Abstract
Serotonergic (5-HT) receptors are upregulated following spinal cord transection. Stimulation by administration of serotonergic receptor agonists has been successful in improving hindlimb function. We tested whether this strategy would be successful in incomplete injury models (moderate or severe thoracic contusion) where descending projections are partially spared which should produce less denervation-induced receptor upregulation. Adult rats received midthoracic moderate (MOD: 25 mm drop) or severe (SEV: 50 mm drop) contusion injuries. Distribution of 5-HT and its transporter and expression of 5-HT2C receptors were evaluated in lumbar spinal cord and motor response to 5-HT receptor activation was assessed using open field locomotion (BBB) score, percent weight supported treadmill stepping (%WS) and evaluation of hindlimb muscle activation (tremor and serotonin syndrome).
5-HT immunostaining 3 months post-contusion revealed few 5-HT fibers caudal to the severe contusion, and more spared caudal to the moderate contusion. The distribution of 5-HT transporter paralleled 5-HT staining, but was more greatly reduced. Thus serotonin reuptake may be less efficient in the injured spinal cord. Immunostaining for the 5-HT2C receptor in the dorsal and ventral horns at L5 showed significant upregulation in SEV, compared to sham or MOD rats.
Neither 5-HT2C nor 5-HT1A receptor agonists, alone or in combination, nor the serotonin transporter inhibitor d-fenfluramine modified BBB scores or %WS in either group. Despite the increased sensitivity of postsynaptic targets, agonist treatment did not improve function in SEV rats. We conclude that selective 5-HT2C or 5-HT1A receptor activation was not effective in improving hindlimb function after incomplete lesions. In contrast, the 5-HT precursor 5-hydroxytryptophan (L-5-HTP), which activates all classes of 5-HT receptors, increased both %WS and hindlimb activity in the MOD group. While no side effects were observed in normal or MOD rats, SEV rats displayed hindlimb tremors and 33% mortality, indicating hypersensitivity to the precursor.
Keywords: mCPP, fenfluramine, DPAT, L-5-HTP, 5-HT transporter, 5-HT2C receptor, spinal cord contusion
Introduction
Serotonin (5-HT) is a neuromodulator supplied by supraspinal neurons that activates spinal locomotor pathways, including neurons contributing to the central pattern generator (CPG) for locomotion. Serotonergic axons project to all regions of the spinal gray matter but are particularly densely distributed in the superficial dorsal horn, the commissural region and the ventral horn. Released 5-HT binds to 5-HT receptors, also located throughout the spinal gray matter. Seven families of 5-HT receptors have now been characterized and several studies of spinal cord injury have demonstrated enhanced motor performance through stimulation of the 5-HT2C, 5-HT1A and 5-HT7 subtypes (Antri et al., 2002, 2003, 2005; Dugan et al., 2009; Kao et al., 2006; Kim et al., 2001; Landry et al., 2004, 2006; Nothias et al., 2005; Zhou et al., 2001; Zimmer and Goshgarian, 2006; Ichiyama et al., 2008; Ung et al., 2008). 5-HT receptor subtypes have different regional distributions. 5-HT2C receptors are particularly dense in the ventral horn and 5-HT1A receptors are dense in the dorsal horn (reviewed in Hochman et al., 2003). Serotonin transporter (SERT), located on serotonergic axons, provides a mechanism for reuptake and inactivation of released 5-HT. The distribution of SERT parallels that of 5-HT immunoreactivity (Sur et al., 1996; Hains et al., 2002) and their loss and return following injury is correlated with behavioral recovery (Saruhashi et al., 2009).
Thoracic spinal cord injury reduces or eliminates descending projections in lumbar spinal cord and results in changes in receptor properties and expression caudal to the injury. 5-HT1A receptors are transiently upregulated (Giroux et al., 1999), Hoffman (H) reflex amplitude becomes elevated, correlated with upregulated 5-HT2 receptors (Lee et al., 2007), and behavioral effects of serotonergic compounds can be substantially altered (Shumsky et al., 2005; Dugan et al., 2009). 5-HT agonists improve hindlimb motor function (as assessed with the BBB score) in rats spinalized as neonates (Kim et al., 1999; Kao et al., 2006) or adults (Hayashi et al. 2003; Nothias et al., 2005; Dugan et al., 2009), whereas they have no effect in normal rats at similar doses and at higher doses reduce motor activity (Lucki et al., 1989). 5-HT2C receptors below the level of the transection are also upregulated in rats spinalized at neonates (Kao et al., 2006) or adults (Hayashi et al. 2003; Dugan et al., 2009). Other receptors are also affected. For example, alpha1 and alpha2 noradrenergic receptors are transiently upregulated (Giroux et al 1999) and alternative splicing of NR1 subunit mRNA is increased (Prybylowski et al., 2001), associated with changes in AMPA and NMDA receptors (Grossman et al., 1999, 2000). These results suggest several possible pharmacologic targets for treatment of severe spinal injuries.
Our working hypothesis was that adult rats with incomplete (contusion) injuries would, like spinal rats, exhibit upregulation of receptors below the injury and show functional hindlimb improvement after treatment with 5-HT agonists. Stimulation with either 5-HT precursor or 5-HT2 agonists has been shown to increase recovery of phrenic motoneuron activity in rats with cervical hemisections (Zhou and Goshgarian, 2000; Zhou et al., 2001), another incomplete injury model. We therefore predicted that rats with contusion injuries that were treated with 5-HT precursor would also demonstrate functional improvement, because the treatment would stimulate release of 5-HT by spared serotonergic axons. Thus, in adult rats with moderate (MOD) or severe (SEV) contusion injuries, we examined the effects on motor function elicited by administration of : 1) two direct agonists (mCPP and DPAT) which stimulate 5-HT2C (Conn and Sanders-Bush, 1987; Hamik and Peroutka, 1989; Callahan and Cunningham, 1994) and 5-HT1A (Middlemiss and Fozard, 1983; Smith and Peroutka, 1986) receptors, respectively; 2) an indirect agonist (D-FEN) which blocks 5-HT reuptake by serotonin transporters (Garattini et al., 1986; Vickers et al., 2001); and 3) a 5-HT precursor (L-5-HTP) which increases synthesis and release of 5-HT (Moir and Eccleston, 1968; Okada et al., 1972; Gartside et al., 1992).
Anatomical outcome measures included density and distribution of 5-HT, SERT, and 5-HT2C receptor immunoreactivity in lumber cord. Behavioral outcome measures included the open field locomotor (BBB) score and percent weight supported hindlimb stepping on a treadmill. Since administration of serotonergic agents can have harmful effects we also evaluated expression of the serotonin syndrome (Martin et al., 1996) and of hindlimb myoclonic tremors.
Materials and Methods
Subjects
Adult female Sprague-Dawley rats weighing 200–250 g (Taconic, Germantown, NY) received either a severe contusion injury (SEV, n=12), a moderate contusion injury (MOD, n=9) or a sham lesion (n=6) and were tested with all drug administrations. A second group of moderately contused rats was created in order to replicate the positive precursor effects (n=9) and control for order effects by administration of only the precursor drug. A third group of rats received a thoracic transection (n=3) and were compared to normal rats (n=3) for evaluation of 5-HT2C receptor immunohistochemistry at 15 weeks post injury.
Surgical Procedure
A) Thoracic Contusion
Rats were anesthetized with an intraperitoneal injection of acepromazine maleate (0.5 mg/kg, Fermenta Animal Health Co., Kansas City, MO), ketamine (63 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (6.3 mg/kg, Bayer Co., Shawnee Mission, KS), a laminectomy was performed to expose the spinal cord, and spinal cord injury was produced using a weight-drop device. A 10 g weight was dropped from the height of 25mm (MOD) or 50mm (SEV) onto the exposed dura of the spinal cord between the vertebrae at T9/T10 using the MASCIS impactor device (Constantini and Young, 1994). The muscle and skin were closed in layers and the animals recovered in their home cages. Animals received behavioral evaluation for the next 12 weeks.
B) Thoracic Transection (TX)
Rats were anesthetized with 5% isoflurane in 2L of oxygen and maintained at 2–3% isoflurane with 1L oxygen for the duration of the surgery. A laminectomy at the T8/T10 level exposed one spinal cord segment at T9. A #10 scalpel blade was used to open the dura and pia mater and #11 scalpel blade was used to transect the spinal cord. A fine-tipped microaspiration device was then used to remove 2 to 3 mm of spinal cord. A collagen matrix, Vitrogen, was injected into the site of the transection. Following the surgery, each animal was given an IM injection of the antibiotic Pen-G (0.5ml) and 5 ml of lactated Ringer’s solution subcutaneously. Animals recovered on heating pads, and when they regained sternal recumbency, were returned to their home cage. Bladders were expressed 3 times daily for 2 weeks or until bladder control was regained. Animals were housed with Alpha-Dri bedding (Shepherd Specialty Papers Inc. Kalamazoo, MI) and kept on warm water blankets throughout the experiment.
