Abstract
Objective
The impact of increased fructose consumption on carbohydrate metabolism is a topic of current interest, but determination of serum level has been hindered due to low concentration and interference from serum glucose. We are reporting a method for the quantification of glucose and fructose in clinical samples using gas chromatography/mass spectrometry (GC/MS). The accuracy and precision of GC/MS and an enzymatic assay were compared.
Design and methods
Mass spectrometry fragmentation patterns of methyloxime peracetate derivatized aldose and ketose were determined. Unique fragments for glucose and fructose were used for quantitative analysis using isotope labeled recovery standards.
Results
Methyloxime peracetate derivatives of glucose and fructose showed characteristic loss of acetate (M-60) or ketene (M-42) under chemical ionization (CI). Under electron impact (EI) ionization, a unique C1-C2 fragment of glucose was formed, while a C1-C3 fragment was formed from keto-hexoses. These unique fragments were used in the quantitative assay of glucose and fructose in clinical samples. In clinical samples, the GC/MS assay has a lower limit of detection than that of the enzymatic assay. In plasma samples from patients evaluated for diabetes the average serum glucose and fructose were 6.19±2.72 mM and 46± 25.22 μM. Fructose concentrations in many of these samples were below the limit of detection of the enzymatic method.
Conclusion
Derivatization of aldose and ketose monosaccharides to their respective O-methyloxime acetates for GC/MS analysis is a facile method for determination of serum/plasma glucose and fructose samples.
Keywords: Fragmentography, mass isotopomer, plasma fructose
Introduction
It has been suggested that the obesity epidemic in the United States and other developed countries is directly or indirectly linked to the increased consumption of fructose and sucrose[1]. High consumption of fructose has been associated with insulin resistance,[2] elevated plasma triglyceride levels[2], and hyperuricemia[3] in experimental animals. The impact of the increased consumption of fructose in the form of sucrose and high fructose corn syrup on carbohydrate metabolism is an increasingly important topic of investigation, but determination of fructose level in serum has been hindered due to low concentration of fructose and interference from glucose in the serum sample.
Currently plasma glucose and fructose are determined using enzyme assays from commercially available kits [4]. The assay is based on the oxidation of glucose as glucose-6-phosphate (G6P) using glucose-6-phosphate dehydrogenase (G6PDH). Fructose-6-phosphate is converted to glucose-6-phosphate by the phosphoglucose isomerase enzyme and subsequently oxidized by the G6PDH in the assay mixture. Fructose concentration is determined as the difference in G6P concentration before and after phosphoglucose isomerase treatment. Given the large difference in serum concentration of glucose and fructose, the analytical variance is expected to be high. Previously, GC/MS methods have been used for the determination of glucose and fructose in serum. Kawasaki et al analyzed fructose as its O-ethylhydroxylamine per-acetate derivative[5, 6]. To avoid interference from glucose, fructose is first separate from glucose using HPLC. In the method of Mao et al.[7], fructose was converted to its methoxylamine before silylation and silylated glucose and fructose are then separated in GC/MS analysis.
We are reporting a method for the determination of monosaccharides (aldoses and ketoses) in clinical sample as methoxime per-acetate (MOA) derivatives. Under electron impact ionization (EI) or chemical ionization (CI) conditions, typical fragmentation patterns of aldo-MOA and keto-MOA of various hexoses and pentoses were observed. Based on the unique retention time and mass fragment of the derivatized D-glucose and D-fructose, we were able to develop a quantitative analysis method for serum glucose and fructose. As a demonstration, the method was applied to determined glucose and fructose concentrations in plasma samples from patients evaluated for diabetes. We also compared the application of the method as clinical serum fructose assay with the reported enzymatic methods that is already utilized in a published clinical research [8].
Experimental Procedure
Reagents
Methoxylamine hydrochloride (CH5NO.HCl), pyridine (C5H5N), D-arabinose, D-galactose, D-xylulose, D-ribulose, D-ribose, 2-D-deoxyribose, D-fructose, D-glucose, zinc sulfate (0.3 N) and barium hydroxide (0.3 N) were purchased from Sigma Aldrich (Missouri, USA). D-mannose was obtained from Fluka (Missouri, USA). Acetic anhydride was purchased from Supelco (Missouri, USA). Ethyl acetate was purchased from Fisher Chemicals (Philadelphia, USA). All materials were used without further purification. For fragmentation analysis, glucose and fructose labeled in specific positions with 13C stable isotope were used. [1,2-13C2] D-glucose (99%), [U-13C6] D-glucose (99%), [1,2,3-13C3] D-fructose (99%), [U-13C6] D-fructose (99%) were obtained from Cambridge Isotope Laboratories, Inc. (Massachusetts, USA). [6-13C1] D-glucose (99%) was obtained from Isotech, Inc. (Missouri, USA). Delipidized, defibrinated human serum was obtained from Golden West Biological (California, USA Cat#SP1070-4). Enzymatic assay of fructose was carried out using commercially available kit by Megazyme obtained from Cedarlane Labs (North Carolina, USA, Cat# K-FRUGL).
Monosaccharides standards
For each listed monosaccharides and stable isotope analogs, aqueous solution of 1 mg/mL were prepared and stored in 4°C. For fragmentation studies, 100 μL was used for analysis.
Plasma samples
Blood samples were initially collected for glycolated hemoglobin analysis from patients being evaluated for diabetes control. After separating hemoglobin from plasma, the plasma to be discarded was saved for the study. Plasma samples were obtained from the Clinical Laboratory of Harbor-UCLA Medical Center and received without patient identifier with Institutional Review Board approval.
