Abstract
Schizophrenia is a life-long, severe, and disabling brain disorder that requires chronic pharmacotherapy. Because current antipsychotic drugs do not provide optimal therapy, we have been developing novel treatments that focus on receptors for the neuropeptide neurotensin (NT). NT69L, an analog of neurotensin(8–13), acts like an atypical antipsychotic drug in several dopamine-based animal models used to study schizophrenia. Another current animal model utilizes non-competitive antagonists of the NMDA/glutamate receptor, such as the psychotomimetic phencyclidine (PCP). In the present study, we investigated the effects of NT69L on PCP-induced behavioral and biochemical changes in the rat. The top of an activity chamber was modified to allow us to perform microdialysis in rat brain, while simultaneously recording the locomotor activity of a rat. PCP injection significantly increased activity as well as the extracellular concentration of norepinephrine (NE), 5-HT, dopamine (DA), and glutamate in the medial prefrontal cortex (mPFC). Pretreating with NT69L blocked the PCP-induced hyperactivity as well as the increase of DA, 5-HT, NE, and glutamate in mPFC. Interestingly and unexpectedly, NT69L markedly increased glycine levels, while PCP was without effect on glycine levels. Thus, NT69L showed antipsychotic-like effects in this glutamate-based animal model for studying schizophrenia. Previous work from our group suggests that NT69L also has antipsychotic-like effects in dopaminergic and serotonergic rodent models. Taken together, these data suggest that NT69L in particular and NT receptor agonists in general, will be useful as broad-spectrum antipsychotic drugs.
Keywords: schizophrenia, NT69L, monoamine, glutamate, glycine, medial prefrontal cortex
1. Introduction
Neurotensin (NT), a tridecapeptide, was first isolated from bovine hypothalami (Carraway and Leeman, 1973). It is widely distributed throughout the central nervous system, with the highest concentration in the amygdale, lateral septum, substantia nigra and ventral tegmental area (Uhl, 1982). In the CNS, NT acts as a neurotransmitter or a neuromodulator (Nemeroff, 1986). The NT system is associated with several neurotransmitter systems including dopaminergic, serotonergic, GABAergic, glutamatergic, and cholinergic. Biochemical studies have shown that antipsychotic drug treatments increase NT neurotransmission, and centrally administered NT produces biochemical and behavioral effects similar to those observed following administration of antipsychotic drugs (Tamminga et al, 1985). Moreover, a large amount of evidence suggests that targeting the neurotensin system may provide a novel treatment for schizophrenia (Breslin et al., 1994), (Garver et al., 1991) and (Sharma et al., 1997).
Our group has been developing analogs of NT that can be administered peripherally, pass into brain, and induce behavioral effects and neurochemical changes similar to those of native NT directly injected into brain (Boules et al., 2003) and (Boules et al., 2006). Since all known activity of NT is mediated by the 8–13 fragment, we have focused our efforts on this part of the molecule. Similar to the prototypical new generation, atypical, antipsychotic drug, clozapine, NT69L, as one of these analogs of NT(8–13), has been shown to be effective in several animal models that predict antipsychotic effects in humans. These include dopamine-based animal models used to study schizophrenia such as apomorphine-induced climbing (Cusack et al., 2000) and amphetamine-induced hyperactivity (Boules et al., 2001). Its effect on monoamine systems involved in schizophrenia has also been studied (Liang et al., 2008) and (Wang Rui, 2004).
Previous studies suggest that the investigation of dysfunction of the prefrontal cortex (PFC) is very important for understanding the pathophysiology of schizophrenia, particularly cognitive dysfunction (Weinberger, 1987) and (Pearlson et al., 1996). Nonschizophrenic patients with prefrontal lesions show schizophrenia-like signs, especially negative and cognitive signs and symptoms (Fuster, 1989). Additionally, it has been consistently reported that schizophrenic patients show decreased metabolism, decreased blood flow, and impaired function of the PFC (Park and Holzman, 1992) (Bertollo et al., 1996), (Cleghorn et al., 1992), and (McGuire et al., 1993). Glutamatergic terminals in the PFC arise from different brain regions i.e. hippocampus, thalamus, amygdala, and other cortical areas, while dopaminergic terminals in the PFC arise from the ventral tegmental area (VTA). Several lines of evidence suggest that glutamatergic and dopaminergic systems play an important role in the physiology and pathology of this area of the brain. An imbalance of glutamate-dopamine interactions in the PFC might underlie behavioral and neurochemical abnormalities that occur in psychiatric disorders such as schizophrenia (Thierry et al., 1990), (Goldman-Rakic et al., 2000), (Moghaddam, 2002), (Steketee, 2003) and (Sesack et al., 2003).
Phencyclidine (PCP), a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA)/glutamate receptor, induces schizophrenia-like cognitive dysfunction and psychotic behavior in otherwise normal individuals (Snyder, 1980) and (Javitt and Zukin, 1991). Thus, its administration to rodents has proven useful as an animal model for studying schizophrenic psychosis (Jentsch and Roth, 1999). The present study was conducted to determine whether NT69L alters PCP- induced behavioral and biochemical changes in a glutamate-based animal model.
