Abstract
DNA damage checkpoints are essential for maintenance of genome integrity. We report here that inducible overexpression of the transcription factor Sharp-1 results in an S and G2/M cell cycle arrest, concomitant with the upregulation of Brca1 and GADD45α expression. In addition, we show that endogenous Sharp-1 mRNA is increased by DNA-damaging agents. Consistently, Sharp-1 overexpressing cells exhibit reduced apoptosis in response to chemotherapeutic drugs along with lower p53 expression and activity. Our studies identify a novel function for Sharp-1 in cell cycle arrest and DNA damage-induced apoptosis. Inappropriate Sharp-1 expression may therefore be associated with tumorigenesis.
Keywords: apoptosis, DNA damage, growth arrest, Dec2
Introduction
Genome integrity is dependent on the activation of checkpoints that block cell cycle progression (1–5). In response to cellular stress such as DNA-damage, mammalian cells arrest in the G1, S, or G2/M phase that allows them to repair DNA. The G2 checkpoint plays a pivotal role in this process by inhibiting the entry of cells harboring DNA-damage into mitosis. If the damage cannot be repaired, programmed cell death (apoptosis) is initiated to eliminate genetically altered cells from the proliferative pool. The tumor suppressor p53 plays a key role in the DNA-damage response. In addition to its established role in G1 arrest through regulation of p21cip1/waf1 expression, p53 plays a role in G2/M arrest through regulation of GADD45α, 14-3-3, and cyclin B1 (5). p53 also regulates the expression of genes required for apoptosis which include Puma, Noxa and Bax (6,7). A failure of this surveillance response results in genetic alterations that lead to genome instability and development of cancers, immunodeficiency, neurodegenerative diseases, and premature ageing (1–5).
We have previously demonstrated that the transcription factor Sharp-1/Dec2 is widely expressed, and plays key roles in cellular differentiation (8–10). The hitherto unstudied role of Sharp-1 in regulation of cellular growth arrest and apoptosis are addressed in this present report. We demonstrate that endogenous Sharp-1 mRNA is up-regulated by genotoxic agents, and its overexpression results in S and G2/M cell cycle arrest. Sharp-1 upregulates the expression of Brca1 and GADD45α, that control cell cycle arrest and DNA repair (11,12). Consistent with its ability to block cell cycle progression, Sharp-1 overexpressing cells exhibit an abrogated apoptotic response in presence of DNA damaging triggers and express reduced levels of p53 and its transcriptional targets. Together, our studies demonstrate that Sharp-1 plays a key role in protection of cells from anti-cancer drugs, and suggest that its altered function or expression may be associated with tumorigenesis and or tumor progression.
Materials and Methods
Cell culture
Doxycycline inducible Sharp-1 expressing cell lines were generated using the rtTA inducible system. NIH3T3 fibroblast cells (ATCC) were transfected with EF1prtTA (13) and selected with G418. After selection, cells were co-transfected with myc-tagged Sharp-1 in pUHD10-3 and a puromycin resistance vector (14). Individual colonies were analyzed for Sharp-1 expression in response to doxycycline (2 µg/ml). Cells containing both vectors but not induced with doxycycline were used as controls. Cells were cultured in DMEM supplemented with 10% bovine serum.
Colony formation assay
Colony formation assays were done as described (14). Colonies were stained with crystal violet. The dye was extracted and the absorbance read at 570 nm.
Cell cycle analysis
Cell cycle analysis was done as described (14). Flow cytometry was performed in a BD Coulter flow cytometer and analyzed using WINMDI software.
Caspase-3 activity
Caspase-3 activity assays were based on cleavage of the substrate Ac-DEVD-pNA [Biomol International]. Cleavage of the substrate was measured spectrophotometrically at 405 nm and the activity calculated.
Reverse transcriptase PCR (RT-PCR)
Semi-quantitative, and quantitative RT-PCR was done as described (10, 15). Primers sequences are listed in Supplementary Table I.