Extraction From Clinical Serum Sample
Internal standard, [1, 2-13C2] D-glucose (0.208 μmol) and [1, 2, 3-13C3] D-fructose (0.069 μmol), was added to each serum sample (200μL). The proteins in the serum were precipitated with addition of 0.3 N barium hydroxide (300μL) and 0.3 N zinc sulfate (300μL). The supernatant containing glucose and fructose was collected for derivatization procedure.
Derivatization
Capped glass test tubes were used for derivatization process. Each aqueous monosaccharide standards or supernatant collected from serum extraction process were transferred into a clean tube and slowly air dried at room temperature. Methoxylamine hydrochloride (100 μL, 0.18 M in pyridine) was added into each test tube at 70°C for 60 minutes. Acetic anhydride (100 μL) was then added into the test tube and allowed to react at 45°C for another 60 minutes. The sample was air dried and redissolved in 50μL ethyl acetate prior to GC/MS analysis.
GC/MS setup
Fragmentation analysis
Two microliter of sample was injected by an auto-sampler (HP 7683) into HP 6890 Gas Chromatogram equipped with Phenomenex Zebron-5® capillary column (30.0 m × 0.25 mm ID, 0.25 μm film thickness). The GC conditions were: injector temperature 180°C; helium as carrier gas with flow rate of 1 ml/minute and a split ratio of 2; and temperature programming. Column temperature was held initially at 180°C for 2 minutes, and ramped up 5°C per minute to 250°C before final temperature increased of 50°C/min to reach 300°C. GC effluent was introduced to the HP 5973 Mass Spectrometer for both CI and EI mass spectrometry analysis where data were recorded in full scan or linear mode in the mass range of 50–500 m/z. Methane gas was used as the reactant gas for chemical ionization (CI).
GC/MS Quantitative Analysis of glucose and fructose
For D-glucose and D-fructose serum analysis, GC/MS data was collected in EI mode. Due to the comparatively high level of glucose toward serum fructose, assays for each sample were run separately. The inlet temperature was set at 200°C for glucose assay with gas split ratio of 10. Column temperature was held at 205°C for 15 minutes prior to 50°C/min ramp to reach the final temperature of 250°C. Analysis was carried out by monitoring m/z = 131 and the respective internal standard ([1, 2-13C2] D-glucose) value of m/z =133. Fructose assay was carried out higher inlet temperature at 250°C and gas split ratio of 2. Keeping the column temperature programming the same, analysis was carried out by monitoring fructose fragment of m/z =203 and the corresponding isotope peak m/z = 206 for the respective internal standard ([1, 2, 3-13C3] D-fructose). Quantification was carried out by establishing linear regression of the average from triplicate isotopomer ratio measurements of D-glucose and D-fructose (m/z 203 and m/z 206) to that of the internal standard (for glucose, m/z 131 to m/z 133 and for fructose, m/z 203 to m/z 206) in delipidized, defibrinated human serum.
Enzymatic Quantitative Assay of fructose
For fructose quantitative measurement via enzymatic assay, standard curve of fructose in water and in delipidized, defibrinated human serum were established from triplicate absorbance readings at 340 nm (Victor3 1420 Multilabel Counter, Perkin Elmer) following the procedure reported by Adams, et al[4] and Teff, et al[8]. The standard curve was derived from the difference of absorbance due to fructose conversions by phosphoglucose isomerase treatment.
Results
Mass spectroscopy of various aldo-MOAs was analyzed both in chemical ionization (CI) and electron impact (EI) mode. Three aldo-hexoses (D-glucose, D-galactose, D-mannose) and two aldo-pentoses (D-arabinose and D-ribose) were examined. In both cases, gas chromatography was able to resolve the cis and trans structures of the monosaccharides derivatives, where the two structures have very similar fragmentation pattern.
Fragment pattern of Aldo-MOA and Keto-MOA
Electron Impact (EI) Mode
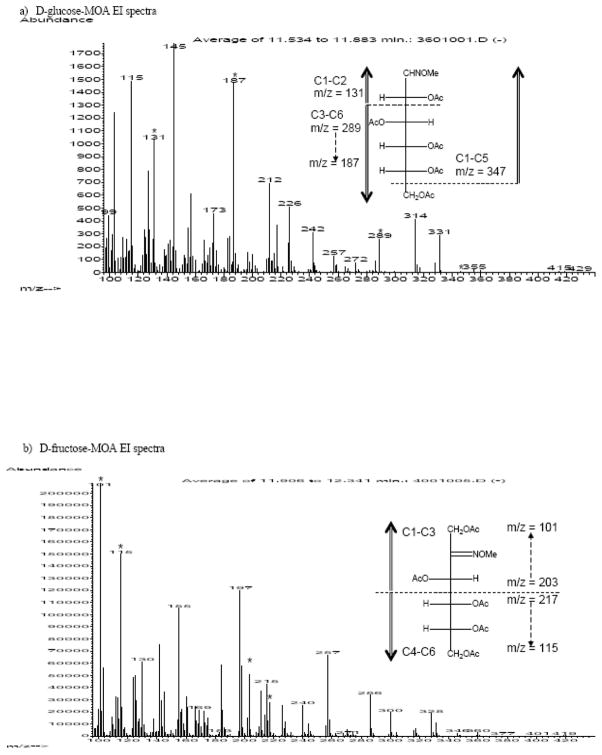
The preparation and fragmentation of aldonitrile peracetate has been extensively studied and reported previously[9–11]. We utilized this information as the starting point in analyzing the aldo-MOA fragmentation. In deducing the fragmentation pattern of aldo-MOA, similarities were observed between aldo-MOA and the reported pattern of aldonitrile peracetate[11, 12]. For each molecular ion (M) of D-glucose-MOA with intact carbon backbone, the corresponding m/z fragments from the isotopomers of [1,2-13C2] D-glucose-MOA, [6-13C1] D-glucose-MOA, and [U-13C6] D-glucose-MOA, were M+2, M+1 and M+6 respectively. The origin of the observed fragments was confirmed by comparison to isotopomer spectra. The fragmentation pattern of the aldo-MOAs was dominated by the loss of acetate (M - 60) and ketene (M - 42) groups from the main carbon structure (Figure 1a).