2. Results
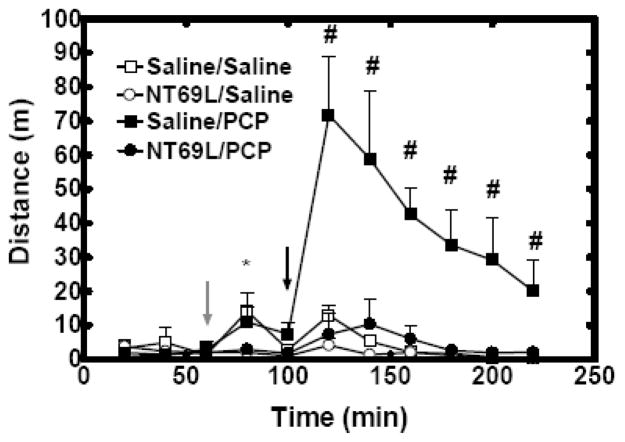
Effect of NT69L on the PCP-induced hyperlocomotion
Locomotor activity was recorded for 220 mins. At 60 mins, rats received either Saline or NT69L injection. At 100 mins, rats received either Saline or PCP injection. Thus, there were four groups: Saline/Saline, NT69L/Saline, Saline/PCP and NT69L/Saline involved in all the experiments.
Both NT69L/Saline treatment and NT69L/PCP treatment had no effect on the locomotor activity (all P>0.57). An overall Time by Group interaction (P<0.001) was observed. Saline injection significantly increased locomotor activity by 4 fold in both Saline/Saline group and Saline/PCP group at 80 mins (P=0.01). In Saline/PCP group, PCP injection significantly increased locomotor activity by 9 fold as compared with no change in NT69L/PCP group (at all time points since 120 mins, P<0.001) (Fig. 2). Insert Figure 2 and Table 1 about here.
Figure 2.
NT69L blocked PCP-induced hyperactivity. The data are shown as the mean of total travel distance measured every 20 min (±S.E.M.). (N=5 for Saline/PCP group, Saline/Saline group and NT69L/Saline group, N=6 for NT69L/PCP group). *, P<0.05 NT69L treated groups versus Saline treated groups. #, P<0.05 versus Saline/Saline, NT69L/Saline and NT69L/PCP groups. Gray arrow indicates when rats received either saline or NT69L injection. Black arrow indicates when rats received either saline or PCP injection.
Table 1.
Basal concentration of Monoamines and amino acids in mPFC.
| Saline/Saline | NT69L/Saline | Saline/PCP | NT69L/PCP | |
|---|---|---|---|---|
| Dopamine (nM) | 0.75±0.3 | 0.82±0.15 | 0.80±0.10 | 0.7±0.2 |
| 5-HT (nM) | 0.68±0.09 | 0.74±0.14 | 0.74±0.07 | 0.7±0.1 |
| NE (nM) | 1.9±0.83 | 2.4±0.4 | 2.6±0.2 | 2±1 |
| DOPAC (nM) | 39±8 | 46±5 | 43±5 | 47±7 |
| Glutamate (μM) | 3.5±0.6 | 2.8±0.8 | 3.0±0.3 | 2.1±0.6 |
| Glycine (μM) | 5.8±1.2 | 4.7±0.9 | 4±1 | 5.2±0.8 |
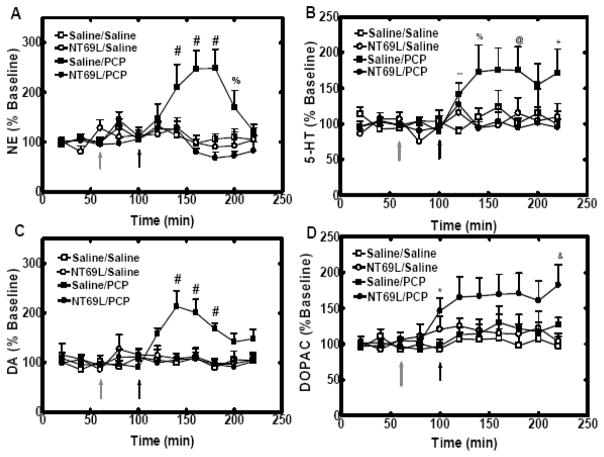
Effects of NT69L on monoamine levels in the mPFC after PCP injection
The top of an activity chamber was modified to allow us to monitor the extracellular concentration of monoamines and amino acids in mPFC by using in vivo microdialysis while simultaneously recording locomotor activity for a rat.
During the whole experiment, there was no change in the release of NE (all P>0.078), 5-HT (all P>0.14) and DA (all P>0.10) in Saline/Saline group, NT69L/Saline group and NT69L/PCP group. From 120 mins to 220 mins, an overall Time by Group interaction was observed for NE (P=0.003) and DA (P=0.05). In the Saline/PCP group, systemic administration of PCP (5 mg/kg i.p) significantly increased NE level to 247±37% (at 140mins P=0.049; at 160mins P=0.007; at 180mins P=0.005; at 200mins P=0.024), 5-HT level to 176±30%, DA level to 214±31% (at 140mins P<0.001; at 160mins P=0.005; at 180mins P<0.001). Pretreatment of NT69L (1 mg/kg i.p) blocked PCP-induced increase of NE, 5-HT and DA release in the mPFC in NT69L/PCP group (Fig. 3A-C). The basal concentration of NE, 5-HT and DA is shown in Table 1.
Figure 3.