Statistical analysis
Error bars indicate mean ± standard error (SE). Statistical analysis was performed by student’s t test and p values <0.05 were considered statistically significant.
Results and Discussion
Sharp-1 causes growth arrest
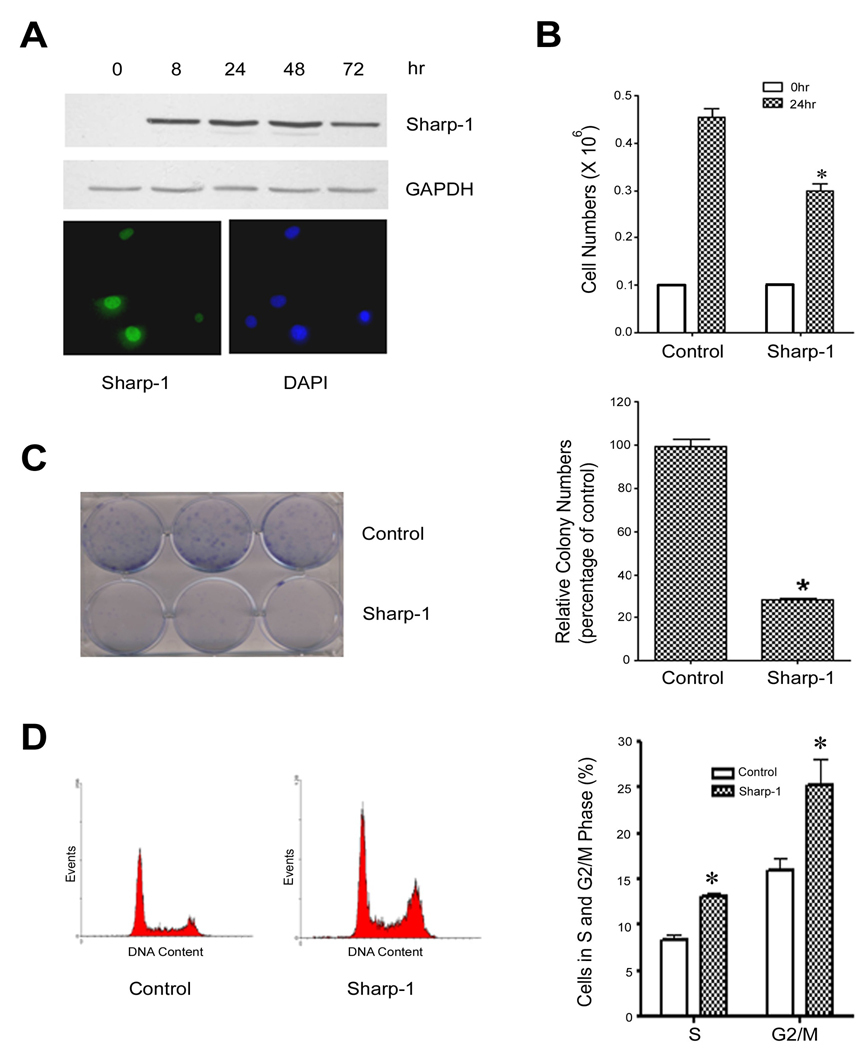
To investigate the role of Sharp-1 in growth arrest and apoptosis, we established stable cell lines in NIH3T3 cells that express myc-tagged Sharp-1 in a doxycycline inducible manner. Cells containing the Sharp-1 construct, but not induced with doxycycline, were used as controls. To examine the kinetics of Sharp-1 induction, cells were left untreated (time 0) or treated with doxycycline for various time points and analyzed by western blot analysis. In the absence of doxycycline, Sharp-1 expression was not detectable using anti-myc antibody (Fig. 1A, time 0 hr). Treatment of cells with doxycycline resulted in the induction of Sharp-1 within 8 hr, which was apparent until 72 hr (Fig. 1A). Immunofluorescence analysis indicated that Sharp-1 was localized in the nucleus (Fig 1A, lower panel). Compared to control cells, the induction of Sharp-1 expression resulted in a significant reduction of cell numbers (Fig. 1B). To confirm the growth inhibitory effect of Sharp-1, colony formation assays were performed. NIH3T3 cells were cultured in the absence or presence of doxycycline and colony formation was visualized by crystal violet staining [Fig. 1C (left panel)]. Sharp-1 expression resulted significant decrease in colony numbers compared to control cells (Fig. 1C, right panel). Together, these results demonstrate that Sharp-1 is a potent growth suppressor. To investigate whether Sharp-1 mediated growth suppression is due to an effect on the cell cycle, or on apoptosis, we first performed cell cycle analysis (Fig. 1D). Interestingly, 24 hr after induction of Sharp-1, a significant increase in the percentage of cells in the S and G2/M phases was evident, with no obvious increase in the sub-G1 population (Fig. 1D; left panel).