Figure 1.
Representative mass spectra of aldo-MOA and keto-MOA of hexoses, a) D-glucose-MOA and b) D-fructose-MOA. Fragments due to main chain C-C bond cleavage from electron ionization are indicated with (*). Suggested fragmentation pattern were illustrated in the inserts.
Loss of terminal carbon due to C-C bond fragmentation was observed as one of the minor fragments byproduct, yielding C1-C5 fragment (m/z = 347). The C1-C5 fragment of D-glucose-MOA will have the same exact m/z values with that of [6-13C1] D-glucose-MOA since the 13C isotope is not presence in this fragment. For [1,2-13C2] D-glucose-MOA and [U-13C6] D-glucose-MOA, the corresponding C1-C5 fragment will have m/z values of m + 2 and m + 5 (Table 1). Another main backbone fragmentation was observed at C2-C3 position. The resulting C1-C2 fragment of aldo-MOA (m/z = 131) was easily identified in both hexoses and pentoses monosaccharide derivatives along with the C3-C6 fragments for hexoses (m/z = 289). The corresponding m/z values of the isotopomers confirm the position of the C-C fragmentation, in which no mass shift was observed in the [1,2-13C2] D-glucose-MOA spectra and a +4 shift in the [U-13C6] D-glucose-MOA. Bond cleavage resulting in C1-C2 fragments in EI mode (m/z = 131) was found to be unique for aldo-MOA and was not observed in any of the keto-MOA spectra.
Table 1.
Isotopomer analysis of fragments formed by C-C bond cleavage at the main chain backbone of aldose-MOA (D-glucose-MOA) and ketose-MOA (D-fructose-MOA) observed in GC/MS EI mode spectra. (See inserts of Figure 1a and 1b for suggested fragmentation patterns).
| Methyloxime-peracetate | Fragments Observed | Isotopomer m/z values | |||
|---|---|---|---|---|---|
| D-glucose | [1,2-13C2] D-glucose | [U-13C6] D-Glucose | [6-13C1] D-glucose | ||
| Glucose | C1-C6 | m/z = m | m/z = m+2 | m/z = m+6 | m/z = m+1 |
| C1-C5 | 347 (m) | 349 (m+2) | 352 (m+5) | 347 (m) | |
| C1-C2 | 131 (m) | 133 (m+2) | 133 (m+2) | 131 (m) | |
| C3-C6 | 289 (m) | 289 (m) | 293 (m+4) | 290 (m+1) | |
| Fructose | D-Fructose | [1,2,3-13C3] D-fructose | [U-13C6] D-fructose | ||
| C1-C6 | m/z = m | m/z = m+3 | m/z = m+6 | ||
| C1-C3 | 101 (m) | 104 (m+3) | 104 (m+3) | ||
| C1-C3 | 203 (m) | 206 (m+3) | 206 (m+3) | ||
| C4-C6 | 115 (m) | 115 (m) | 118 (m+3) | ||
| C4-C6 | 217 (m) | 217 (m) | 220 (m+3) | ||
The formation of oxime was particularly helpful for GC/MS analysis of ketoses, which are not accessible with the aldonitrile peracetate method. Similar to the aldoses, gas chromatography was able to resolve the cis and trans structures of the ketose mononsaccharides derivatives, where the two structures have very similar fragmentation pattern. The common method to analyzed ketoses is to form ketoxime with TMS pendant group[13–19]. Similar to derivatization with TMS, conversion of ketose into keto-MOA also allows distinct gas chromatography isolation with an added benefit as the method offers a robust two-step synthesis with minimum work-up procedure [9, 10, 12, 20]. EI mode analysis was also carried out for the keto-MOAs. The spectra were once again dominated by the loss of acetate and/or ketene groups. Major structural information offered in EI mode with the observed C1-C3 fragments at m/z = 203 and m/z = 101 (after further cleavage of acetate and ketene groups), as confirmed by the m+3 values for corresponding fragments from both [1,2,3-13C3] D-fructose-MOA and [U-13C6] D-fructose-MOA. Presence of the C4-C6 fragments were observed at m/z = 217 and at m/z = 115 which have no mass shift in the [1,2,3-13C3] D-fructose-MOA, and a mass shift of M+3 for [U-13C6] D-fructose-MOA. These aldo-MOA and keto-MOA fragments were summarized in Table 1.