In panels A, NT69L blocked the PCP-induced increase of NE levels in mPFC. In panels B, NT69L blocked the PCP-induced increase of 5-HT levels in mPFC. In panels C, NT69L blocked PCP-induced increases of DA levels in mPFC. In panels D, NT69L increased DOPAC levels in mPFC. All data are shown as the % baseline of monoamines measured every 20 min (±S.E.M.). (N=5 for Saline/PCP group, Saline/Saline group and NT69L/Saline group, N=6 for NT69L/PCP group). %, P<0.05 versus NT69L/Saline and NT69L/PCP groups. *, P<0.05 NT69L treated groups versus Saline treated groups. &, P<0.05 versus NT69L/Saline and Saline/Saline groups. #, P<0.05 versus Saline/Saline, NT69L/Saline and NT69L/PCP groups. @, P<0.05 versus Saline/Saline and NT69L/PCP groups. **, P<0.05 versus Saline/Saline group. +, P<0.05 versus NT69L/PCP group. Gray arrow indicates when rats received either saline or NT69L injection. Black arrow indicates when rats received either saline or PCP injection.
There was no change in the release of DOPAC in Saline/Saline group, NT69L/Saline group and Saline/PCP group (all P>0.18). In NT69L/PCP group, NT69L injection significantly increase DOPAC release to 146±19% at 100mins (P=0.02), and the following PCP injection exacerbated NT69L-induced increase of DOPAC in NT69L/PCP group (Fig. 3D) (at 220 mins, P=0.01). The basal concentration of DOPAC is shown in Table 1.
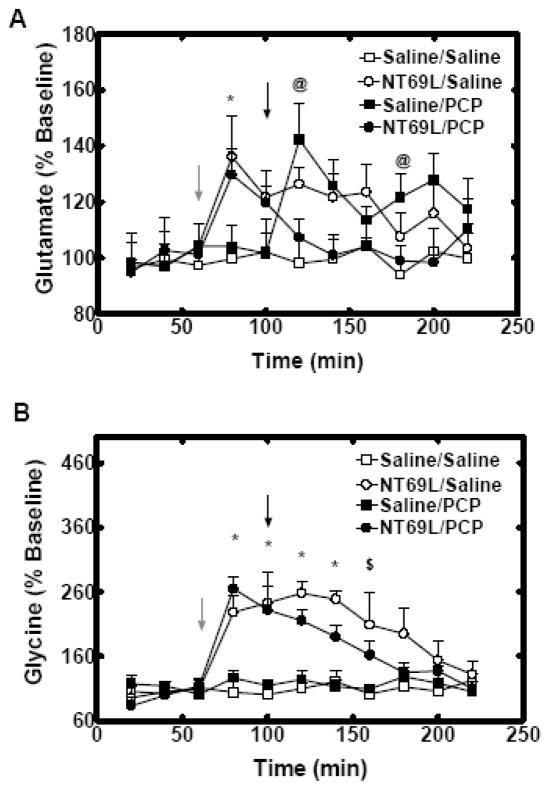
Effects of NT69L on the amino acid levels in the mPFC after acute PCP injection
Saline/Saline treatment had no effect on the glutamate release in mPFC. In the first 100 mins, an overall Time by Group interaction (P=0.02) was observed for glutamate release. NT69L injection increased glutamate level to136±15% in NT69L/Saline group and to130±9% in NT69L/PCP group, at 80mins (P=0.02) as compared with no change in Saline treated groups (all P>0.78). From 120 mins to 220 mins, PCP injection increased glutamate release to 142±13% in Saline/PCP group at 120 mins (P=0.008). Pretreatment of NT69L blocked the PCP-induced increase of glutamate in NT69L/PCP group (Fig. 4A). On the other hand, PCP injection decreased NT69L-induced increase of glutamate release in NT69L/PCP group vs NT69L/Saline group (P=0.03). The basal concentration of glutamate is shown in Table 1.
Figure 4.
In panels A, NT69L blocked the PCP-induced increase of glutamate levels in mPFC. In panels B, NT69L significantly increased glycine levels in mPFC in both NT69L/Saline group and NT69L/PCP group. All data are shown as % baseline of amino acids measured every 20 min (±S.E.M.). (N=5 for PCP group, Saline/Saline group and NT69L/Saline group, N=6 for NT69L pretreated group). *, P<0.05 NT69L treated groups versus Saline treated groups. @, P<0.05 versus Saline/Saline and NT69L/PCP groups. $, P<0.05 versus Saline/Saline, and Saline/PCP groups. Gray arrow indicates when rats received either saline or NT69L injection. Black arrow indicates when rats received either saline or PCP injection.
There was no change in glycine release in mPFC in both Saline/Saline group and Saline/PCP group (P>0.39). An overall Time by Group interaction (P<0.001) was observed for glycine re lease. NT69L injection significantly increased glycine release (to 228 ± 27% in NT69L/Saline group and to 265 ± 19% in NT69L/PCP group, at 80 mins–140 mins P<0.001; at 160mins P=0.01) (Fig. 4B). Additionally, there was a significant difference between NT69L/Saline group and NT69L/PCP group since PCP injection gradually decreased NT69L-induced glycine release in NT69L/PCP group (at 120 mins P=0.04; at 140 mins P=0.02). The basal concentration of glycine is shown in Table 1.
3. Discussion
NT69L, an analog of NT (8–13), has atypical, antipsychotic-like effects in several DA-based animal models. This peptide mediates its effect through both NTS1 and NTS2, the two NT G-protein coupled receptors that are thought to mediate most, if not all, effects of NT and other NT receptor agonists. Because NT69L only binds to NT receptors in a large screen of binding sites (Richelson, unpublished data), the pharmacological effects of this peptide are mediated by NT receptors. The question is whether NTS1, NTS2, or both receptors are involved in the antipsychotic-like effects of NT69L. PFC is rich in NTS1 binding sites mostly on the cell bodies of pyramidal cells and on GABA-ergic interneurons (Petrie et al., 2005). This region also contains immunoreactivity for NTS2 (Asselin et al., 2001).