Figure 1. Sharp-1 causes cell cycle arrest.
(A) Sharp-1 expression was induced with doxycycline for 0–72hr and analyzed by western blot using anti-myc antibody. GAPDH was used as a loading control. Cells were immunostained 24 hr after doxycline treatment and analyzed by fluorescence microscopy. Nuclei were stained with DAPI (lower panel). (B) Cells were plated in triplicates at a density of 0.1×106 and left untreated (control) or treated with doxycycline (Sharp-1). 24 hr later, cell numbers were counted by trypan blue assay. Error bars indicate mean ± standard error (SE). (C) NIH3T3 cells without or with doxycycline treatment were cultured at a low density for 10 days, and colonies were stained with crystal violet. The panel on the left shows representative plates from two independent experiments. For quantification, the dye was extracted and the absorbance read at 570nm (right). Sharp-1 expressing cells showed a significant reduction in colony formation (*p<0.05). (D) Control and cells treated with doxycycline (24 hr) were analyzed by FACS. The left panel show representative histograms of cell cycle profiles in cells without (control) and with Sharp-1 overexpression. Quantification (right panel) of the percentage cells undergoing S and G2 arrest indicated a significant increase in Sharp-1 over-expressing cells compared to controls (*p<0.05). Results are mean ± SE from 4 independent experiments.
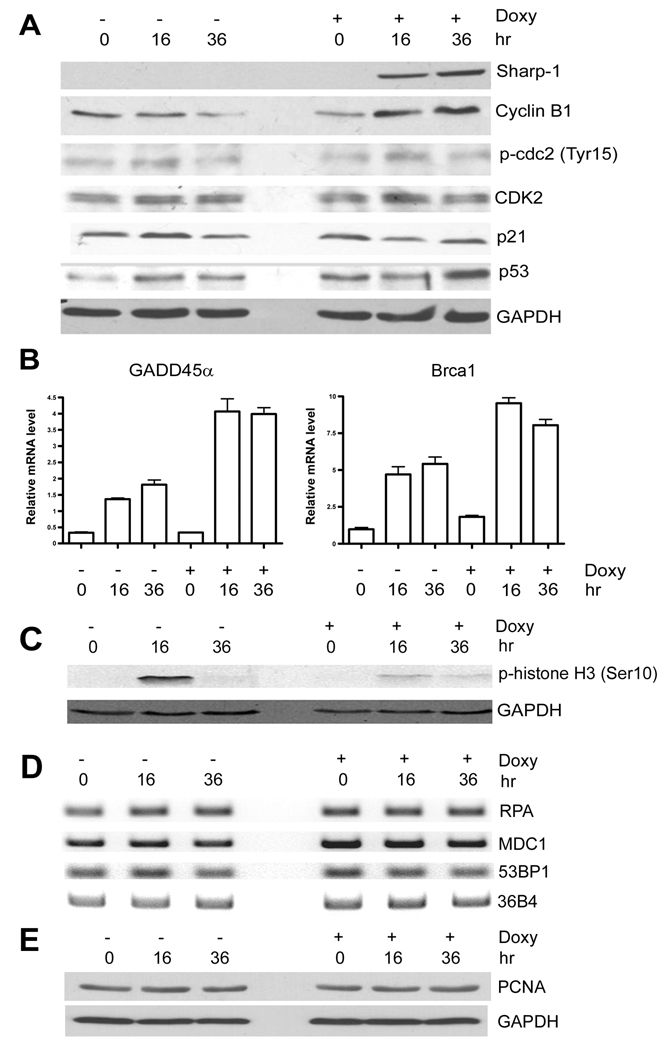
We then analyzed the mechanisms underlying growth arrest induced by Sharp-1. The G2/M checkpoint is controlled by the cyclinB/Cdc2 complex. Compared to control cells, cyclin B1 expression was somewhat increased after induction of Sharp-1, whereas the levels of p-cdc2-Tyr15, which inhibits CyclinB/Cdc2 activity (16) remained unchanged (Fig. 2A). Moreover, no changes in the levels of p21 and Cdk2 were apparent. However, p53 levels were increased 36 hr after Sharp-1 expression compared to control cells. One of the key p53 target genes involved in G2/M arrest is GADD45α (11). In control cells, GADD45α was slightly increased over time. Interestingly, GADD45α mRNA was up-regulated by Sharp-1 even prior to the induction of p53, indicating that GADD45α may be directly regulated by Sharp-1 (Fig. 2B). Similarly, Brca1, which is required for S-phase arrest and DNA-repair was induced by Sharp-1 (Fig. 2B). A hallmark of entry into mitosis is the phosphorylation of histone H3 (17). In control cells, histone H3 phosphorylation (ser 10) was increased at 16 hr (Fig. 2C). In contrast, Sharp-1 overexpressing cells showed a marked reduction in phosphor H3 levels confirming that Sharp-1 disrupts G2 progression. Given the S-phase arrest induced by Sharp-1, we further examined genes involved in DNA-replication or repair (1–5, 12). However, the expression of RPA, 53BP1, MDC1 and PCNA was not altered by Sharp-1 overexpression (Fig. 2D, E).
Figure 2. Sharp-1 induces GADD45α and Brca1 expression.
(A) Cells were left untreated (−) or treated with doxycycline (+ Doxy) for 16 and 36 hr. Sharp-1, cyclin B1, phosphorylated cdc2 (Tyr 15), CDK2, p21waf/cip1 and p53 were analyzed by western blot. (B) GADD45α and Brca1 mRNA was analyzed by quantitative RT-PCR. GAPDH was used as an internal control. (C). The level of the mitotic marker H3 (ser 10) was determined before after doxycycline treatment by western blot. (D) Expression of RPA, 53BP1, and MDC1 was analyzed by RT-PCR. The results are representative of two independent experiments. (E) PCNA and GAPDH levels were analyzed by western blot.