C) Sham lesions
Control animals received the same surgical procedures including laminectomy, but did not receive a spinal contusion, thus their spinal cords were normal. All procedures were carried out in accordance with a protocol approved by Drexel University College of Medicine Institutional Animal Care and Use Committee and followed the NIH guidelines for the care and use of laboratory animals.
Histological Analysis
Three animals from each group (sham, MOD, SEV) were sacrificed at 15 weeks post injury to allow 3 weeks of wash out from the last drug administration. They were perfused with 4% paraformaldehyde in 0.1M phosphate buffer (PB) pH 7.4 for histological analysis. Spinal cords were removed and washed with PB for 2 hr, then placed in PB containing 30% sucrose for 72 hr. Specimens were frozen in OCT compound (Tissue Tek, Sakura, Inc, Japan) and sectioned on a freezing microtome at 20 μm. The lesion segment was sectioned parasagittally and alternate sections were Nissl-myelin stained to confirm size of lesion or used for 5-HT or 5-HT transporter immunocytochemistry. Transverse sections rostral (C7) and caudal (L2 and L5) to the lesion were also stained for 5-HT and 5-HT transporter. Three additional animals from each group were decapitated without perfusion, their spinal cords removed, frozen and transversely sectioned for 5-HT2C receptor immunocytochemistry.
5-HT immunoreactivity
Sections through the lesion site and rostral (C7) and caudal (L2 and L5) to the injury were stained with a polyclonal antibody to 5-HT. Frozen sections mounted on slides were incubated at 4 °C with the primary antibody (diluted 1:40,000 Immunostar, Stillwater, MN) for 16 hr, with biotinylated goat anti-rabbit IgG for 2 hr, and with avidin-biotinylated horseradish peroxidase complex for 2 hr, as specified by the manufacturer (ABC Standard Kit; Vector Laboratories, Burlingame, CA). Peroxidase reactivity was visualized with 0.05% diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide in 0.05mM Tris buffer.
5-HT transporter immunoreactivity
The lesion site and sections rostral (C7) and caudal (L2 and L5) to the lesion site were stained with a polyclonal antibody to 5-HT transporter (SERT). Frozen sections mounted on slides were incubated at 4°C with the primary antibody (diluted 1:10,000, Immunostar, Stillwater, MN) for 16 hr, with biotinylated goat anti-rabbit IgG for 2 hr, and with avidin-biotinylated horseradish peroxidase complex for 2 hr, as specified by the manufacturer (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA). Peroxidase reactivity was visualized with 0.05% diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide in 0.05mM Tris buffer.
5-HT and 5-HT transporter double labeling
Some sections from the lesion site and segments from regions rostral (C7) and caudal (L2 and L5) to the lesion were stained with a polyclonal antibody to 5-HT and a monoclonal antibody to 5-HT transporter to evaluate colocalization. Frozen sections mounted on slides were incubated at 4 °C with both the primary 5-HT antibody (diluted 1:20,000 Immunostar, Stillwater, MN) and the primary 5-HT transporter antibody (diluted 1:1,000) for 16 hr and then with both FITC goat anti-rabbit IgG and rhodamine X goat anti-mouse IgG for 2 hr.
5-HT2C immunoreactivity
At 15 weeks post-injury, three animals from each group (sham, MOD, SEV, normal, TX) were decapitated without perfusion. Their spinal cords were removed and sections caudal to the lesion site (L5) were stained with an antibody to the 5-HT2C receptor. Consecutive 20 μm fresh frozen sections were mounted on slides and fixed with cold acetone for 10 minutes prior to being incubated at room temperature with the primary antibody (diluted 1:50 Santa Cruz Biotechnology Inc., Santa Cruz, CA) for 24 hr, and then with Rhodamine Red X-conjugated AffiniPure donkey anti-goat IgG secondary antibody for 2 hr. Mounting medium was applied (Vectashield Mounting Medium for Fluorescence; Vector Laboratories, Burlingame, CA). Slides were then stored in 4 °C after visualization under fluorescence microscope.
Quantification of immunocytochemical reactions
Stained sections were examined under a Leica DMRBE microscope (Wetzlar, Germany), and images were captured using a DC-330 color video camera (DAGE-MTI, Inc., Michigan City, IN). Images were then processed on a Power Mac G4 computer with an IP-Lab program (Scanalytics Inc., Fairfax,VA) to quantitate immunopositive staining.
5-HT and SERT immunoreactivity was quantified at both L2, the level containing elements of the CPG, and L5, the level containing motor neurons innervating the hindlimb, on 6 sections from each animal. Regions of interest in the dorsal horn, ventral horn, dorsalateral part of the lateral funiculus, and ventral funiculus were captured from both sides of the slide. Threshold values were chosen so that only immunopositive axons and cells were identified. Total labeled pixels were divided by the area of the region of interest to obtain mean density per unit area.
5-HT2C immunoreactivity was quantified at L5 on 6 sections from each animal. Images were captured within a fixed box size of 768 × 494 pixels at 200X. One region of interest in the dorsal horn (Laminae I and II) and two in the ventral horns (Lamina IX) were captured on each side (Figure 4C) on two sections each on four consecutive slides. The number of pixels occupied by immunolabeled structures within this box was measured. Thresholding values were chosen from sham lesioned animals and applied to all slides so that only immunopositive structures were measured. Total labeled pixels were divided by the sample box size (768 × 494 pixels) to obtain mean density per pixel.
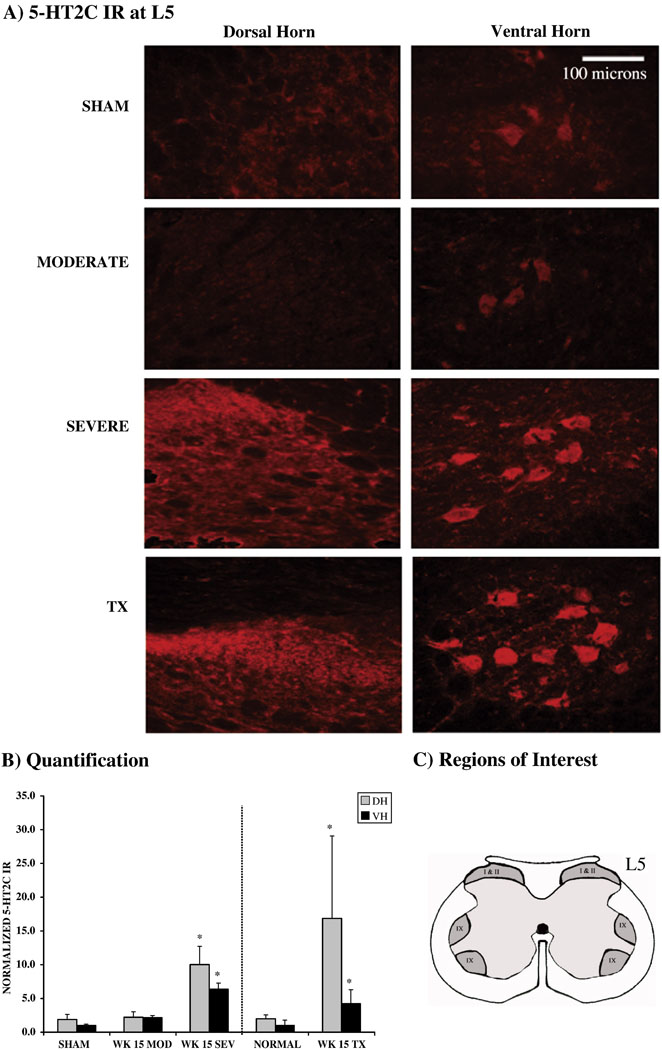
Figure 4.
A) 5-HT2C receptor immunoreactivity in dorsal and ventral horns at L5 from normal, MOD, SEV and TX rats (n=3 per group). In normal and moderately contused rats, cells in dorsal horn and ventral horn show similar levels of immunoreactivity. In SEV rats, dorsal horn and ventral horn cells show increased immunoreactivty. B) Quantification of 5-HT2C receptor immunocytochemistry in dorsal and ventral horns demonstrating significantly increased staining in both dorsal and ventral horn at 15 wks after SEV injury and after TX (* p < 0.05). No such upregulation was found after moderate contusion injury. Data were normalized to VH area fraction of sham injured animals for contusion analysis and to VH area fraction of normal animals for transection analysis. C) Diagram of rodent L5 spinal segment with shaded areas indicating regions of interest collected for analysis.