Animal models for studying schizophrenic psychosis that are DA-based only relate to the positive symptoms of the disease and not the negative and cognitive signs and symptoms. Moreover, several studies have reported that amphetamine may actually alleviate negative and cognitive signs and symptoms in schizophrenia patients (Goldberg et al., 1991). On the other hand, PCP-based animal models may reflect both the positive and negative symptoms of schizophrenia, including cognitive dysfunction that can be found in normal human subjects given PCP (Snyder, 1980) and (Javitt and Zukin, 1991). The present studies show that systemic administration of PCP significantly increased locomotor activity as expected and that pretreatment with NT69L blocked PCP-induced hyperactivity. These results support previous work with an analog of PCP, dizocilpine (MK801), showing that NT69L reverses the disruption of prepulse inhibition of the startle response caused by this NMDA non-competitive antagonist (Shilling et al., 2003). Previous work on another NT(8–13) analog (Feifel et al., 1999) supports the hypothesis that NT receptor agonists in general exhibit antipsychotic-like effects at least in part through the NMDA/glutamate system.
In the current study, PCP caused similar neurochemical changes in the mPFC as observed in schizophrenic patients. In the PFC, a complex interaction exists between various neurotransmitters including DA, NE, 5-HT, acetylcholine, glutamate, GABA, and NT (Steketee, 2003). Most of these neurotransmitters are involved in neurochemical changes observed in schizophrenic patients. DA levels appear to be elevated in brains of schizophrenic patients (see review by Heritch, 1990) and have also been reported in schizophrenic patients by using brain imaging techniques. Levels of 5-HT have consistently been reported to be elevated in the putamen, caudate, globus pallidus, and prefrontal cortex in schizophrenic patients (Ohuoha et al., 1993). An apparent relationship has been demonstrated between elevated NE and certain schizophrenic symptoms (Breier et al., 1994). NE has been shown to reduce distractibility by strengthening PFC function and to improve cognitive dysfunction seen in schizophrenia (Arnsten et al., 2004). Additionally, some form of a glutamate dysfunction has been demonstrated in schizophrenia. It includes increased levels of both glutamate and NMDA receptors in the amygdala and hippocampal regions (Zavitsanou et al., 2002), (Gao et al., 2000) and (Kerwin et al., 1990).
Previous studies have clearly shown that acute administration of PCP to animals profoundly increases forebrain dopaminergic (Doherty et al., 1980), (Verma and Moghaddam, 1996) and (Jentsch et al., 1997), serotonergic (Amargos-Bosch et al., 2006), and noradrenergic (Marwaha, 1982) transmissions. Our results confirmed these findings where systemic administration of PCP significantly increased extracellular concentration of DA, 5-HT, and NE in the mPFC. Pretreatment with NT69L significantly decreased the PCP-induced increase of DA, 5-HT, and NE release in the mPFC, a result that is similar to the effect of NT69L on DA, 5-HT, and NE release caused by nicotine (Liang et al., 2008).
A recent in vivo microdialysis study shows that NT69L (1 mg/kg) increases extracellular concentrations of DA by about 35–40% in mPFC (Prus et al., 2007). However, we did not observe an increase of DA levels in mPFC after NT69L injection. This discrepancy may be due to the difference of probe location, flow rate, or temporal resolution. The results of NT69L on DOPAC levels were interesting. Our previous work showed that NT69L elevates DOPAC levels in the hypothalamus (Boules et al., 2000). This also appeared to be the case for the mPFC (Fig. 3D). However, the data suggest that the combination of PCP and NT69L were synergistic (and not additive) with respect to DOPAC release. Since DOPAC is a major metabolite of DA, whose release was markedly increased by PCP (Fig. 3D), these results suggest that NT69L decreases PCP-induced release of DA by increasing DA metabolism.
As a noncompetitive antagonist of the NMDA receptor, PCP has consistently been shown to increase levels of glutamate in the mPFC (Abekawa et al., 2006) and (Abekawa et al., 2007). Our present findings again reproduce these previous results. A possible explanation is that in mPFC, PCP blocks the NMDA receptors’ function on GABA neurons, which indirectly disinhibits cortico-cortical glutamate neurons. These neurons synapse to glutamatergic pyramidal cells via non-NMDA types of glutamate receptors (Adams and Moghaddam, 1998). The disinhibition increases extracellular concentrations of glutamate in mPFC. The elevated glutamate binds to non-NMDA receptors, causing the activation of cortico-accumbal glutamatergic pathway which then induces hyperactivity (Berendse et al., 1992). Previous studies have shown that NT treatment increases glutamate levels in several brain regions such as striatum (Ferraro et al., 2001) and the mPFC (Sanz et al., 1993). The present study is the first to show that NT69L can increase extracellular concentrations of glutamate in mPFC. It has been proposed that NT increases glutamate levels through interactions with NTS1 and DA D2 receptors on presynaptic membranes. Thus, binding of NT69L to NTS1 receptors would inhibit D2 receptor function on the glutamate neuron, further stimulating glutamate release (Antonelli et al., 2007). Moreover, pretreatment of NT69L blocked the PCP-induced glutamate release in the mPFC. In the current study, there was no change in GABA release in mPFC in all groups (data not shown). A previous study shows that NT increases extracellular concentration of GABA in the PFC (Petrie et al., 2005). The discrepancy is likely due to the way these compounds were administered. NT was directly injected into brain of NT (Petrie et al., 2005), while in this and all our other studies, NT69L was given outside the brain. We have discussed previously that conclusions about the effects of a NT receptor agonist given outside the brain cannot be predicted from studies in which a NT receptor agonist is injected into discrete areas of the brain (Richelson et al., 2003).