Chemical Ionization (CI) Mode
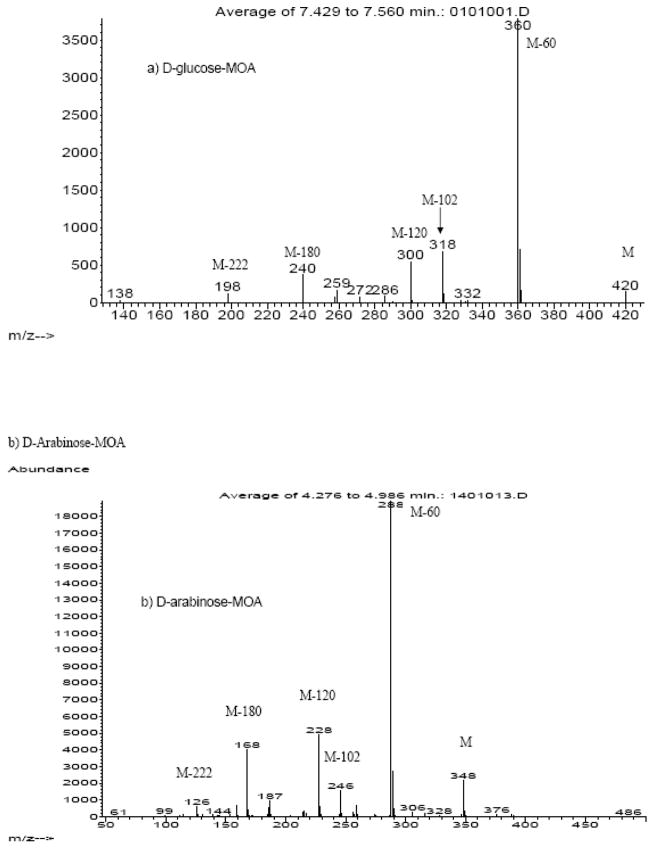
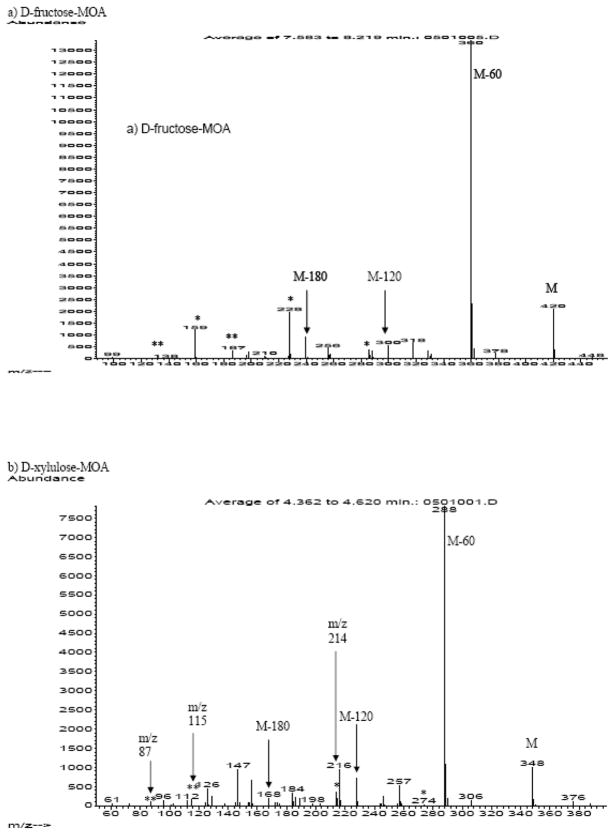
The fragmentation pattern of the aldo-MOAs in the CI mode (Figure 2) was dominated by the loss of acetate (M - 60) and ketene (M - 42) groups from the main carbon structure (Table 2). Similarly, keto-MOA mass fragmentation pattern were dominated by fragments resulted from the loss of acetate and ketene group (Figure 3). Even with a gentler ionization method, mass spectra of keto-MOA collected in CI mode showed fragments resulted from C-C bond cleavage of the ketose main chain. From analysis of fructose and its isotopomer, two different C-C bond cleavages were observed (Table 2). The first bond cleavage resulted in the formation of fragments with 5 carbons in the main chain backbone for keto-hexose (m/z = 288, m/z = 228, and m/z =256). We confirm the length of these fragments by comparison of the corresponding fragment in the spectra of [U-13C6] D-fructose-MOA, which shows a shift of M+5. The [1,2,3-13C3] D-fructose-MOA spectra shows m/z values of m/z+2 instead of m/z+3 for the corresponding fragments suggesting loss in one of the 13C from the C1, C2 or C3 position. As the mass fragments are known to contain 5 carbons backbone, we can conclude that the C-C bond cleavage occurred in the C1-C2 position. In keto-pentose spectra, the 4 carbon fragment products of this C-C bond cleavage were observed at very low signal intensity (m/z = 274 and m/z = 214). The loss of C1 from the main chain could be the consequences of the molecule trying to stabilize the C2 position by converting the oxime into nitrile group. The presence of m/z = 256 in keto-hexose and m/z = 244 for keto-pentose support this argument as the mass fragments indicate the formation of 5 and 4 carbons chain aldonitrile fragment. The second main chain backbone fragmentation was identified with the observance of m/z values that corresponds to fragments with 4 carbons backbone (m/z = 187, m/z= 159, and m/z =138) in D-fructose-MOA. Analysis of the [1,2,3-13C3] D-fructose-MOA isotopomer spectra shows a shift of +1, suggesting that the fragmentation occurred in C2-C3 position.
Figure 2.
Representative mass spectra (CI Mode) of aldoses-MOA: (a) D-glucose-MOA and (b) D-arabinose-MOA. Fragmentation was dominated by loss of acetate (m/z = 60) and ketene (m/z = 42) pendant groups, with no C-C cleavage of the main chain carbons.
Table 2.