Drugs
All drugs were dissolved in sterile saline. Saline was also used as the vehicle injection for behavioral testing. 1-(m-chlorophenyl)-piperazine hydrochloride (mCPP, 0.15 mg/kg, IP) and 8-dihydroxy-2-di-n-propylaminotetralin (DPAT, 0.1 mg/kg, SC) were injected 5 min before testing. D-fenfluramine (D-FEN, 1.0 mg/kg, IP) was given 30 min before testing. Carbidopa (25 mg/kg, IP) was injected 30 min before L-5-hydroxtryptamine (L-5-HTP, 100 mg/kg, IP), which was injected 30 min before testing. All drugs were purchased from Sigma. These doses were based on those shown to enhance locomotor function in spinal rats for mCPP (Kim et al., 2001; Hayashi et al., 2002; Nothias et al., 2005) and to enhance motor activity in normal rats for DPAT (Evenden and Angeby-Möller, 1990) and L-5-HTP (Schlosberg and Harvey, 1979; Clarke et al., 1984; Simansky and Schechter, 1988). We did not attempt to identify a dose-response relationship because of the high mortality associated with some of these drugs in the SEV group (see below).
Behavioral tests
MOD (n=9) and SEV (n=12) rats were habituated to the behavioral testing preoperatively and evaluated after drug administration for up to 12 weeks postoperatively. Each week of drug testing began with behavioral testing following saline injection. A two day wash out period separated different drug administrations. The rats were tested with mCPP and D-FEN at 4 weeks post-injury, and with DPAT and mCPP alone and in combination (counterbalanced with saline injections for double injection controls) at 6 weeks post-injury. At 9 weeks post-injury, they were tested with L-5-HTP (plus carbidopa, given in order to facilitate central drug delivery (Schlosberg and Harvey, 1979; Clarke et al., 1984; Simansky and Schechter, 1988)). Due to unexpected mortality from the precursor drug, the SEV group lost 3 rats (their data were excluded from the study) leaving 9 for behavioral assessment at 12 weeks. Finally, the rats were retested with mCPP and D-FEN at 12 weeks post-injury. A second group of MOD rats (n=9) was tested at 9 weeks only with L-5-HTP (plus carbidopa) in order to replicate the positive results without potential influence from prior drug exposure. Performance was videotaped where appropriate and mirrors were used for observation of contralateral limbs. All tests were scored by trained observers who had interrater-reliability scores greater than 95%. All observers were unaware of the group identity of the animals.
Open Field Locomotor (BBB) Test
Hindlimb function was assessed in an open field (5 × 2 feet) using the BBB locomotor rating scale (Basso et al., 1995). Rats were observed for 2 min by two trained observers and scored from 0 (no observable movements) to 21 (normal locomotion).
Treadmill
Rats were trained for 3 min/day in the Eco 3/6 Treadmill apparatus (Columbus Instruments, Columbus, OH). The treadmill measures 33”L × 20”W × 20”H with a running surface that is 17”L × 2.375”W. In order to optimize data collection for these studies, the treadmill was set to a slow speed of 6.5 m/min. Locomotor function (% Weight Supported steps) was assessed by counting the number of weight supported steps taken on a treadmill in 3 min and expressed as a percentage of total steps. Non-weight supported steps were those in which neither knee nor hindquarters were elevated above the surface. Data are expressed as the difference in %WS between drug and saline administration.
Hindlimb Tremors
Myoclonus (tremor) in the hindlimbs was reliably elicited by treadmill stimulation. Following treadmill training, the presence of hindlimb tremors was determined by observation during the behavioral tests and hindlimb palpation (Nothias et al, 2005; Kao et al., 2006; Dugan et al.; 2009). Tremors were rated on a 4 point scale (0 = no tremors, 1 = mild (detectable on palpation), 2 = moderate (visually noticeable but does not interfere with function), 3 = intense (interferes with function).
Serotonin Syndrome
Depletion of serotonin followed by administration of serotonergic agents, especially 5-HT1A agonists, has been shown to elicit a group of stereotypies referred to as “the serotonin syndrome” (Martin, 1996). The serotonin syndrome consists of a constellation of full body motor responses, but since we were interested in reorganization after spinal injury, we focused on motor behavior expressed caudal to the injury, i.e., hindlimb activation, which consists of coordinated hindlimb sweeping with alternating rhythmic movements. Animals were observed in the home cage after drug administration and rated on a 4 point scale [0 = none, 1 = mild (1–2 bouts of short duration), 2 = moderate (1–2 bouts of long duration or 3–5 bouts of short duration), 3 = severe (3 or more bouts of long duration)] for each element of the syndrome (Shumsky et al., 2005). These data are reported as the difference between drug effect and baseline score.
Statistics
Data for BBB tests were analyzed by two-way ANOVA between group (sham, MOD, SEV) and drug (saline, mCPP and D-FEN) at both 4 and 12 weeks post-surgery. Data for BBB tests at week 6 were analyzed by two-way ANOVA between group and drug (saline+saline, DPAT+saline, saline+mCPP, DPAT+mCPP) with drug taken as a repeated measure. Post hoc analysis was performed, where appropriate, using Dunnett’s test. Analysis of tremor expression over time was performed using the Chi square test. Differences in serotonin syndrome intensities were compared using the paired sign test. All comparisons were considered to be significant at the 0.05 alpha level.
Immunocytochemical data from receptor studies were analyzed by ANOVA for the 3 groups of animals with each tissue section taken as an individual data point from 3–5 replicate slides per animal. Power analyses confirmed that a robust reliable difference in these outcome measures at the 0.05 alpha level could be determined from 5–6 sections on a minimum of 3 replicate slides each obtained from three animals per group.
Results
1. ANATOMICAL RESULTS
Anatomical studies were performed caudal to the lesion at both L2 and L5, where CPG neurons and hindlimb motoneurons are located, respectively. Because data were very similar at both levels (see Fig. 3), only L5 data is described below.
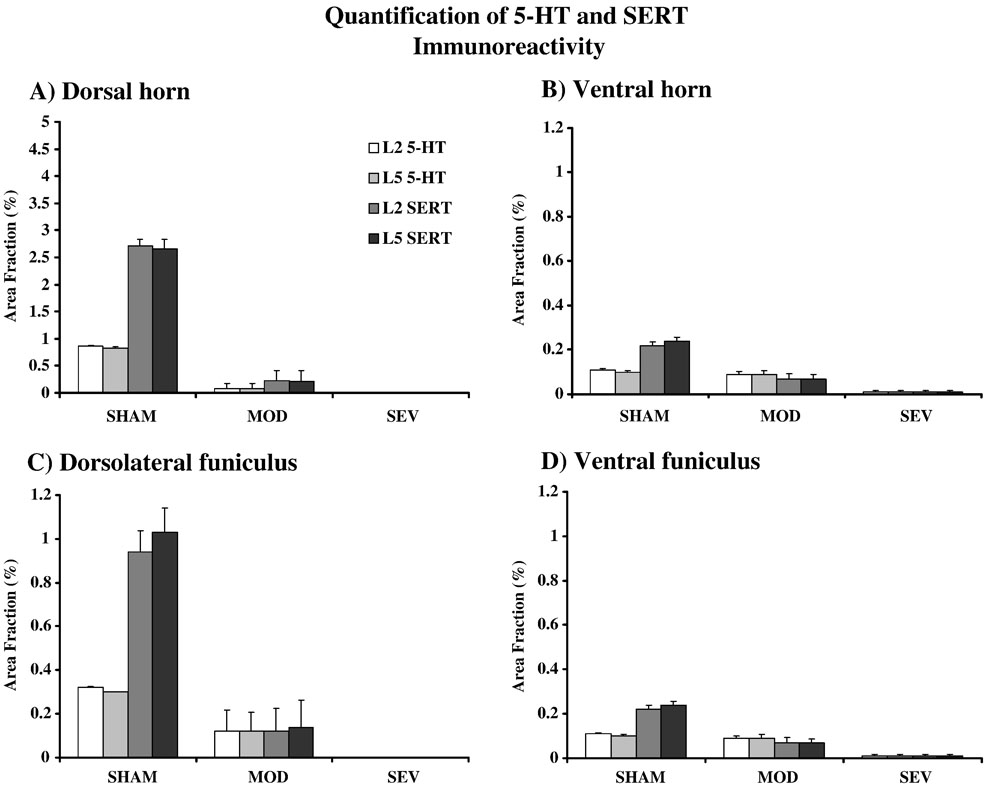
Figure 3.
Quantification of immunostaining for 5-HT and SERT in A) dorsal horn, B) ventral horn, C) dorsolateral funiculus, and D) ventral funiculus for normal MOD and SEV and both L2 and L5 (n = 3 per group).