Perhaps most interesting are our results showing for the first time that a NT receptor agonist robustly increased extracellular concentrations of glycine in the mPFC. This was an unexpected finding that opens up a new direction for the investigation of a possible mechanism of atypical antipsychotic-like activity of NT69L and other NT agonists. Normal NMDA receptor function depends on not only the binding of glutamate to the receptor, but also the binding of glycine to an allosteric site on this receptor (GLYB). The source of endogenous ligands for GLYB, such as glycine and D-serine, are largely from glial cells. Studies have reported low levels of D-serine in schizophrenic patients (Hashimoto et al., 2003)), which suggests hypofunction of NMDA receptors in brains of patients with schizophrenia. In addition, clinical studies using GLYB agonists (such as D-serine and D-cycloserine) have shown very promising results for treatment of schizophrenia. A significant decrease in negative and cognitive symptoms has been reported in the GLYB agonist treated group (Heresco-Levy et al., 1999) and (Coyle and Tsai, 2004). Additionally, as mentioned previously, our unpublished studies suggest that the effects of NT69L in animal models are more similar to clozapine than to the typical antipsychotic drug haloperidol. Interestingly, clozapine alters the glycine modulatory site on NMDA receptors (Coyle and Tsai, 2004). The mechanism whereby NT69L elevates brain levels of glycine in the mPFC is uncertain. However, it could possibly involve transport mechanisms for glycine into glial cells.
Recent work utilizing pharmacological magnetic resonance imaging (phMRI) shows that agents stimulating NMDA receptors via the GLYB co-agonist site (e.g., D-serine) can potentiate NMDA receptor activity in the living rat brain (Gozzi et al., 2008). This preclinical work along with the clinical studies mentioned supports the idea that glycine receptor agonists hold promise for treatment of schizophrenia. More importantly, this phMRI study (Gozzi et al., 2008) showed that the effects of PCP could be completely inhibited by pretreatment with GLYB receptor agonists. These data suggest that in our studies NT69L blocked the effects of PCP by increasing glycine release.
Several studies have shown that PCP affects NT systems. However, the results are not consistent. PCP (2.5 mg/kg, s.c) causes a short lasting elevation of extracellular NT-LI levels in the mPFC (Hertel et al., 1995). While in another study both acute and chronic administrations of PCP significantly decreased the content of NT-LI in the frontal cortex (Wachi et al., 1985).
In summary, we report some novel observations with the NT receptor agonist NT69L: 1) it blocked PCP-induced biochemical changes, while blocking its behavioral effects; and 2) NT69L increased both glutamate and glycine levels in the mPFC. These observations open up new lines of investigation focusing on the mechanism of glycine release by NT69L, the role of this release in the antipsychotic-like effects of this compound, and the relationship between changes in glutamate and glycine release. Ongoing studies of the effect of NT69L on PCP-induced changes in monoamines and amino acids in the mPFC may provide possible neuronal mechanisms for understanding some of the pathophysiology of schizophrenia and may provide further neuronal targets for the pharmacological treatment of schizophrenia.
4. Experimental Procedure
Animals and Surgery
All procedures were approved by the Mayo Foundation Institutional Animal Use and Care Committee. Male Sprague-Dawley rats (approximately 2.5 months of age and weighing 300±25 g at the beginning of the study) (Harlan, Indianapolis, IN, USA), were housed in a temperature controlled room (23±2°C) with a 12/12 light/dark cycle (lights off at 6:00 pm) and free access to food and water. On the day of surgery, each rat was anesthetized with gasiform isoflurane (1% isoflurane in a mixture of 20% oxygen and 80% nitrogen gas), and placed in a stereotaxic apparatus (KOPF Ins., Tujunga, CA, USA) for surgical implantation of a guide cannula. The guide cannula (CMA Microdialysis Inc., Acton, MA, USA) was positioned to mPFC according to the coordinates (AP 3.2, ML 0.5, DV 2.0). The guide cannula was secured to the skull with dental cement anchored by two stainless steel screws. After surgery, each rat was individually housed and allowed to recover for at least 3 days before the microdialysis experiment.
Microdialysis Procedure and Locomotor Activity
Microdialysis experiments were carried out on conscious, freely moving rats. On the day of the experiment, the stylet in the guide cannula was replaced with the microdialysis probe (CMA/12 with 2 mm membrane active length, CMA Microdialysis Inc., Acton, MA, USA). The probe was perfused at 2 μl/min with artificial cerebrospinal fluid (146 mM NaCl, 1.2 mM, CaCl2, 3 mM KCl, 1.0 mM MgCl2, 1.9 mM Na2HPO4, 0.1 mM, NaH2PO4, pH 7.4). The rat was placed into a Plexiglas Opto-Varimex Minor motility chamber (Columbus Instruments, Columbus, OH, USA). The chamber has a modified top to allow us to perform microdialysis in rat brain, while simultaneously recording the locomotor activity of a rat. After at least 2 h equilibration, dialysate samples were automatically collected every 20 min into vials containing 2 μl perchloric acid (0.5 M) to retard oxidation of monoamines. Three baseline fractions were collected before NT69L or saline injection. Pretreatment of NT69L (1 mg/kg, ip) or saline occurred 40 min before PCP (5mg/kg, ip) or saline injection during microdialysis. Locomotor activity was recorded during 1 hr baseline collection, 40 min after saline/NT69L injection and 2 hrs following PCP/Saline injection. Half of the volume of all dialysate samples was immediately analyzed via the HPLC-electrochemical detector for the determination of monoamines and their metabolites. The other half was analyzed via CE-LIF for measuring amino acid levels. Results were reported as % increase over baseline. At the end of each experiment, the animals were sacrificed and the brains were removed and sectioned to verify the correct position of the probe (Figure 1). Only the data from rats with accurate probe placement were included in data analyses (One animal in saline/PCP group was excluded, due to inaccurate cannula placement).