Sharp-1 is induced by genotoxic agents and protects cells from apoptosis
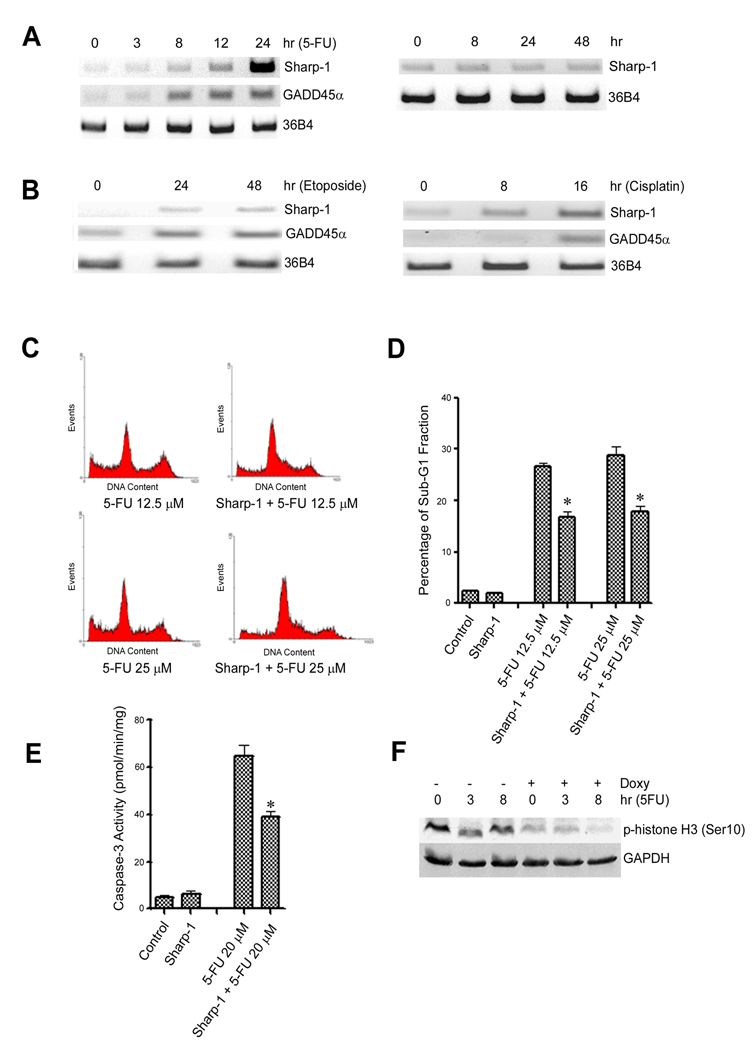
The DNA-damage pathway activates the G2/M checkpoint, which provides a survival mechanism in mammalian cells (18). Since Sharp-1 expression results in a pronounced G2/M arrest, we investigated its role in the DNA-damage response. We first analyzed whether Sharp-1 expression is modulated by DNA-damage. NIH3T3 cells were treated with the anticancer drugs 5-FU, cisplatin, and etoposide (Fig. 3A,B). Interestingly, similar to GADD45α, Sharp-1 mRNA was elevated by all genotoxic stimuli (Fig. 3A, left panel; and Fig. 3B) suggesting that it may modulate the cellular response to chemotherapeutic drugs. Sharp-1 expression however, was not increased by prolonged culture in the absence of drug treatment (Fig. 3A, right panel).
Figure 3. Sharp-1 is up-regulated by DNA-damaging stimuli and inhibits apoptosis.
(A). NIH3T3 cells were treated with 30 µM 5FU for 0, 3, 8, 12 and 24 hr. Sharp-1 and GADD45α expression was analyzed by RT-PCR. 36B4 was used as an internal control (left panel). Sharp-1 mRNA was examined in cells cultured for various time points by RT-PCR (right panel) (B). NIH3T3 cells were treated with 2 µM etoposide or 20 µM cisplatin for 0, 24 and 48 hr or 0, 8 and 16 hr, respectively. Sharp-1 and GADD45α expression was analyzed by RT-PCR. (C) NIH3T3 cells were pre-treated with doxycycline for 8 hr to induce Sharp-1, and were then co-treated with 12.5 µM or 25 µM 5FU for 72 hr. Representative histograms of cell cycle profiles without and with Sharp-1 overexpression are shown. (D) The percentage of sub-G1 fraction in cells treated with 5-FU in the absence and presence of Sharp-1 overexpression was determined. Sharp-1 overexpression resulted in a significant decrease (*p<0.05) in the percentage of apoptotic cells. Error bars indicate mean ± SE. (E). Caspase-3 activity was determined in control and Sharp-1 expressing cells under conditions described in (C). Sharp-1 expressing cells exhibited a significant (*p<0.05) decrease in caspase-3 activity compared to controls. (F). Control and doxycycline induced cells (16 hr) were treated with 5-FU and phospho H3 (ser10) levels was determined.