Fragments observed in CI mode GC/MS spectra of aldose-MOA and ketose-MOA. For both aldose-MOA and ketose-MOA, loss of acetate and ketene pendant groups dominated the spectra. Main chain C-C bond cleavage only observed for ketose-MOA.
| Molecular ion (m/z) | Fragment m/z values resulted from loss of acetate (m/z = 60) and/or ketene (m/z = 42) | Loss of main chain carbon Only observed in ketose-MOA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | M-OAc | M-2OAc | M-2Ac | M-OAc-Ac | M-3OAc | M-2OAc-Ac | M-3OAc-Ac | M-C1 | M-(C1+C2) | |
| Hexose* | M-60 | M-2(60) | M-2(42) | M-60-42 | M-3(60) | M-2(60)-42 | M-3(60)-42 | |||
| Aldose | 420 | 360 | 300 | 331 | 318 | 240 | 258 | 198 | - | - |
| Ketose | 420 | 360 | 300 | - | 318 | 240 | - | 198 | 288 256 228 |
187 159 138 |
| Pentose** | ||||||||||
| Aldose | 348 | 288 | 228 | 259 | 246 | 168 | 187 | 126 | - | - |
| Ketose | 348 | 288 | 228 | - | 246 | 168 | - | 126 | 274 214 |
115 87 |
For hexoses-MOA (*), aldose-MOA are D-glucose-MOA, D-galactose-MOA, D-mannose-MOA, and ketose-hexose-MOA is D-fructose-MOA.
For pentoses-MOA (**), aldose-MOA are D-arabinose-MOA, D-ribose-MOA, and ketose-MOA are D-xylulose-MOA, D-ribulose-MOA
Figure 3.
Representative of CI mass spectra of ketoses-MOA: a) D-fructose-MOA and b) D-xylulose-MOA. Unique keto-MOA fragments due to loss of C1 and C1+C2 are noted with (*) and (**) respectively.
Serum Samples Analysis
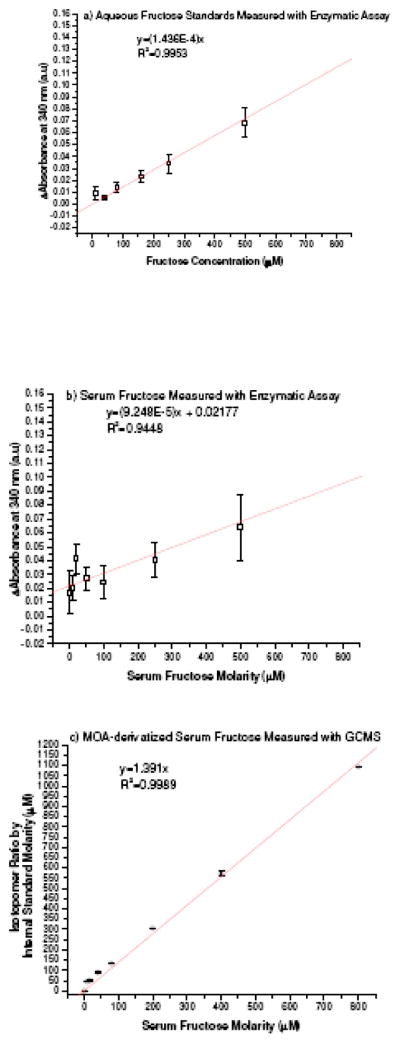
Knowledge on the fragmentation pattern of aldo-MOA and keto-MOA is particularly helpful in analysis of D-glucose and D-fructose mixture. The m/z values of unique fragments from each molecule (m/z = 131 and m/z = 203, respectively) can be used as peak identifier along with their respective gas chromatography retention time, thus associated problems with qualitative identification and/or analysis of the derivatized mixture can be alleviated. Linear response for the range of interest was examined for D-glucose-MOA EI measurement with selective ion monitoring of m/z = 131 and m/z=133 for the [1,2-13C2] D-glucose-internal standard. Similarly, fructose assay utilized selective ion monitoring of the unique C1-C3 fragments with m/z= 203 and with m/z = 206 for the [1,2,3-13C3] D-fructose internal standard. Quantification was determined by linear regression correlation of the average isotopomer ratio values to the mass ratio of the monosaccharide and the respective internal standard. Percent recover for the whole work-up procedure was determined by adding known amount of D-glucose or D-fructose to serum samples with known serum level values. The average percent recovery from 4 different assays was calculated to give 98.41±2.33 % for D-glucose and 99.12±3.88 % for D-fructose. The limit of detection of our method is 0. 3 μM, and the limit of quantification is 15 μM in serum matrix. Standard curves of D-fructose in water (Figure 4a) and in serum (Figure 4b) based on commercially available enzymatic assay kit were established based on the previously reported method [4]. Standard curves of serum D-fructose-MOA assay with GC/MS is presented in Figure 4c showing lower limit of quantification and an improved assay precision.
Figure 4.
Standard curves of fructose prepared either in water (aqueous D-fructose) or in dialyzed serum (serum D-fructose) analyzed using enzymatic or GC/MS methods. a) Aqueous D-fructose, and b) Serum D-fructose using enzymatic assay; and c) Serum D-fructose with MOA-derivatization and GC/MS assay
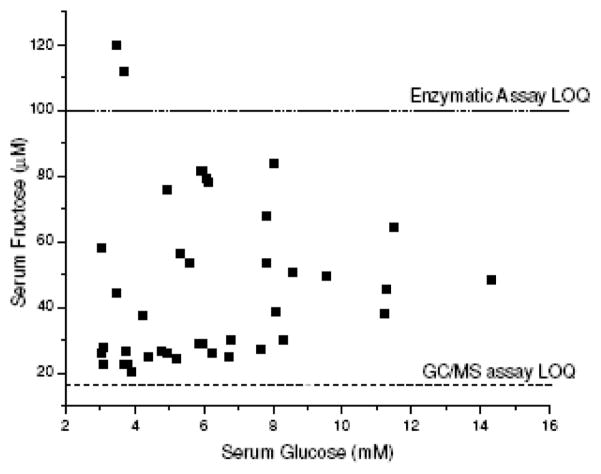
Glucose values from 39 clinical samples (Figure 5) were measured to give an average of 6.19 mM and standard deviation of 2.72 mM. The measured fructose serum level was found to be 2 magnitudes lower, with an average of 46.26±25.22 μM.
