muscarinic acetylcholine receptors. Brain Res. 1994;652:263–272. doi: 10.1016/0006-8993(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Anzalone SJ, Savage LM. Impaired, Spared, and Enhanced acetylcholine Efflux Across the Hippocampus and Striatum in Diencephalic Amnesia is Dependent on Task Demands. Neurobiol Learn Mem. 2008 doi: 10.1016/j.nlm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Hasselmo ME. Functional transitions between epileptiform-like activity and associative memory in hippocampal region CA3. Brain Res Bull. 1997;43:485–493. doi: 10.1016/s0361-9230(97)00003-8. [DOI] [PubMed] [Google Scholar]
- Wu H, Hajszan T, Xu C, Leranth C, Alreja M. Group I Metabotropic Glutamate Receptor Activation Produces a Direct Excitation of Identified Septohippocampal Cholinergic Neurons. J Neurophysiol. 2004;92:1216–1225. doi: 10.1152/jn.00180.2004. [DOI] [PubMed] [Google Scholar]
- Wu M, Newton SS, Atkins JB, Xu C, Dunman RS, Alreja M. Acetylcholinesterase Inhibitors Activate Septohippocampal GABAergic Neurons via Muscarinic but Not Nicotinic Receptors. J Pharmacol Exp Ther. 2003;307:535–543. doi: 10.1124/jpet.103.052514. [DOI] [PubMed] [Google Scholar]
- Wu M, Shanabrough M, Leranth C, Alreja M. Cholinergic Excitation of Septohippocampal GABA But Not Cholinergic Neurons: Implications for Learning and Memory. J Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. J Psychopharm. 2002;16:313–319. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]