Since Sharp-1 is up-regulated by genotoxic agents and triggers arrest, we hypothesized that Sharp-1 expression may favor cell survival in face of DNA-damage. We therefore examined the effect of Sharp-1 on DNA-damage induced apoptosis. Sharp-1 was induced with doxycycline for 8 hr, and cells were then treated with 5-FU or cisplatin. The extent of apoptosis was assayed by flow cytometry using the sub-G1 fraction as a surrogate marker. In control cells treated with 12.5µM or 25µM 5-FU alone, 27% and 30% of cells underwent apoptosis respectively. In Sharp-1 overexpressing cells however, apoptosis was significantly reduced to 16% and 18 % respectively (Fig 3C–D). Similar effects were seen with cisplatin treatment (Supplementary Fig. 1). Consistent with the above results, Sharp-1 overexpressing cells exhibited a significant decrease in caspase-3 activity compared to control cells (Fig. 3E). Moreover, compared to control cells, Sharp-1 overexpressing cells showed a striking reduction of the mitotic marker phospho-histone H3 (Fig. 3F), confirming that the cells are arrested in the G2 phase.
Sharp-1 attenuates the p53 response to DNA-damage
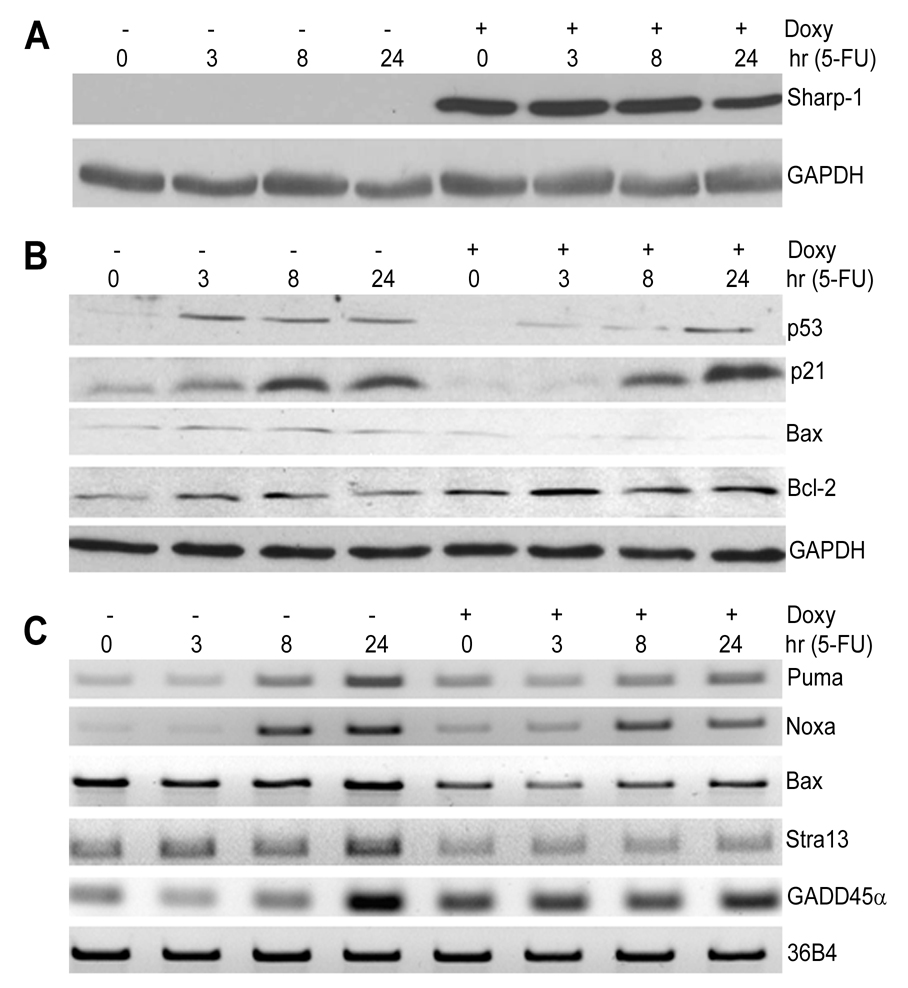
Activation of the p53 pathway is essential for the response to DNA-damage. To study the mechanisms underlying apoptosis-resistance conferred by Sharp-1, we analyzed p53 expression levels and its transcriptional activity. NIH3T3 cells were pre-treated with doxycycline for 16 hr to induce Sharp-1 expression. Cells were then co-treated with 5-FU for 0, 3, 8 and 24 hr. Sharp-1 was expressed for the duration of drug treatment (Fig. 4A). In control cells, p53 was significantly up-regulated as early as 3 hr (Fig. 4B). In contrast, the p53 response was attenuated three and 8 hr after 5-FU treatment in Sharp-1 expressing cells, although at late stages there was no obvious difference. Concomitantly the expression of p21waf1/cip1 and Bax was reduced suggesting that p53 transcriptional activity is attenuated by Sharp-1 expression. In contrast, Bcl2 levels were increased in Sharp-1 overexpressing cells.
Figure 4. Sharp-1 inhibits expression of pro-apoptotic p53 targets.
(A,B). Cells were left untreated (−) or induced with doxycycline (+) for 16 hr prior to addition of 15 µM 5-FU for 0, 3, 8 and 24 hr. Sharp-1, p53, p21, Bax, and Bcl2 were analyzed by western blot. (C). The expression of Noxa, Puma, Bax, Stra13 and GADD45α was analyzed by RT-PCR in control and doxycycline induced cells after various time points of 5-FU treatment as indicated.