A. Serotonergic axon density decreases in lumbar spinal cord in contused animals
As expected, longitudinal sections show marked depletion of serotonin caudal to the injury, with some increased 5-HT immunoreactivity just rostral to the lesion (Fig. 1). The depletion in 5-HT is greater in the dorsal horn and dorsal lateral funiculus than in the ventral horn and ventral funiculus (Fig. 2 and Fig. 3) because the contusion injury is inflicted on the dorsal surface of the spinal cord. Quantification of myelin stains has shown that about 3% of axons remain in caudal spinal cord following severe contusion and about 10% of axons survive following a moderate contusion (Young, 2002). 5-HT axons are among those partially spared by contusion injury, with more spared by a moderate than a severe lesion (Faden et al., 1988). We performed a similar quantification of area fraction that was restricted to the 5-HT immunoreactive axons in the lateral and ventral funiculi and the dorsal and ventral horns (Fig. 3). Our results show a reduction of 55% of 5-HT immunoreactivity in the ventral horn of MOD rats and a 97% reduction in serotonergic immunoreactivity in the ventral horn in the lumbar spinal cord of SEV rats.
Figure 1.
5-HT immunostaining rostral and caudal to the T9 lesion site in normal, moderately and severely contused spinal cords (n = 3 per group). Spinal cords were sectioned longitudinally. In uninjured spinal cord, the density of 5-HT in the gray and white matter was similar from rostral to caudal. In the moderately contused spinal cord, the density of 5-HT immunoreactivity was increased rostral to the lesion (* p < 0.05), very much decreased just caudal to the lesion, but further caudally, appears less depleted (* p < 0.05). In the severely contused spinal cord, the density of 5-HT is increased rostral to the lesion (* p < 0.05) and greatly decreased at all levels caudal to the lesion.
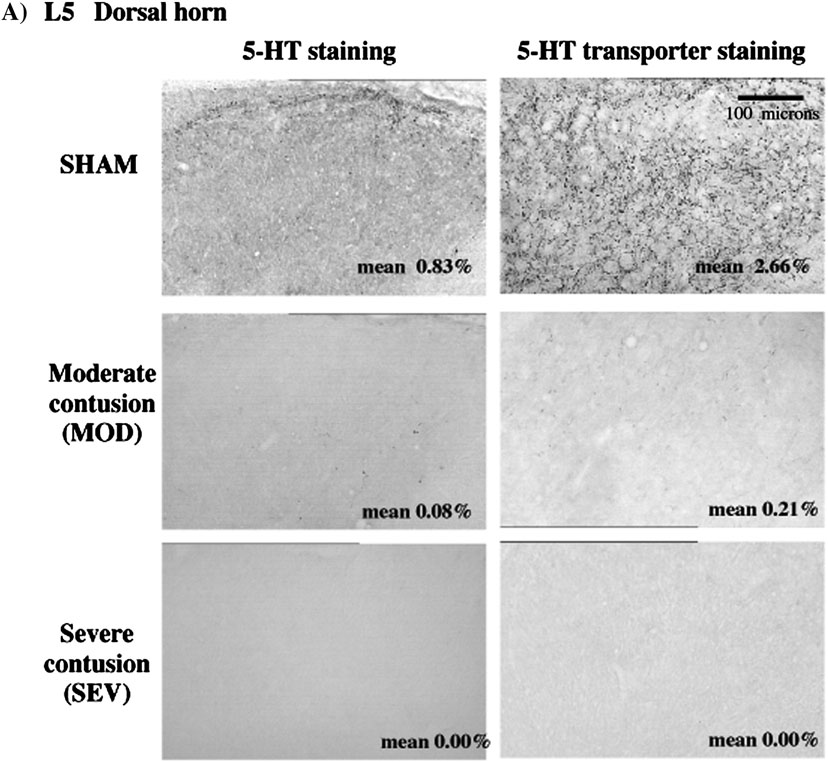
Figure 2.
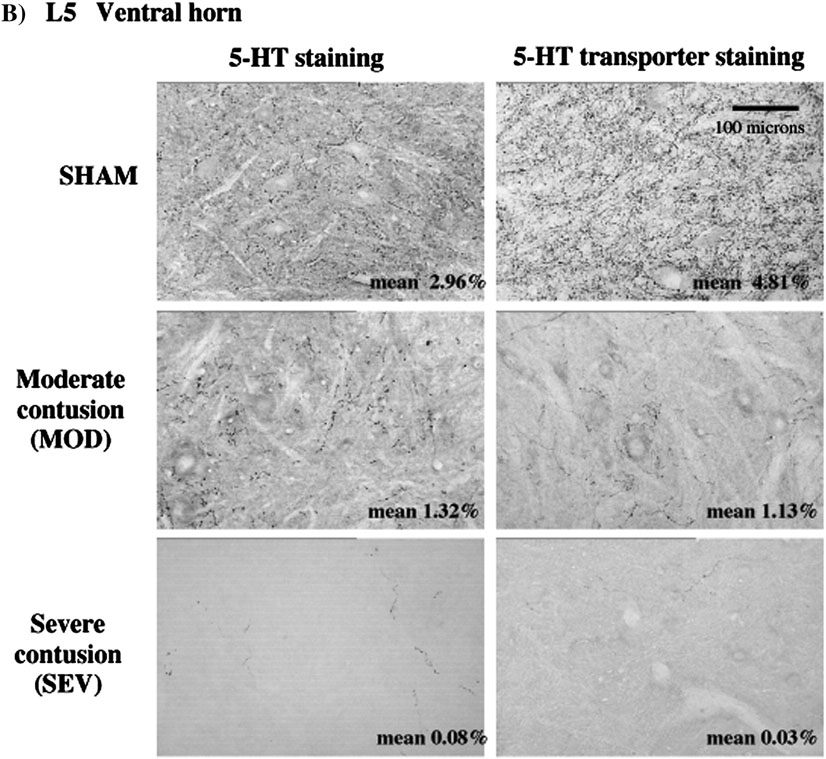
Serotonin and SERT immunoreactivity. A) Staining in dorsal horn in normal, MOD and SEV L5 spinal cord. Area fraction of immunoreactivity is indicated in bottom right corner of each micrograph. In normal rats, the area fraction of SERT staining is greater than that of 5-HT. Note the marked decrease in both 5-HT and SERT immunostaining in MOD and absence in SEV animals. B) Staining in ventral horn in normal, MOD and SEV L5 spinal cord. Area fraction of immunoreactivity is indicated in bottom right corner of each micrograph. In normal spinal cord, area fraction of SERT staining is greater than for 5-HT staining. Note decrease in immunoreactivitiy for both 5-HT and SERT in both contusion models.
B. Serotonin transporter is even more depleted
In normal animals, the distribution of the serotonin transporter, SERT, parallels that of 5-HT immunostaining but SERT is significantly denser than 5-HT immunoreactivity. Following contusion injury, the distribution of SERT staining still paralleled 5-HT staining in caudal spinal cord but was markedly less dense than 5-HT-immunoreactivity (Fig. 2 and Fig. 3). SERT immunoreactivity in the ventral horn was decreased by 77% in MOD and 99% eliminated in SEV rats. Double staining experiments showed some 5-HT axons in the injury groups with no apparent SERT immunoreactivity (data not shown). Thus there appears to be a relatively greater loss of transporter in spared and/or sprouting serotonergic axons that remain in caudal spinal cord. This should result in diminished reuptake and drugs such as D-FEN that are dependent on reuptake mechanisms should be less effective (see below).
C. 5-HT2C receptors are upregulated after severe, but not moderate, contusion injuries
The density of 5-HT2C receptor immunoreactivity was quantified in the caudal spinal cord at L5 in both the dorsal and ventral horns (Fig. 4). 5-HT2C receptor immunostaining was detectable at L5 in controls, localized mainly in lamina I/II of the dorsal horn and around motoneuron pools of the ventral horn in lamina IX. There was no difference in the receptor binding between control and MOD animals. Receptor upregulation was significant in the dorsal and ventral horns in the SEV group at 15 wks post injury. The upregulation in the dorsal horn was greater than that in the ventral horn in the SEV group. This is presumably because the contusion injury most severely damages dorsal spinal cord structures and therefore would cause greater denervation of the 5-HT targets in the dorsal horns. In order to further explore the effects of denervation, a group of rats that received a complete thoracic transection was also evaluated at 15 wks post injury compared to a normal control group that was processed together. Both data sets were normalized to the area fraction of the ventral horn in sham or normal controls (Fig 4B). This separate quantification of the area fraction revealed a significant increase in 5-HT2C receptor immunostaining that was equivalent in both dorsal (p < 0.05) and ventral (p < 0.05) horns at L5 following complete thoracic spinal transection.
