Abstract
The H-reflex habituates at relatively low frequency (10 Hz) stimulation in the intact spinal cord, but loss of descending inhibition resulting from spinal cord transection reduces this habituation. There is a return towards a normal pattern of low-frequency habituation in the reflex activity with cycling exercise of the affected hind limbs. This implies that repetitive passive stretching of the muscles in spinalized animals and the accompanying stimulation of large (Group I and II) proprioceptive fibers has modulatory effects on spinal cord reflexes after injury. To test this hypothesis, we induced pyridoxine neurotoxicity that preferentially affects large dorsal root ganglia neurons in intact and spinalized rats. Pyridoxine or saline injections were given twice daily (IP) for 6 weeks and half of the spinalized animals were subjected to cycling exercise during that period. After 6 weeks, the tibial nerve was stimulated electrically and recordings of M and H waves were made from interosseous muscles of the hind paw. Results show that pyridoxine treatment completely eliminated the H-reflex in spinal intact animals. In contrast, transection paired with pyridoxine treatment resulted in a reduction of the frequency-dependent habituation of the H-reflex that was not affected by exercise. These results indicate that normal Group I and II afferent input is critical to achieve exercise-based reversal of hyper-reflexia of the H-reflex after spinal cord injury.
Keywords: spinal cord injury, H-reflex, sensory neuropathy, exercise, plasticity
1 Introduction
Spinal cord injury (SCI) results in the exaggeration of segmental reflexes caudal to the level of injury. It is probable that the loss of inputs from descending supraspinal pathways leads to a reorganization of spinal circuitry that promotes spasticity or hyper-reflexia. Numerous mechanisms responsible for hyper-reflexia following SCI have been proposed (Nielsen, et al., 2007), including changes in motoneuron properties (i.e. hyper-excitability) (Li and Bennett, 2003), synaptic growth (Little, et al., 1999), decreased presynaptic inhibition of Ia terminals (Calancie, et al., 1993, Schindler-Ivens and Shields, 2000, Skinner, et al., 1996) and changes in electrical coupling of spinal motoneurons and interneurons (Reese, et al., 2006, Yates, et al., 2008). Hyper-reflexia can be quantified by measuring the Hoffman or H-reflex, the electrical analogue of the tendon jerk reflex. The H-reflex is a compound muscle action potential elicited by the synaptic activation of motoneurons by muscle afferents following stimulation of muscle nerves. Any change in H-reflex amplitude under a given condition thus reflects changes in the excitability of the reflex pathway. Under normal conditions, the magnitude of the H-reflex is subject to depression by repetitive activation at relatively low frequency (10 Hz) stimulation (Ho and Waite, 2002, Reese, et al., 2006, Schindler-Ivens and Shields, 2000, Skinner, et al., 1996). This phenomenon is believed to be due to presynaptic mechanisms and to be dependent on descending control. After SCI, this frequency-dependent depression/habituation of the H-reflex is progressively reduced, and accompanied by the development of hyper-reflexia and spasticity in humans as well as in animals (Schindler-Ivens and Shields, 2000, Thompson, et al., 1992).
Passive exercise has been shown to restore this frequency-dependent depression of spinal reflexes in a time-dependent manner (Kiser, et al., 2005, Reese, et al., 2006), with significant returns in reflex depression occurring by 30 days of exercise in rats (Reese, et al., 2006). These investigators assumed that exercise helped reorganize or preserve local neuronal circuits subserving presynaptic inhibition (Skinner, et al., 1996) by providing recurrent signaling leading to an intrasegmental reorganization (or plasticity) and a restitution of low frequency-dependent depression of the H-reflex (Reese, et al., 2006), although a direct test of this was not performed. This implies that passive stretching of the muscles and ensuing stimulation of proprioceptive fibers can have functional effects on spinal cord reflexes in spinalized animals.
To test this hypothesis, we induced pyridoxine neurotoxicity in spinal cord injured rats and measured H-reflexes in those animals and control cohorts. Pyridoxine (or vitamin B6) administration induces dose-dependent metabolic injury (Krinke and Fitzgerald, 1988) predominately affecting large neurons of the dorsal root ganglia (DRG). The primary site of injury is the soma of DRG neurons of the dorsal root ganglion and the injury has pathophysiological consequences to the integrity of their long myelinated fibers. The cell body manifests this damage as cytoplasmic alterations including vacuolization, increased dense bodies, neurofilament aggregates and chromatolysis. More advanced neurotoxic changes lead to neuronal death with phagocytosis by satellite cells (Perry, et al., 2004). This leads to cell body and axonal atrophy, decrease in the number of large caliber axons (Perry, et al., 2004) and argyrophilic axonal profiles in the dorsal columns (Helgren, et al., 1997). The anatomical and electrophysiological data presented here indicate normalization of the H-reflex depression with exercise, but this activity-dependent plasticity is considerably compromised if proprioceptive input to the spinal cord is altered.
2 Methods
2.1 Animal groups
Fifty adult female Sprague-Dawley rats (225–250 g) were used in this study. Animals were divided into six groups; 7 uninjured controls + saline treatment (Sal), 5 uninjured controls + pyridoxine treatment (Pyr), 18 transected animals + saline treatment (Tx-Sal), 5 transected animals + pyridoxine treatment (Tx-Pyr), 9 transected animals + saline treatment + exercise (Tx-Sal-Ex) and 6 transected animals + pyridoxine treatment + exercise (Tx-Pyr-Ex). Exercise consisted of daily passive cycling (see below). Intraperitoneal injections of Pyridoxine (300mg/kg/day) or saline were given twice daily for 6 weeks. Six weeks post-transection, the tibial nerve was stimulated (see below) and recordings of M and H waves were made from interosseous muscles of the hind paw. The number of animals in the Tx-Sal group was increased after we noticed two distinct subgroups of responses in H-reflex responses to repeated stimulation for this control group (see Discussion). Differences in animal numbers for the other groups are due to technical or surgical difficulties in obtaining electrophysiological measurements from some of the animals. All experiments were performed at Drexel University College of Medicine, Philadelphia PA. The experimental protocol was approved by Drexel University’s Institutional Animal Care and Use Committee (IACUC) and animal care followed National Institute of Health (NIH) guidelines.