To further examine p53 activity, we analyzed the mRNA levels of several p53 target genes (Fig. 4C). Consistent with reduced apoptosis and p53 levels, the expression of Puma, Noxa, Bax, and Stra13/Dec1 (6,7,15) were not appropriately induced by 5-FU in Sharp-1 overexpressing cells. In contrast, GADD45α was elevated compared to control cells at early time points and not induced further. It is noteworthy that Sharp-1 expression alone is sufficient to down regulate Bax and Stra13 expression even in the absence of 5-FU, and upregulate GADD45α levels (Fig. 2 and Fig. 4; time 0, +doxycycline). On the other hand, the reduced expression of Puma and Noxa may be due to reduced p53 activity as the expression of these genes is not altered by Sharp-1 alone. We have previously reported that Stra13 is repressed by Sharp-1, and regulates DNA-damage-induced apoptosis (9,15). Consistent with these studies Stra13 is down-regulated in Sharp-1 expressing cells that are resistant to apoptosis. Similar to the responses seen with 5-FU, Sharp-1 overexpressing cells also showed reduced levels of p53, p21 and Bax in response to cisplatin (data not shown).
Taken together, our studies indicate a novel regulatory axis through which Sharp-1 regulates survival of normal cells via activation of cell cycle checkpoints. This function provides protection from the cytotoxic effects of DNA damaging agents. This survival mechanism is applicable to normal cells (this study). It is conceivable that inappropriate Sharp-1 expression plays a role tumorigenesis and/or tumor progression and other disorders such as premature ageing arising from cell survival subsequent to DNA-damage.
Supplementary Material
Sharp-1 inhibits cisplatin induced apoptosis. (A). Representative histograms of cells treated with 10µM and 20µM cisplatin without (left panel) and with Sharp-1 overexpression (right panel). (B) The percentage of cells in the sub-G1 fraction after treatment with 10µM and 20µM cisplatin without and with Sharp-1 over-expression were calculated. Treatment of control cells with 10µM and 20µM cisplatin resulted in 16% and 19% apoptosis respectively. In cells pre-treated with doxycycline, apoptosis was decreased to 10% and 13% (*p<0.05) confirming that Sharp-1 protects cells from genotoxic stress induced cell death. Results are mean ± SE.