2. MOTOR FUNCTION FOLLOWING CONTUSIONS
MOD rats plateaued by 4 weeks post contusion with average baseline BBB scores of 9.6 ± 0.4 and 22.2 ± 13.6% weight supported hind limb stepping on the treadmill. SEV plateaued at average BBB scores of 8.0 ± 0.1 with no weight supported stepping by 4 weeks post contusion. These results are consistent with previous reports (Young, 2002).
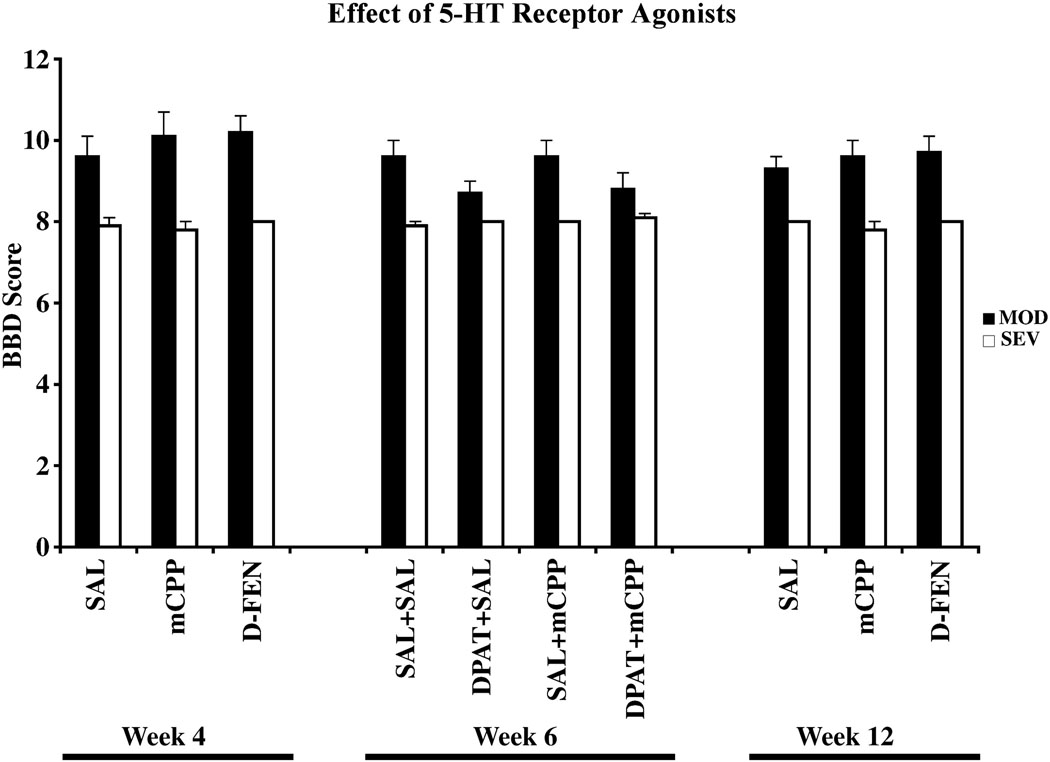
A. Neither direct nor indirect 5-HT agonists improve function after contusion lesions
mCPP administration at 4 weeks post injury did not modify the BBB score (Fig. 5) or weight supported stepping in either MOD (Difference %WS = +2.2 ± 2.5%) or SEV (Difference %WS = 0.0 ± 0.0%) groups. This result persisted, as mCPP administration at 12 weeks post injury also did not modify BBB score (Fig. 5) or weight supported stepping in either MOD (Difference %WS = +1.7 ± 1.8%) or SEV (Difference %WS = 0.0 ± 0.0%) groups. DPAT alone did not improve the BBB score (Fig. 5) or weight support in either MOD (Difference %WS = +0.2 ± 6.0% after DPAT, −4.9 ± 3.4% after mCPP) or SEV (Difference %WS = 0.0 ± 0.0% after DPAT).
Figure 5.
BBB Behavioral data for sham (n = 6), MOD (n = 9), and SEV (n = 9) rats. Following precursor administration at Week 9, 3 SEV rats died reducing the SEV group to n = 6 for subsequent mCPP and D-FEN testing at Week 12. Behavioral data from Wk 4 and Wk 6 for these rats were included in the analysis.
We then tested the combined effects of DPAT with mCPP at 6 weeks postcontusion to stimulate both 5-HT1A and 5-HT2C receptors. The combined agonist treatment also did not improve either BBB (Fig. 5) or weight support in either MOD (Difference %WS = −3.0 ± 3.3%) or SEV groups (Difference %WS = 0.0 ± 0.0%).
Finally, we tested the effects of the indirect 5-HT agonist D-FEN which blocks serotonin uptake (1.0 mg/kg, IP). D-FEN also did not change BBB scores in either MOD or SEV groups at 4 or 12 wks postoperatively (Fig. 5). We again found no change in percent weight supported steps on the treadmill (Difference %WS = −3.7 ± 1.9% after 4 wks, +1.2 ± 0.7% after 12 wks). Thus neither direct nor indirect agonists improved motor function following either MOD or SEV lesions and our working hypothesis that they would do so was therefore rejected.
B. 5-HT precursor increases motor function after contusion injury
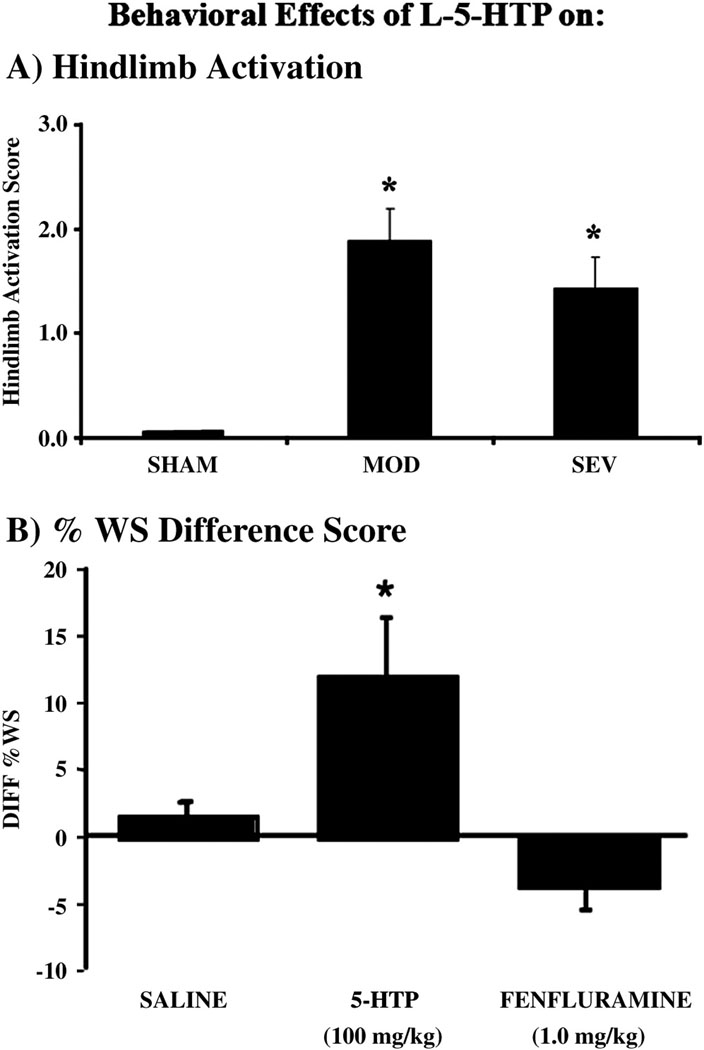
Because stimulation by agonists which target receptors on postsynaptic neurons or blockade of reuptake mechanisms to increase levels of 5-HT was ineffective, we next asked whether stimulating the spared serotonergic axons to synthesize and release more 5-HT would improve function. Administration of the 5-HT precursor L-5-HTP (100 mg/kg, IP), combined with carbidopa (25 mg/kg, IP) to block peripheral L-5-HTP degradation thereby facilitating precursor delivery centrally (Schlosberg and Harvey, 1979; Clarke et al., 1984; Simansky and Schechter, 1988), increased hindlimb motor function in both MOD and SEV groups. As hindlimb activation is expressed as sweeping and alternating rhythmic movements of the hindlimb, but not as myoclonus, it can potentially contribute to useful movements. Both MOD (n=18) and SEV (n=9) groups showed significant increases in the intensity of hindlimb activation (p < 0.05) in response to L-5-HTP (Fig. 6A). About 60% of the animals in both contusion groups expressed hindlimb activation, while only about 33% expressed stereotypies rostral to the injury. This same dose produced no hindlimb activation and only minimal expression of other stereotypies in sham lesioned control animals. For the MOD group, we also measured weight supported stepping in the 13 of 18 rats that had BBB scores of 9 or higher (indicating weight support in stance). The 5-HT precursor significantly increased weight supported stepping on the treadmill (p < 0.05) by a difference of 15% from the previous day’s saline baseline (Fig. 6B). This increase occurred in 11 of 13 animals, 3 of which had shown weight support in stance but no weight supported treadmill stepping without the drug. The average BBB score was not changed (BBB score = 10.0 ± 0.6) as a result of drug administration because in most rats the resultant average weight supported stepping (37%) did not translate from occasional (>50%) weight supported plantar steps (BBB score =10) to frequent (50–95%) weight supported plantar steps (BBB score = 11). Rats with SEV contusions showed no improvement in BBB score (BBB score = 8.0 ± 0.0) and did not achieve weight supported stepping on the treadmill following precursor administration.