Figure 5.
Distribution of glucose and fructose in sera determined using MOA-derivatization and GC/MS. Average serum glucose concentration was 6.19±2.72 mM and average fructose concentration, 46±25.22 μM. The limit of quantification (LOQ) for the assay and the commercially available enzymatic assay are presented as dashed line (see Figure 4).
Discussion
In analysis of monosaccharides with GC/MS is one of the most common practices in carbohydrates research [18, 19]. Various chemical reactions were employed to convert molecules of interest into a more volatile derivative that can be easily isolate with gas chromatography prior to the mass spectrometry analysis. The most popular method for monosaccharides derivatization is to convert the aldose or ketose into the corresponding alditol acetate [18, 19, 21–23]. However, significant loss of information could occur as certain aldose and ketose will be reduced into the same exact alditol acetate, thus limiting the possibilities for mixture analysis [22].
To overcome this obstacle, aldose and ketose was reacted to form their respective oximes. For aldoses, the conversion started by reacting the aldehyde group with hydroxylamine, followed by reaction of the hydroxyl groups with acetic anhydride. In the presence of excess acetic acid, the hydroxyl-oxime was easily converted into the more stable cyano (nitrile) functional group. The method has been extensively studied for optimization of experimental procedure [9, 24] and fragmentation pattern of aldonitrile has been reported [10]. Unfortunately, this approach is not as suitable for analysis of ketoses because the volatile nitrile group cannot be form. Known alternative approaches toward ketose derivatization are to create methyl-oxime (MO) or benzoyl-oxime derivatives of the ketone group [12], forming a more stable protecting group at the C2 position [9, 12–14, 25].
Conversion of the aldehyde and ketone to the corresponding aldonitrile or methyloxime alone is not sufficed for GC/MS analysis due to the low molecular weight and poor volatility. The remaining hydroxyl pendant groups are silylated with trimethylsilyl ether (TMS) or acetylated with acetic anhydride to improve volatility [12–14, 17, 25, 26]. Derivatization of aldose and ketose into the respective methyloxime derivatives with TMS pendant groups have been reported [13, 25]. The drawbacks for TMS application are the efficiency of the derivatization steps and the stability of the resulting product in high moisture environment. Combination of MO formation with the conversion of hydroxyl groups into peracetate pendant groups form methyloxime-peracetate (MOA) have also been reported for various aldoses [9, 10, 14, 20], with limited scope on the gas chromatography and minimum insight into the mass spectroscopy data, even though the synthetic steps are more robust in comparison to MO-TMS.
In this paper, we are reporting the effectiveness of MOA-derivatization method for GC/MS analysis of aldose and ketoses. By analyzing the spectra of aldose (D-glucose) and ketose (D-fructose) isotopomer, we can study the fragmentation path of aldo-MOA and keto-MOA both under EI and CI condition. Spectra collected from electron ionization (Figure 1) show fragmentation of the molecules due to loss of pendant group and cleavage of the main chain backbone. The molecular structure differences between the aldo-MOA and the keto-MOA at the first three carbon position resulted in generation of unique C1-C2 fragment (m/z 131) for aldo-MOA and C1-C3 fragment (m/z 203) for keto-MOA under EI analysis. We observed that using chemical ionization (Figure 2 and Figure 3), fragmentation of the derivatized monosaccharide analytes was mainly due to the loss of loss of acetate (M-60) or ketene (M-42). The isotopomer study also allows identification and explanation for the unique fragmentation products of ketose-MOA under chemical ionization (Figure 3) due to the cleavage at C1-C2 position and at C2-C3 position as described in the result section.
With increasing interest in the health effects of high fructose diet [1, 2, 27–30], it is beneficial to have a practical sample preparation method that allows for assay of glucose and fructose without having to carry out differential derivation of a single sample [15], or extraneous isolation of fructose [5, 6] to minimize glucose interference [31, 32]. By utilizing the specific GC retention time and MS fragments m/z values of D-glucose-MOA and D-fructose-MOA, the method provides a promising utilization as assay of glucose and fructose with high selectivity and sensitivity.
We compare our assay method the commercially available enzymatic assay, as seen in Figure 4. As stated in the enzymatic assay kit manual, the smallest differentiating absorbance for the assay is 0.010, with standard deviation of 0.005 to 0.010 may occurred in duplicate assay runs of a single sample. We observed that the enzymatic assay is acceptable for quantification of aqueous fructose at 50 μM-1 mM concentration range (Figure 4a). In the serum matrix where glucose concentration at mM range was present, the quantification of fructose was greatly affected as the low limit of quantification range increase to 100 μM with poor precisions observed over the entire range of the quantification standard curve (Figure 4b). Utilizing the enzymatic assay for clinical serum fructose assay is of concern as the assay could provide inflated fructose levels for samples with high glucose concentration and/or fructose level lower than the assay limit of quantification. In comparison to the commercially available enzymatic assay, our assay method has lower limit of detection (0.3 μM) and quantification (15 μM in serum). Our assay provides access to quantify lower serum fructose concentration with the improvement of the assay sensitivity while preserving the precision of the measurements (Figure 4c).