Figure 1.
Coronal sections showing microdialysis probe placement within mPFC for all animals. Panel A: A picture of real brain coronal section. Black arrow points out probe location. Panel B-C, Brain coronal section figures. Solid lines indicate the active dialysis regions. Numbers below the figure represent the position of the slice relative to Bregma. The figure was adapted from Paxinos and Watson (2005).
Monoamine Assay
Monoamines and their metabolites were measured on an ESA HPLC coupled with Coulochem II electrochemical detector system (ESA Inc., Chelmsford, MA, USA) with a 20 μl sample loop. They were separated on an MD-150 analytical column (3 × 150mm, 3μm C18, ESA Inc., Chelmsford, MA, USA) with MDTM mobile phase (ESA Inc., Chelmsford, MA, USA) at 0.6 ml/min. Potential settings for detection were E1 at −175 mV, E2 at 250 mV, GC at 350 mV. Peaks were displayed, integrated, and stored with ESA 501 Chromatography data system (ESA Inc., Chelmsford, MA, USA). The detection limits of NE, DA, 5-HT and DOPAC are 263.8 pM, 22.6pM, 37.8pM and 259.4 pM, respectively.
Amino Acid Assay CE
Amino acids were measured utilizing the procedure established by (Lada and Kennedy, 1996) and refined by (Bowser and Kennedy, 2001) and (Smith et al., 2004). Briefly, analytes in the dialysate containing primary amine moieties were derivatized on-line by mixing dialysate flow with a 1μl/min stream of OPA derivatization solution (10 mM OPA, 40 mM BME, 36 mM borate, 0.81 mM HPBCD, 10% MeOH (vol/vol), pH 10.5), with a reaction time of approximately 30 s. Samples of the dialysate were injected onto the capillary (separation buffer = 40 mM borate, 0.9 mM HPBCD) using a flow-gate interface as described previously (Lada and Kennedy, 1996; Bowser and Kennedy, 2001; Smith et al., 2004). The OPA labeled analytes were detected using LIF in a sheath-flow detector cell. Fluorescence was excited using the 351 nm line (20 mW total UV) of an argon-ion laser (Enterprise II 622; Coherent Laser Group, Santa Clara, CA, USA). Emission was filtered and collected at 90° from the incident beam on a photomultiplier tube (PMT, R9220; Hamamatsu, Bridgewater, NJ, USA). Current from the PMT was amplified and sampled with the same software and hardware used for controlling the injections. The detection limits of glutamate and glycine are 84.5nM and 28.7nM, respectively.
Statistical Analysis
Two-way repeated-measure ANOVA followed by Tukey test was used to compare the percentage of increase over baseline in all microdialysis experiments as well as distance in locomotor activity tests between groups and time as independent factors, and time as the repeated factor using Sigma Stat software (SPSS, Inc., Chicago, IL, USA). The interaction between the time and groups has also been considered. Difference between groups was analyzed by one-way ANOVA using the same software in each time point. P<0.05 was considered significant.
Acknowledgments
We would like to thank Dr. Tomfumi Miura and Irfan K. Fauq, for technical support.