Figure 6.
A). Administration of the 5-HT precursor (L-5-HTP) increases hindlimb activation in both moderate (n = 18) and severely (n =9) contused rats (* p < 0.05), but has no effect on sham rats (n=6). B). Administration L-5-HTP increases weight supported steps on a treadmill in moderately contused rats by 15% over vehicle administration (* p < 0.05). The indirect agonist D-FEN has no effect on weight supported stepping. Saline injection has no effect. Data are presented as difference scores between drug response and baseline behavior.
C. Deleterious effects of treatment with serotonergic agents
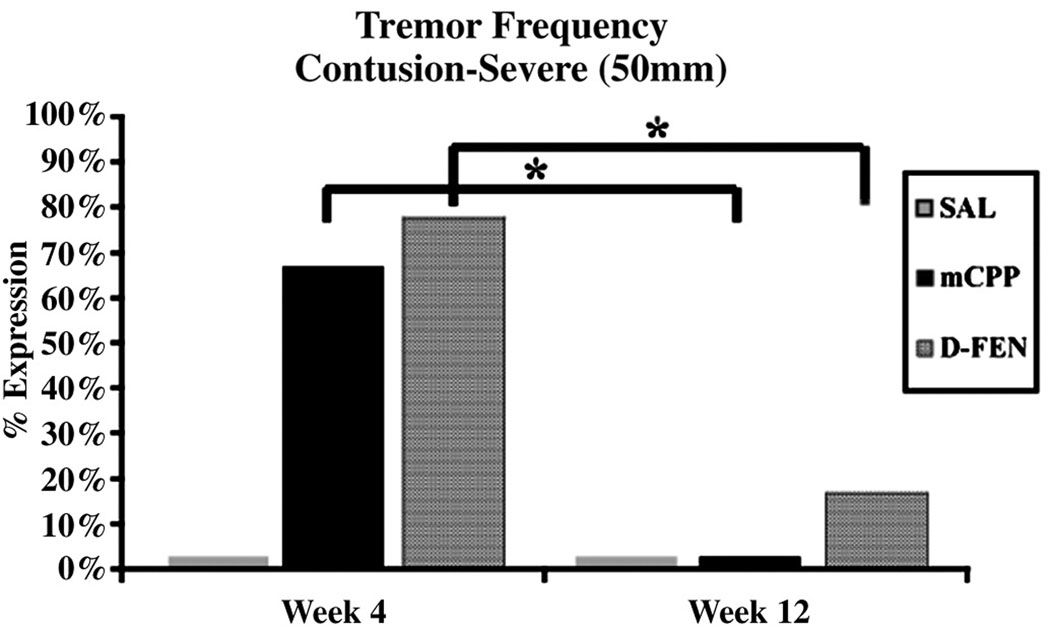
Providing serotonergic agonists in humans or animals with serotonin depletion can elicit potentially harmful side effects of tremors and the serotonin syndrome. Neither sham nor MOD rats developed hindlimb tremors in response to D-FEN or mCPP given alone. Animals in the SEV group, however, expressed hindlimb tremors in response to either mCPP (67%) or D-FEN (78%) at 4 weeks, although this response was reduced over time as fewer (p < 0.05) expressed hindlimb tremors in response to mCPP (0%) or D-FEN (17%) at 12 weeks (Fig. 7). In both MOD and SEV rats, DPAT elicited significant hindlimb activation (p < 0.05) resulting in mild serotonin syndrome intensities (Table 1). In MOD rats, the administration of mCPP did not modify the significant expression of hindlimb activation by DPAT. In SEV rats, however, the combination of DPAT and mCPP significantly reduced hindlimb activation (p < 0.05) compared with DPAT and SAL.
Figure 7.
Administration of both mCPP and D-FEN induces hindlimb tremors in severely contused rats (n=9) at 4 wks. Significantly fewer tremors are seen at Wk 12 (* p < 0.05).
TABLE 1. Hindlimb activation following drug administration.
Hindlimb activation scores following drug administration to MPD (n=9) and SEV (n=12) rats
| SAL+SAL | SAL+mCPP | DPAT+SAL | DPAT+mCPP | |
|---|---|---|---|---|
| MOD | 0.0±0.0 | 0.2±0.2 | 1.0±0.2* | 0.9±0.3 |
| SEV | 0.0±0.0 | 0.0±0.2 | 1.2±0.0* | 0.4±0.2§ |
mCPP (0.15 mg/kg, IP) did not elicit a significant effect. DPAT (0.1 mg/kg, SC) produced mild hindlimb activation (p < 0.05).
mCPP blocked this DPAT effect in the SEV rats (p < 0.05), but not the MOD rats.
Perhaps more importantly SEV rats appeared to be hypersensitive to the 5-HT precursor, because 4 out of 9 of these rats expressed hindlimb tremors, and several hours after injections 3 of those 4 died. These animals were excluded from the study.
Discussion
We investigated the effects of contusion injuries on the integrity of the serotonergic system in the spinal cord and the effect of serotonergic drugs on associated motor deficits. The type, severity, and duration of motor deficits were comparable to those previously reported (Young, 2002). Counter to our hypothesis, serotonergic agonists did not elicit substantial improvements in motor activity in an incomplete spinal injury.
Changes in density and distribution of serotonergic system
Thoracic contusions greatly reduced 5-HT immunoreactivity below the injury, and the degree of decrease was correlated with the severity of the contusion, as previously described (e.g., Basso et al., 1996; Faden et al., 1988; Young, 2002). Not surprisingly, the loss of 5-HT axons and terminals was correlated with the loss of serotonin transporter (SERT), but the contusions produced an even greater proportional loss of SERT than of the serotonergic axonal processes. This may reflect an energetic choice by the 5-HT neurons to produce fewer transporters in surviving axons. Following SEV, but not MOD contusions, 5-HT2C receptors caudal to the injury were upregulated. Interestingly, the upregulation seen after SEV included an increase in both intensity of immunofluorescence and spatial distribution of the receptors in the motor neuron pool, which was similar to what we observed following thoracic transection. In addition, we have previously observed a similar upregulation of 5-HT2C receptors in adult rats that had received a thoracic spinal transection as neonates (Kao et al., 2006) using these methods. Interestingly, autoradiographic methods, which typically label only receptors expressed at the cell surface, detected an upregulation of 5-HT2C receptors in only ventral horn of adult rats that had received thoracic spinal transection as neonates (Kim et al., 1999). Furthermore, an increase of 5-HT2C mRNA (as well as 5-HT2A mRNA) has been shown in ventral horn caudal to the injury after another incomplete lesion, cervical spinal hemisection (Basura et al., 2001). The upregulation of receptors after TX and SEV, but not MOD, suggests that a threshold denervation is required to increase compensatory postsynaptic 5-HT2C expression to a detectable level.