We measured 39 serum samples for serum glucose and fructose level. Serum fructose was found to be in the micromolar range, in agreement with the reported values by Macdonald et al.[33]. The concentration of glucose in the serum was found to be at the millimolar range. As seen in Figure 5, we are able to measure the serum fructose level in samples with varied glucose concentration including samples in the high end of glucose serum level, thus allowing the method usage in fructose metabolism study in diabetic population. In comparison to the published data by Kawasaki et al.[6], we observed a higher average serum fructose values. The increase in serum fructose value might be due to the difference in population or dietary fructose intake.
Conclusion
We have developed a quantitative method for measuring serum glucose and fructose concentration. The method requires minimum work-up procedure and simultaneous derivatization reactions to form the respective glucose-MOA and fructose-MOA. The method is precise and sensitive, capable of detecting and quantifying serum fructose level in the micromolar range in the presence of the highly abundance millimolar range serum glucose. This method should prove to be useful for monitoring fructose level in health and diseases.
Acknowledgments
The authors would like to thank Dr. Paul Fu Sr. of the Department of Pathology at Harbor-UCLA Medical Center for his help in obtaining the discard plasma samples. Dr. Mary E. Patterson is the recipient of an NIH Endocrine, Metabolism and Nutrition Fellowship award (2 T32 007571-21-A1). This study is supported by the Biomedical Mass Spectrometry Laboratory of the GCRC (PHS M01-RR00425) and the Metabolomics Core Laboratory of the UCLA Center of Excellence in Pancreatic Diseases (P01 AT003960).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray GA, Nielsen SJ, Popkin BM. Consumption of High-Fructose Corn Syrup in Beverages May Play a Role in The Epidemic of Obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 2.Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW. Fructose-Induced in vivo Insulin Resistance and Elevated Plasma Triglyceride Levels in Rats. Am J Clin Nutr. 1989;49:155–163. doi: 10.1093/ajcn/49.6.1155. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa T, Hu H, Zharikov S, et al. A Causal Role for Uric Acid in Fructose-Induced Metabolic Syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 4.Adams SH, Stanhope KL, Grant RW, Cummings BP, Havel PJ. Metabolic and Endocrine Profiles in Response to Systemic Infusion of Fructose and Glucose in Rhesus Macaques. Endocrinology. 2008;149:3002–3008. doi: 10.1210/en.2007-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawasaki T, Akanuma H, Yamanouchi T. Increased Fructose Concentrations in Blood and Urine in Patients With Diabetes. Diabetes Care. 2002;25:353–357. doi: 10.2337/diacare.25.2.353. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki T, Ogata N, Akanuma H, et al. Postprandial Plasma Fructose Level Is Associated With Retinopathy in Patients With Type 2 Diabetes. Metabolism. 2004;53:583–588. doi: 10.1016/j.metabol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Mao Y-y, Bai J-q, Chen J-h, et al. A Pilot Study of GC/MS-based Serum Metabolic Profiling of Acute Rejection in Renal Transplantation. Transpl Immunol. 2008;19:74–80. doi: 10.1016/j.trim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and Metabolic Effects of Consuming Fructose- and Glucose-Sweetened Beverages with Meals in Obese Men and Women: Influence of Insulin Resistance on Plasma Triglyceride Responses. J Clin Endocrinol Metab. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrant GO, Moss CW. Determination of Monosaccharides as Aldononitrile, O-Methyloxime, Alditol, and Cyclitol Acetate Derivatives by Gas Chromatography. Anal Chem. 1984;56:633–638. [Google Scholar]
- 10.Szafranek J, Pfaffenberger CD, Horning EC. The Mass Spectra of Some Per-O-Acetylaldononitrile. Carbohydr Res. 1974;38:97–105. doi: 10.1016/s0008-6215(00)82341-1. [DOI] [PubMed] [Google Scholar]
- 11.McGinnis GD, Biermann CJ. Analysis of Monosaccharides as Per-O-Acetylated Aldononitrile (PAAN) Derivatives by Gas-Liquid Chromatography (GLC) In: Biermann CJ, McGinnis GD, editors. Analysis of Carbohydrates by GLC and MS. Boca Raton, Florida: CRC Press, Inc; 1989. pp. 119–125. [Google Scholar]
- 12.Neeser J-R, Schweizer TF. Analysis of Carbohydrates as O-Alkyloxime Derivatives by Gas-Liquid Chromatography (GLC) In: Biermann CJ, McGinnis GD, editors. Analysis of Carbohydrates by GLC and MS. Boca Raton, Florida: CRC Press, Inc; 1989. pp. 143–155. [Google Scholar]