This work was supported by NIMH grant MH71241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature reference
- Abekawa T, Ito K, Koyama T. Role of the simultaneous enhancement of NMDA and dopamine D1 receptor-mediated neurotransmission in the effects of clozapine on phencyclidine-induced acute increases in glutamate levels in the rat medial prefrontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:177–193. doi: 10.1007/s00210-006-0115-9. [DOI] [PubMed] [Google Scholar]
- Abekawa T, Ito K, Koyama T. Different effects of a single and repeated administration of clozapine on phencyclidine-induced hyperlocomotion and glutamate releases in the rat medial prefrontal cortex at short- and long-term withdrawal from this antipsychotic. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:261–271. doi: 10.1007/s00210-007-0154-x. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargos-Bosch M, Lopez-Gil X, Artigas F, Adell A. Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int J Neuropsychopharmacol. 2006;9:565–573. doi: 10.1017/S1461145705005900. [DOI] [PubMed] [Google Scholar]
- Angrist B, Peselow E, Rubinstein M, Corwin J, Rotrosen J. Partial improvement in negative schizophrenic symptoms after amphetamine. Psychopharmacology (Berl) 1982;78:128–130. doi: 10.1007/BF00432248. [DOI] [PubMed] [Google Scholar]
- Antonelli T, Fuxe K, Tomasini MC, Mazzoni E, Agnati LF, Tanganelli S, Ferraro L. Neurotensin receptor mechanisms and its modulation of glutamate transmission in the brain: relevance for neurodegenerative diseases and their treatment. Prog Neurobiol. 2007;83:92–109. doi: 10.1016/j.pneurobio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Adrenergic targets for the treatment of cognitive deficits in schizophrenia. Psychopharmacology (Berl) 2004;174:25–31. doi: 10.1007/s00213-003-1724-3. [DOI] [PubMed] [Google Scholar]
- Asselin ML, Dubuc I, Coquerel A, Costentin J. Localization of neurotensin NTS2 receptors in rat brain, using. Neuroreport. 2001;12:1087–1091. doi: 10.1097/00001756-200104170-00044. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bertollo DN, Cowen MA, Levy AV. Hypometabolism in olfactory cortical projection areas of male patients with schizophrenia: an initial positron emission tomography study. Psychiatry Res. 1996;60:113–116. doi: 10.1016/0165-1781(96)02619-4. [DOI] [PubMed] [Google Scholar]
- Boules M, Cusack B, Zhao L, Fauq A, McCormick DJ, Richelson E. A novel neurotensin peptide analog given extracranially decreases food intake and weight in rodents. Brain Res. 2000;865:35–44. doi: 10.1016/s0006-8993(00)02187-9. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Bioactive analogs of neurotensin. Peptides. 2006;27:2523–2533. doi: 10.1016/j.peptides.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Boules M, Iversen I, Oliveros A, Shaw A, Williams K, Fredrickson P, Richelson E. The neurotensin agonist, NT69L, suppresses sucrose-reinforced operant behavior in the rat. Brain Res. 2007;1127:90–98. doi: 10.1016/j.brainres.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Boules M, McMahon B, Wang R, Warrington L, Stewart J, Yerbury S, Fauq A, McCormick D, Richelson E. Selective tolerance to the hypothermic and anticataleptic effects of a neurotensin analog that crosses the blood-brain barrier. Brain Res. 2003;987:39–48. doi: 10.1016/s0006-8993(03)03227-x. [DOI] [PubMed] [Google Scholar]
- Boules M, Warrington L, Fauq A, McCormick D, Richelson E. A novel neurotensin analog blocks cocaine- and D-amphetamine-induced hyperactivity. Eur J Pharmacol. 2001;426:73–76. doi: 10.1016/s0014-2999(01)01197-9. [DOI] [PubMed] [Google Scholar]
- Bowser MT, Kennedy RT. In vivo monitoring of amine neurotransmitters using microdialysis with on-line capillary electrophoresis. Electrophoresis. 2001;22:3668–3676. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Waltrip RW, 2nd, Listwak S, Holmes C, Goldstein DS. The effect of clozapine on plasma norepinephrine: relationship to clinical efficacy. Neuropsychopharmacology. 1994;10:1–7. doi: 10.1038/npp.1994.1. [DOI] [PubMed] [Google Scholar]
- Breslin NA, Suddath RL, Bissette G, Nemeroff CB, Lowrimore P, Weinberger DR. CSF concentrations of neurotensin in schizophrenia: an investigation of clinical and biochemical correlates. Schizophr Res. 1994;12:35–41. doi: 10.1016/0920-9964(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Cleghorn JM, Franco S, Szechtman B, Kaplan RD, Szechtman H, Brown GM, Nahmias C, Garnett ES. Toward a brain map of auditory hallucinations. Am J Psychiatry. 1992;149:1062–1069. doi: 10.1176/ajp.149.8.1062. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 2004;174:32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- Cusack B, Boules M, Tyler BM, Fauq A, McCormick DJ, Richelson E. Effects of a novel neurotensin peptide analog given extracranially on CNS behaviors mediated by apomorphine and haloperidol. Brain Res. 2000;856:48–54. doi: 10.1016/s0006-8993(99)02363-x. [DOI] [PubMed] [Google Scholar]
- Doherty JD, Simonovic M, So R, Meltzer HY. The effect of phencyclidine on dopamine synthesis and metabolic in rat striatum. Eur J Pharmacol. 1980;65:139–149. doi: 10.1016/0014-2999(80)90386-6. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. J Pharmacol Exp Ther. 1999;288:710–713. [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Fernandez M, Bebe BW, O’Connor WT, Fuxe K, Glennon JC, Tanganelli S, Antonelli T. Nigral neurotensin receptor regulation of nigral glutamate and nigroventral thalamic GABA transmission: a dual-probe microdialysis study in intact conscious rat brain. Neuroscience. 2001;102:113–120. doi: 10.1016/s0306-4522(00)00448-6. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. 2. New York: Raven Press; 1989. [Google Scholar]
- Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Garver DL, Bissette G, Yao JK, Nemeroff CB. Relation of CSF neurotensin concentrations to symptoms and drug response of psychotic patients. Am J Psychiatry. 1991;148:484–488. doi: 10.1176/ajp.148.4.484. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Bigelow LB, Weinberger DR, Daniel DG, Kleinman JE. Cognitive and behavioral effects of the coadministration of dextroamphetamine and haloperidol in schizophrenia. Am J Psychiatry. 1991;148:78–84. doi: 10.1176/ajp.148.1.78. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Herdon H, Schwarz A, Bertani S, Crestan V, Turrini G, Bifone A. Pharmacological stimulation of NMDA receptors via co-agonist site suppresses fMRI response to phencyclidine in the rat. Psychopharmacology (Berl) 2008;201:273–284. doi: 10.1007/s00213-008-1271-z. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of. 2003 doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- Heritch AJ. Evidence for reduced and dysregulated turnover of dopamine in schizophrenia. Schizophr Bull. 1990;16:605–615. doi: 10.1093/schbul/16.4.605. [DOI] [PubMed] [Google Scholar]
- Hertel P, Mathe JM, Nomikos GG, Iurlo M, Mathe AA, Svensson TH. Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat. Behav Brain Res. 1995;72:103–114. doi: 10.1016/0166-4328(96)00138-6. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Elsworth JD, Redmond DE, Jr, Roth RH. Phencyclidine increases forebrain monoamine metabolism in rats and monkeys: modulation by the isomers of HA966. J Neurosci. 1997;17:1769–1775. doi: 10.1523/JNEUROSCI.17-05-01769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. 1990;39:25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- Lada MW, Kennedy RT. Quantitative in vivo monitoring of primary amines in rat caudate nucleus using microdialysis coupled by a flow-gated interface to capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1996;68:2790–2797. doi: 10.1021/ac960178x. [DOI] [PubMed] [Google Scholar]
- Liang Y, Boules M, Shaw AM, Williams K, Fredrickson P, Richelson E. Effect of a novel neurotensin analog, NT69L, on nicotine-induced alterations in monoamine levels in rat brain. Brain Res. 2008;1231:6–15. doi: 10.1016/j.brainres.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Marwaha J. Candidate mechanisms underlying phencyclidine-induced psychosis: an electrophysiological behavioral, and biochemical study. Biol Psychiatry. 1982;17:155–198. [PubMed] [Google Scholar]
- McGuire PK, Shah GM, Murray RM. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-s. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The interaction of neurotensin with dopaminergic pathways in the central nervous system: basic neurobiology and implications for the pathogenesis and treatment of schizophrenia. Psychoneuroendocrinology. 1986;11:15–37. doi: 10.1016/0306-4530(86)90029-6. [DOI] [PubMed] [Google Scholar]
- Ohuoha DC, Hyde TM, Kleinman JE. The role of serotonin in schizophrenia: an overview of the nomenclature, distribution and alterations of serotonin receptors in the central nervous system. Psychopharmacology (Berl) 1993;112:S5–15. doi: 10.1007/BF02245003. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- Petrie KA, Schmidt D, Bubser M, Fadel J, Carraway RE, Deutch AY. Neurotensin activates GABAergic interneurons in the prefrontal cortex. J Neurosci. 2005;25:1629–1636. doi: 10.1523/JNEUROSCI.3579-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, Huang M, Li Z, Dai J, Meltzer HY. The neurotensin analog NT69L enhances medial prefrontal cortical dopamine and acetylcholine efflux: potentiation of risperidone-, but not haloperidol-, induced dopamine efflux. Brain Res. 2007;1184:354–364. doi: 10.1016/j.brainres.2007.09.092. [DOI] [PubMed] [Google Scholar]
- Richelson E, Boules M, Fredrickson P. Neurotensin agonists: possible drugs for treatment of psychostimulant abuse. Life Sci. 2003;73:679–690. doi: 10.1016/s0024-3205(03)00388-6. [DOI] [PubMed] [Google Scholar]
- Sanz B, Exposito I, Mora F. Effects of neurotensin on the release of glutamic acid in the prefrontal cortex and striatum of the rat. Neuroreport. 1993;4:1194–1196. [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Janicak PG, Bissette G, Nemeroff CB. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. Am J Psychiatry. 1997;154:1019–1021. doi: 10.1176/ajp.154.7.1019. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Richelson E, Feifel D. The effects of systemic NT69L, a neurotensin agonist, on baseline and drug-disrupted prepulse inhibition. Behav Brain Res. 2003;143:7–14. doi: 10.1016/s0166-4328(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Smith A, Watson CJ, Frantz KJ, Eppler B, Kennedy RT, Peris J. Differential increase in taurine levels by low-dose ethanol in the dorsal and ventral striatum revealed by microdialysis with on-line capillary electrophoresis. Alcohol Clin Exp Res. 2004;28:1028–1038. doi: 10.1097/01.alc.0000131979.78003.34. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Phencyclidine. Nature. 1980;285:355–356. doi: 10.1038/285355a0. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, LeWitt PA, Chase TN. Cholecystokinin and neurotensin gradients in human CSF. Arch Neurol. 1985;42:354–355. doi: 10.1001/archneur.1985.04060040064013. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Godbout R, Mantz J, Glowinski J. Influence of the ascending monoaminergic systems on the activity of the rat prefrontal cortex. Prog Brain Res. 1990;85:357–364. doi: 10.1016/s0079-6123(08)62690-4. discussion 364–355. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Distribution of neurotensin and its receptor in the central nervous system. Ann N Y Acad Sci. 1982;400:132–149. doi: 10.1111/j.1749-6632.1982.tb31565.x. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi M, Okuda M, Togashi S, Fuwano S, Miyashita O. Effects of phencyclidine administration on behavior and brain neurotensin-like immunoreactivity in rats. Brain Res. 1985;333:393–396. doi: 10.1016/0006-8993(85)91601-4. [DOI] [PubMed] [Google Scholar]
- Wang Rui MB, Tiner William, Richelson Elliott. Effects of repeated injections of the neurotensin analog NT69L on dopamine release and uptake in rat striatum in vitro. Brain Res. 2004;1025:21–28. doi: 10.1016/j.brainres.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Ward PB, Huang XF. Selective alterations in ionotropic glutamate receptors in the anterior cingulate cortex in schizophrenia. Neuropsychopharmacology. 2002;27:826–833. doi: 10.1016/S0893-133X(02)00347-0. [DOI] [PubMed] [Google Scholar]