Acknowledgements
We are grateful to Drs. H. Bujard and P.B. Fisher for providing tetracycline inducible vectors, and Dr R. Gopalkrishnan for comments on the manuscript. This work was supported in part by NCI, a Scholar Award from the Leukemia and Lymphoma Society, and the National University of Singapore (R.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Motoyama N, Naka K. DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Dev. 2004;14:11–16. doi: 10.1016/j.gde.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 4.Meek DW. Tumor suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 5.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 6.McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 8.Azmi S, Ozog A, Taneja R. Sharp-1/DEC2 inhibits skeletal muscle differentiation through repression of myogenic transcription factors. J Biol Chem. 2004;279:52643–52652. doi: 10.1074/jbc.M409188200. [DOI] [PubMed] [Google Scholar]
- 9.Azmi S, Sun H, Ozog A, Taneja R. mSharp-1/DEC2, a basic helix-loop-helix protein functions as a transcriptional repressor of E box activity and Stra13 expression. J Biol Chem. 2003;278:20098–20109. doi: 10.1074/jbc.M210427200. [DOI] [PubMed] [Google Scholar]
- 10.Gulbagci NT, Li L, Ling B, Gopinadhan S, Walsh M, Rossner M, et al. SHARP1/DEC2 inhibits adipogenic differentiation by regulating the activity of C/EBP. EMBO Rep. 2009;10:79–86. doi: 10.1038/embor.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebermann DA, Hoffman B. Gadd45 in the response of hematopoietic cells to genotoxic stress. Blood Cells Mol Dis. 2007;39:329–335. doi: 10.1016/j.bcmd.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durant ST, Nickoloff JA. Good timing in the cell cycle for precise DNA repair by BRCA1. Cell Cycle. 2005;4:1216–1222. doi: 10.4161/cc.4.9.2027. [DOI] [PubMed] [Google Scholar]
- 13.Gopalkrishnan RV, Christiansen KA, Goldstein NI, DePinho RA, Fisher PB. Use of the human EF-1alpha promoter for expression can significantly increase success in establishing stable cell lines with consistent expression: a study using the tetracycline-inducible system in human cancer cells. Nucleic Acids Res. 1999;27:4775–4782. doi: 10.1093/nar/27.24.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Taneja R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc Natl Acad Sci USA. 2000;97:4058–4063. doi: 10.1073/pnas.070526297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thin TH, Li L, Chung TK, Sun H, Taneja R. Stra13 is induced by genotoxic stress and regulates ionizing-radiation-induced apoptosis. EMBO Rep. 2007;8:401–407. doi: 10.1038/sj.embor.7400912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry LD, Gould KL. Regulation of Cdc2 activity by phosphorylation at T14/Y15. Prog Cell Cycle Res. 1996;2:99–105. doi: 10.1007/978-1-4615-5873-6_10. [DOI] [PubMed] [Google Scholar]
- 17.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J. Cell Sci. 2003;116:3677–3685. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell MJ, Walworth NC, Carr AM. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sharp-1 inhibits cisplatin induced apoptosis. (A). Representative histograms of cells treated with 10µM and 20µM cisplatin without (left panel) and with Sharp-1 overexpression (right panel). (B) The percentage of cells in the sub-G1 fraction after treatment with 10µM and 20µM cisplatin without and with Sharp-1 over-expression were calculated. Treatment of control cells with 10µM and 20µM cisplatin resulted in 16% and 19% apoptosis respectively. In cells pre-treated with doxycycline, apoptosis was decreased to 10% and 13% (*p<0.05) confirming that Sharp-1 protects cells from genotoxic stress induced cell death. Results are mean ± SE.