- 13.Laine RA, Sweeley CC. O-methyloximes of Sugars. Analysis as O-trimethylsilyl Derivatives by Gas-Liquid Chromatography and Mass Spectrometry. Carbohydr Res. 1973;27:199–213. [Google Scholar]
- 14.Pelletier O, Cadieux S. Glass Capillary or Fuse-Silica GAs Chromatography-Mass Spectrometry of Several Monosaccharides and Related Sugars: Improved Resolution. J Chromatogr B Biomed Sci Appl. 1982;231:225–235. doi: 10.1016/s0378-4347(00)81847-2. [DOI] [PubMed] [Google Scholar]
- 15.Ye F, Yan X, Xu J, Chen H. Determination of Aldoses and Ketoses by GC-MS Using Differential Derivatisation. Phytochem Anal. 2006;17:379–383. doi: 10.1002/pca.928. [DOI] [PubMed] [Google Scholar]
- 16.Kakehi K, Honda S. Silyl Ethers of Carbohydrates. In: Analysis of Carbohydrates by GLC and MS. Biermann CJ, McGinnis GD, editors. Boca Raton, Florida: CRC Press, Inc; 1989. pp. 43–85. [Google Scholar]
- 17.Kito Y, Kawakishi S, Namiki M. Radiation-induced Degradation of D-Fructose in Aerated Condition. Agric Biol Chem. 1981;45:1999–2003. [Google Scholar]
- 18.Biermann CJ. Introduction to Analysis of Carbohydrates by Gas-Liquid Chromatography (GLC) In: Biermann CJ, McGinnis GD, editors. Analysis of Carbohydrates by GLC and MS. Boca Raton, Florida: CRC Press, Inc; 1989. pp. 2–17. [Google Scholar]
- 19.Black GE, Fox A. Recent Progress in The Analysis of Sugar Monomers from Complex Matrices Using Chromatography in Conjunction with Mass Spectrometry or Stand-Alone Tandem Mass Spectrometry. J Chromatogr A. 1996;720:51–60. [Google Scholar]
- 20.Murphy D, Pennock CA. Gas Chromatographic Measurement of Blood and Urine Glucose and Other Monosaccharides. Clin Chim Acta. 1972;42:67–75. doi: 10.1016/0009-8981(72)90378-6. [DOI] [PubMed] [Google Scholar]
- 21.Fox A, Morgan SL, Gilbart J. Preparation of Alditol Acetates and Their Analysis by Gas Chromatography (GC) and Mass Spectroscopy (MS) In: Biermann CJ, McGinnis GD, editors. Analysis of Carbohydrates by GLC and MS. Boca Raton, Florida: CRC Press, Inc; 1989. pp. 87–117. [Google Scholar]
- 22.Cowie GL, Hedges JL. Determination of Neutral Sugars in Plankton, Sediments and Wood by Capillary Gas Chromatography of Equilibrated Isomeric Mixtures. Anal Chem. 1984;56:497. [Google Scholar]
- 23.Walla MD, Lau PY, Morgan SL, Fox A, Brown A. Capillary Gas Chromatography-Mass Spectrometry of Carbohydrate Components of Legionelle and Other Bacteria. J Chromatogr. 1984;288:399. doi: 10.1016/s0021-9673(01)93716-1. [DOI] [PubMed] [Google Scholar]
- 24.Mawhinney TP, Feather MS, MArtinez JR, Barbero GJ. A Rapid, Convenient Method for The Determination of Hexosamines as O-acetylated-O-methyloximes by Gas-Liquid Chromatography. Carbohydr Res. 1979;75:C21–C23. [Google Scholar]
- 25.Storset P, Stokke O, Jellum E. Monosaccharides and Monosaccharide Derivatives in Human Seminal Plasma. J Chromatogr B Biomed Sci Appl. 1978;145:351–357. doi: 10.1016/s0378-4347(00)81364-x. [DOI] [PubMed] [Google Scholar]
- 26.DeJongh DC, Radford T, Hribar JD, et al. Analysis of Trimethylsilyl Derivatives of Carbohydrates by Gas Chromatography and Mass Spectrometry. JACS. 1969;91:1728. [Google Scholar]
- 27.Funari VA, Crandall JE, Tolan DR. Fructose Metabolism in The Cerebellum. Cerebellum. 2007;6:130–140. doi: 10.1080/14734220601064759. [DOI] [PubMed] [Google Scholar]
- 28.Harnack LJ, Jeffery RW, Boutelle KN. Temporal Trends In Energy Intake In The United States: An Ecologic Perspective. Am J Clin Nutr. 2000;71:1478–1484. doi: 10.1093/ajcn/71.6.1478. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins M, Gabriely I, Wozniak R, Vilcu C, Shamoon H, Rossetti L. Fructose Improves the Ability of Hyperglycemia Per Se to Regulate Glucose Production in Type 2 Diabetes. Diabetes. 2002;51:606–614. doi: 10.2337/diabetes.51.3.606. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Lozada LG, Tapia E, Jiménez A, et al. Fructose-Induced Metabolic Syndrome is Associated with Glomerular Hypertension and Renal Microvascular Damage in Rats. Am J Physiol Renal Physiol. 2007:292. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 31.Pitkanen E, Kanninen T. Determination of Mannose and Fructose in Human Plasma Using Deuterium Labelling and Gas Chromatography/Mass Spectrometry. Biol Mass Spectrom. 1994;23:590–595. doi: 10.1002/bms.1200230909. [DOI] [PubMed] [Google Scholar]
- 32.Pitkanen E. Mannose, Mannitol, Fructose and 1,5-anhydroglucitol Concentrations Measured by Gas Chromatography/Mass Spectrometry in Blood Plasma of Diabetic Patients. Clin Chim Acta. 1996;251:91–103. doi: 10.1016/0009-8981(96)06284-5. [DOI] [PubMed] [Google Scholar]
- 33.Macdonald I, Keyser A, Pacy D. Some Effects, in Man, of Varying The Load of Glucose, Sucrose, Fructose, or Sorbitol on Various Metabolites in Blood. Am J Clin Nutr. 1978;31:1305–1311. doi: 10.1093/ajcn/31.8.1305. [DOI] [PubMed] [Google Scholar]