Behavioral results
In our previous studies using 5-HT agonists, we found that motor function improved after administration of 5-HT receptor agonists in animals that had received midthoracic transections as neonates (Kim et al.1999; Shumsky et al., 2005; Kao et al., 2006) or adults (Hayashi et al. 2002; Nothias et al., 2005; Dugan et al., 2009). In order to determine whether there was a selective mechanism associated with specific receptors or releasing agents, we tested the 5-HT2C receptor agonist mCPP and the 5-HT1A receptor agonist DPAT. The directly acting 5-HT2C agonist, mCPP, failed to improve motor function in contused rats. In previous studies, mCPP did restore weight supported stepping in adult rats that had received thoracic transections as neonates (Kim et al., 2001; Kao et al., 2006). We attributed that therapeutic action to the ability of mCPP to stimulate 5-HT2C receptors in the spinal motor circuitry. Therefore, the lack of effect of mCPP in adult rats that received contusion injury is surprising given that 5-HT2C receptor upregulation is seen after SEV. However, respiratory recovery following cervical spinal hemisection has been shown to depend more upon increases in 5-HT2A receptors than 5-HT2C (Basura et al., 2001; Zhou and Goshgarian, 2000; Zhou et al., 2001), which we did not test. Thus the manifestations of spinal injury on serotonergic function in adult rats must depend upon both the developmental stage at which damage occurs and the nature of the injury. The indirect 5-HT agonist, D-FEN, failed to improve motor function in contused rats. This result is consistent with the loss of 5-HT axons and apparently even greater loss of SERT on surviving axons that is required for this agent to effect 5-HT release. D-FEN requires adequate releasable stores of endogenous 5-HT to mediate its actions (Vickers et al., 2001), and our data clearly demonstrate that virtually none of the necessary serotonergic terminals—or their transporters--remain to support this drug effect.
5-HT precursor
The major positive finding in this study, which we replicated in a second group of MOD animals that did not receive any prior drug treatment, is that L-5-HTP increased hindlimb activity in both MOD and SEV rats, and increased weight supported stepping in MOD rats. This 5-HT precursor acts indirectly on motor function because it must be converted by decarboxylation to the primary amine. These results with the precursor might seem discrepant with those obtained with the indirectly acting (releasing) agent D-FEN. However, neurons and nonneuronal cells within the spinal cord express decarboxylase activity. Some MOD rats were capable of some weight supported stepping even in the absence of precursor administration. Thus, the motor excitatory response to L-5-HTP enabled the remaining neural drive to the caudal musculature with the resulting improvement in overall function. In contrast, SEV rats failed to display baseline weight support and therefore the same precursor treatment did not enable integrated motor responses of any type.
Although L-5-HTP activated hindlimb muscles, this agent did not improve BBB scores. At the time of testing (9 weeks postcontusion), the baseline BBB scores had plateaued. Clearly, the excitatory effect of the precursor on some aspects of motor output to the hindlimb was insufficient to reflect in the omnibus score of the BBB. Thus, in adults after contusion injuries, increasing 5-HT drive to the serotonergic receptors within the motor circuitry may be a necessary, but not a sufficient, component of therapeutic significance. Indeed, stimulation with precursor treatment in rats with severe contusions evoked excessive, even fatal, consequences. Rats spinalized at a high cervical level also exhibit altered phrenic motoneuron responsiveness along with life threatening side effects following systemic L-5-HTP administration (Mitchell et al., 1992). These observations certainly raise concerns about potential novel therapeutic strategies using L-5-HTP in humans.
The importance of understanding the mechanisms of action is revealed by examining the deleterious effects of these serotonergic agonists in this model. The direct agonist mCPP produced hindlimb tremors, but only mild hindlimb activation in rats with severe contusions. mCPP has high efficacy at 5-HT2C receptors in normal mammalian nervous tissue and high to moderate affinity (with varying efficacy) at several other serotonergic receptors (Hamik and Peroutka, 1989). The combination of mCPP with the 5-HT1A receptor agonist DPAT appears to have interacted to reduce hindlimb activation. The greatest hindlimb activity and the worst deleterious effects were elicited by L-5-HTP, which produces an agonist (5-HT itself) that activates a broader range of serotonergic receptors than mCPP. Further pharmacological analysis employing antagonists should establish whether differences in the serotonergic profiles of these drug treatments reveal the critical, multiple 5-HT receptors responsible for functional motor improvement. Notably, D-FEN produced a time course of tremors similar to mCPP in SEV. We believe this reflects the ability of the primary de-ethylated metabolite (D-norfenfluramine) to interact directly with 5-HT2C receptors in rats (Porter et al., 1999; Vickers et al., 2001). This tremorigenic response dissipated over the course of the 12 week study. The different outcomes at 4 and 12 weeks for these drugs may have revealed the effects of progressive muscular atrophy or receptor adaptations with time. Further studies are required to assess this hypothesis.
In conclusion, serotonergic agonists enhance motor function in the contused spinal cord, but with substantial deleterious effects. Based on our findings with complete injury models, we had expected that we would find greater enhancements in this incomplete injury model. Instead we found that the degree of 5-HT2C receptor changes was insufficient to support major changes in motor function in response to these serotonergic agents. Investigation of the reorganization of the 5-HT system at the receptor level and understanding its interplay with synaptic 5-HT levels is needed to develop further pharmacotherapeutic approaches to the treatment of incomplete spinal cord injury.
Abbreviations
- 5-HT
serotonin
- BBB
open field locomotor test (Basso, Beattie, Bresnahan Locomotor Score)
- CPG
central pattern generator
- D-FEN
fenfluramine, serotonin transporter inhibitor
- DPAT
8—hydroxy-2-(di-n-propylamino)tetraline, serotonin1A receptor agonist
- L-5-HTP
L-5-hydroxytryptophan, serotonin precursor
- mCPP
metachlorophenylpiperazine, serotonin2C receptor agonist
- MOD
moderate contusion (25 mm drop)
- SERT
serotonin transporter
- SEV
severe contusion (50 mm drop)
- TX
transection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antri M, Barthe JY, Moufle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following combined pharmacological stimulation of serotonergic receptors with 8-OH-DPAT and quipazine. Neurosci Lett. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in the spinal rat. Eur J Neurosci. 2003;18:1963–1972. doi: 10.1046/j.1460-9568.2003.02916.x. [DOI] [PubMed] [Google Scholar]
- Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: Effects of long-tern treatment with a 5-HT2 agonist. Eur J Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transaction. Exp. Neurology. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Zhou S-Y, Walker PD, Goshgarian HG. Disrtibution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169:255–263. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Bedard P, Barbeau H, Barbeau G, Filion M. Progressive increase of motor activity induced by 5-HTP in the rat below a complete section of the spinal cord. Brain Res. 1979;169:393–397. doi: 10.1016/0006-8993(79)91040-0. [DOI] [PubMed] [Google Scholar]
- Bregman BS. Development of serotonin immunoreactivity in the rat spinal cord and its plasticity after neonatal spinal cord lesions. Dev Brain Res. 1987;34:245–263. doi: 10.1016/0165-3806(87)90213-6. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Involvement of 5-HT2C receptors in mediating the discriminative stimulus properties of m-chlorophenylpiperazine (mCPP) European Journal of Pharmacology. 1994;257:27–38. doi: 10.1016/0014-2999(94)90690-4. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Parker AJ, Stirk GC. Locomotion in the rat after 5-hydroxy-l-tryptophan. Eur J Pharmacol. 1984;98:255–260. doi: 10.1016/0014-2999(84)90597-1. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. Relative efficacies of piperazines at the phosphoinositide hydrolysis-linked Serotonergic (5-HT2 and 5-HT1C) receptors. J Pharmacol Exp Ther. 1987;242:552. [PubMed] [Google Scholar]
- Constantini S, Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg. 1994;80:97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- Dugan EA, Jacob-Vadakot S, Hanford K, Amato N, Murray M, Shumsky JS. 5-HT2 receptors provide targets for pharmacotherapy after spinal cord injury. Neurorehab Neural Rep. 2009 (submitted) [Google Scholar]
- Evenden JL, Angeby-Möller K. Effects if 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on locomotor activity and rearing of mice and rats. Psychopharmacology. 1990;102:485–491. doi: 10.1007/BF02247129. [DOI] [PubMed] [Google Scholar]
- Faden AI, Gannon A, Basbaum AI. Use of serotonin immunocytochemistry as a marker of injury severity after experimental spinal trauma in rats. Brain Res. 1988;450:94–100. doi: 10.1016/0006-8993(88)91548-x. [DOI] [PubMed] [Google Scholar]
- Garattini S, Mennini T, Bendotti C, Invernizzi R, Samanin R. Neurochemical mechanism of action of drugs which modify feeding via the Serotonergic system. Appetite. 1986;7 (suppl.):15–38. doi: 10.1016/s0195-6663(86)80050-2. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Cowen PJ, Sharp T. Effect of 5-hydroxy-l-tryptophan on the release of 5-HT in rat hypothalamus in vivo as measured by microdialysis. Neuropharmacology. 1992;31:9–14. doi: 10.1016/0028-3908(92)90154-h. [DOI] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA. Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol. 1999;406:402–414. [PubMed] [Google Scholar]
- Grossman SD, Wolff BB, Yasuda RP, Wrathall JR. Alterations in AMPA receptor subunit expression after experimental spinal cord contusion injury. J Neurosci. 1999;19:5711–5720. doi: 10.1523/JNEUROSCI.19-14-05711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Wolff BB, Yasuda RP, Wrathall JR. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem. 2000;75:174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- Guertin PA. Recovery of locomotor function with combinatory drug treatments designed to synergistically activate specific neural networks. Curr Med Chem. 2009;16:1366–1371. doi: 10.2174/092986709787846541. [DOI] [PubMed] [Google Scholar]
- Hains BC, Everhart AW, Fullwood SD, Hulsebosch CE. Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Exp. Neuro. 2002;175:347–362. doi: 10.1006/exnr.2002.7892. [DOI] [PubMed] [Google Scholar]
- Hamik A, Peroutka SJ. 1-(m-chlorophenyl)piperazine (mCPP) interactions with neurotransmitter receptors in the human brain. Biol. Psychiatry. 1989;25:569–575. doi: 10.1016/0006-3223(89)90217-5. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nothias J-M, Antonacci D, Ely J, McBride S, Michael M, Olexa R, Tumolo M, Murray M, Simansky K, Shumsky JS. Direct and indirect 5-HT agonists enhance motor function in models of adult spinal cord injury. Society for Neuroscience. 2002 Abstracts 28:Program No. 851. [Google Scholar]
- Hayashi Y, Jacob S, Nothias J, Murray M, Simansky K, Shumsky JS. Distribution of serotonergic fibers, 5-HT transporter, and 5-HT2C receptors in lumbar cord correlates with degree of motor function following various spinal cord injuries. Soc Neurosci. 2003 Abstr 29:Program No. 498.8. [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. 5-HT receptors and the neuromodulatory control of spinal cord function. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. Washington, DC: CRC Press; 2003. pp. 47–87. [Google Scholar]
- Ichiyama RM, Gerasimenko Y, Jindrich DL, ZHong H, Roy RR, Edgerton VR. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neurosci Lett. 2008;438:281–285. doi: 10.1016/j.neulet.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Jacob-Vadakot S, Himes BT, Murray M, Moxon KA. Role of the 5-HT2C Receptor in Improving Weight Supported Stepping in Adult Rats Spinalized as Neonates. Brain Res. 2006 doi: 10.1016/j.brainres.2006.07.020. (in press) [DOI] [PubMed] [Google Scholar]
- Kim D, Murray M, Simansky KJ. The serotonergic 5-HT(2C) agonist mchlorophenylpiperazine increases weight-supported locomotion without development of tolerance in rats with spinal transections. Exp Neurol. 2001;169:496–500. doi: 10.1006/exnr.2001.7660. [DOI] [PubMed] [Google Scholar]
- Kim D, Adipudi V, Shibayama M, Giszter S, Tessler A, Murray M, Simansky KJ. Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection. J Neurosci. 1999;19:6213–6224. doi: 10.1523/JNEUROSCI.19-14-06213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry ES, Geurtin PA. Differential effects of 5-HT1 and 5-HT2 receptor agonists on hindlimb movements in paraplegic mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1053–1060. doi: 10.1016/j.pnpbp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur J Neurosci. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- Lee JK, Johnson CS, Wrathall JR. Upregulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp Neurol. 2007;203:502–511. doi: 10.1016/j.expneurol.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Ward HR, Frazer A. Effect of 1-(m-chlorophenyl)piperazine and 1-(m-trifluoro-methylphenyl) piperazine on locomotor activity. J Pharm Exp Ther. 1989;249:155–164. [PubMed] [Google Scholar]
- Martin TG. Serotonin Syndrome. Ann Emerg Med. 1996;28:520–526. doi: 10.1016/s0196-0644(96)70116-6. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. 8-Hydroxy-2-(di-n-propylamino)tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Giradrdon S, Bervoets K. 8-OH-DPAT-induced spontaneous tail-flicks in the rat are facilitated by the selective serotonin (5-HT)2C agonist, RO 60-0175: Blockade of its actions by the novel 5-HT2C receptor antagonist SB 206,553. Neuropharmacol. 1997;36:743–745. doi: 10.1016/s0028-3908(97)00071-3. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Sloan HE, Jiang C, Miletic V, Hayashi F, Lipski J. 5-Hydroxytryptophan (5-HTP) augments spontaneous and evoked phrenic motoneuron discharge in spinalized rats. Neurosci Lett. 1992;141:75–78. doi: 10.1016/0304-3940(92)90338-8. [DOI] [PubMed] [Google Scholar]
- Moir ATB, Eccleston D. The effects of precursor loading in the cerebral metabolism of 5-hydroxy-yindoles. J Neurochem. 1968;15:48–54. doi: 10.1111/j.1471-4159.1968.tb06827.x. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Sullivan SA, Winterson BJ. Behaviors induced by 5-hydroxytryptophan in neonatal, preweaning, postweaning, and adult Sprague-Dawley rats. Pharmacol Biochem Beh. 1992;42:413–419. doi: 10.1016/0091-3057(92)90134-2. [DOI] [PubMed] [Google Scholar]
- Nothias J-M, Mitsui T, Shumsky JS, Antonacci MD, Murray M. Combined effects of neurotrophin secreting transplants, exercise and serotonin drug challenge improve function in spinal rats. Neurorehab. Neural Repair. 2005;19:296–312. doi: 10.1177/1545968305281209. [DOI] [PubMed] [Google Scholar]
- Okada F, Saito Y, Fujieda T, Yamashita I. Monoamine changes in the brain of rats injected with L-5-hydroxytryptophan. Nature. 1972;238:1093–1108. doi: 10.1038/238355a0. [DOI] [PubMed] [Google Scholar]
- Pappert EJ, Goetz CG, Stebbins GT, Belden M, Carvey PM. 5-hydroxytryptophan-induced myoclonus in guidea pigs: mediation through 5-HT1/2 receptor subtypes. Eur. J.Pharm. 1998;347:51–56. doi: 10.1016/s0014-2999(98)00086-7. [DOI] [PubMed] [Google Scholar]
- Porter RHP, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT 2A, 5-HT 2B and 5-HT 2C receptors in CHO-K1 cells. British Journal of Pharmacology. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski KL, Grossman SD, Wrathall JR, Wolff BB. Expression of splice variants of the NR1 subunit of the N-methyl-D-aspartate receptor in the normal and injured rat spinal cord. J Neurochem. 2001;76:797–805. doi: 10.1046/j.1471-4159.2001.00069.x. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Giroux N, Chau C, Marcoux J, Brustein E, Reader TA. Pharacological aids to locomotor training after spinal injury in the cat. J Physiol. 2001;533.1:65–74. doi: 10.1111/j.1469-7793.2001.0065b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhashi Y, Matsusue Y, Fujimiya M. The recovery of 5-HT transporter and 5-HT immunoreactivity in injured rat spinal cord. Arch Orthop Trauma Surg. 2009;129:1279–1285. doi: 10.1007/s00402-008-0754-z. [DOI] [PubMed] [Google Scholar]
- Schlosberg AJ, Harvey JA. Effects of L-DOPA and L-5HTP on locomotor activity in the rat after selective or combined destruction of central catecholamine and serotonin receptors.J. Pharmacol. Exp. Ther. 1979;211:296–304. [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Shumsky JS, Kao T, Amato N, Simansky K, Murray M. Partial 5-HT1A receptor agonist activity by the 5-HT2C receptor antagonist SB 206,553 is revealed in rats spinalized as neonates. Exp. Neurol. 2005;191:361–365. doi: 10.1016/j.expneurol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Simansky KJ, Schechter LE. Properties of some 1-arylpiperazines as antagonists of stereotyped behaviors mediated by central serotonergic receptors in rodents. J. Pharmacol.Exp. Ther. 1988;247:1073–1081. [PubMed] [Google Scholar]
- Smith LM, Peroutka SJ. Differential effects of 5-hydroxytryptamine 1A selective drugs on the 5-HT behavioral syndrome. Pharmacology Biochemistry & Behavior. 1986;24:1513–1519. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- Sur C, Betz H, Schloss P. Localization of the serotonin transporter in rat spinal cord. Eur. J. Neurosci. 1996;8:2753–2757. doi: 10.1111/j.1460-9568.1996.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Ung R-V, Landry ES, Rouleau P, Lapointe NP, Rouillard C, Guertin PA. Role of spinal 5-HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur J Neurosci. 2008;28:2231–2242. doi: 10.1111/j.1460-9568.2008.06508.x. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Dourish CT, Kennett GA. Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT 2C receptors. Neuropharmacology. 2001;41:200–209. doi: 10.1016/s0028-3908(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord contusion models. Prog. Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- Zhou S-Y, Basura GJ, Goshgarian HG. Serotonin2 receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]
- Zhou S-Y, Goshgarian HG. 5-Hydroxytryptophan induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]