2.2 Spinal transection
Rats were deeply anesthetized with isoflurane. The adequacy of anesthesia was confirmed by the absence of corneal reflexes and response to paw pinch. Under aseptic conditions, the spinal cord was exposed by laminectomy of the ninth thoracic vertebra (T9). Meningeal membranes were opened longitudinally and a 2 mm length of T10 spinal cord was removed by gentle aspiration. The resulting complete transection lesion cavity was filled with gelfoam to achieve hemostasis. Gelfoam was removed, the dura was closed with 10-0 suture and the overlying muscles and fascia were closed in layers with sutures. Finally, the skin incision was closed with wound clips. After surgery, bladders were manually expressed 2–3 times daily for approximately 2 weeks, until reflex voiding returned. Ampicillin (0.20 ml, 100 mg/kg) was administered twice daily for 7 days to prevent infection. Special absorbent bedding was provided to prevent pressure ulceration or other post operative complications.
2.3 Bicycling exercise
Details of this passive form of hindlimb exercise have been provided previously (Houle, et al., 1999, Skinner, et al., 1996). In brief, animals were supported in an adjustable sling with their hind limbs dangling beneath them through openings in the sling. The hind paws were secured to motor-driven foot pedals with surgical tape so that there was no bending of the foot except at the ankle joint. Exercise began 5 days after spinal transection and continued on a daily basis, 5 days per week. There were two 30 minute exercise sessions separated by a 10 minute rest period each day, at a rate of 45 rpm.
2.4 Electrophysiology experiment
Animals were deeply anaesthetized by intraperitoneal injection of xylazine (8 mg/kg) and ketamine (80 mg/kg), which has been shown to minimally depress the spinal monosynaptic reflex and does not alter the time course of presynaptic inhibition (Ho and Waite, 2002, Lodge and Anis, 1984, Tang and Schroeder, 1973). The tibial nerve was exposed below the popliteal fossa and mounted on a stimulating bipolar hook. Exposed tissues were covered with warm mineral oil to prevent drying. Bipolar EMG electrodes were inserted subcutaneously in the digital interosseous muscles between the fourth and fifth metatarsals. The ground electrode was located in the skin of the leg.
The reflex was first tested with single pulses (100 µs duration) to determine the threshold and the maximal response level for both the M-wave and the H-reflex. The level of stimulation which produced a maximum H-reflex response was then used for 2 series of 16 consecutive stimulations, one at 0.3 Hz, the other at 10 Hz with 2 min rest in between runs. Stimuli were delivered via an isolated pulse stimulator (A–M Systems Model 2100, A–M Systems Inc, Carlsborg WA). EMG responses were recorded with a differential AC amplifier (A–M Systems Model 1700, A–M Systems Inc, Carlsborg WA; Gain ×1000; pass band 10-5K Hz). Data acquisition (sampled at 10 kHz) was controlled by customized software (Labview, National Instruments Corp. Austin, Texas) running on a personal computer.
At the conclusion of the experiment, animals were euthanized with Euthasol (390 mg/kg sodium pentobarbital, 50 mg/kg phenytoin, ip) and perfused through the heart with 4% paraformaldehyde. Lumbar level 5 (L5) dorsal root ganglia (DRGs) and associated dorsal roots were harvested for anatomical analyses.
2.5 Anatomical measures
The effects of pyridoxine treatment on the cross sectional area of L5 sensory neurons and their centrally directed axons were quantified. DRGs were cut at 20 µm on a cryostat and a series of alternating sections was reacted with neurofilament-200 primary antibody (for large caliber fibers; Chemicon AB1989) and a goat anti-rabbit IgG (R2004, Sigma), followed by rabbit peroxidase anti-peroxidase (P2026, Sigma). Diaminobenzidine (DAB, D4293, Sigma) was used for the colorimetric detection of immunopositive neurons. For each animal all immunopositive neurons in three non-consecutive sections were counted and the cross-sectional area measured using the nucleator function of Stereo Investigator v.7.0. Neurons were only counted if a nucleus could be identified. After all neurons were counted, they were binned by size for statistical analysis. Individual dorsal roots were processed for epon embedding and 1 µm semi thin sections were prepared for analysis of axon size by the Myelinated Axon Segmentation Algorithm (MASA) (Chin, et al., 2004, Schwartz, et al., 2005). MASA operates in multiple conceptual stages: 1) initial seeds are identified using a local histogram-based thresholder, 2) a modification of the Waks-Tretiak path search algorithm is used to segment putative axons and their myelin sheath, and 3) each candidate axon is evaluated by a multi-parameter classifier. Cross-sectional areas of axons in the dorsal root (Fig. 1) were collected and binned for statistical analysis. For both neuronal and axonal cross-sectional area studies, a chi-square analysis was run to determine whether there were changes in the overall percentages in the area bins sorted by treatment. Further analysis used ANOVA to determine whether there was a difference between groups in each area bin. A p value of 0.05 or less was deemed significant.
Figure 1.
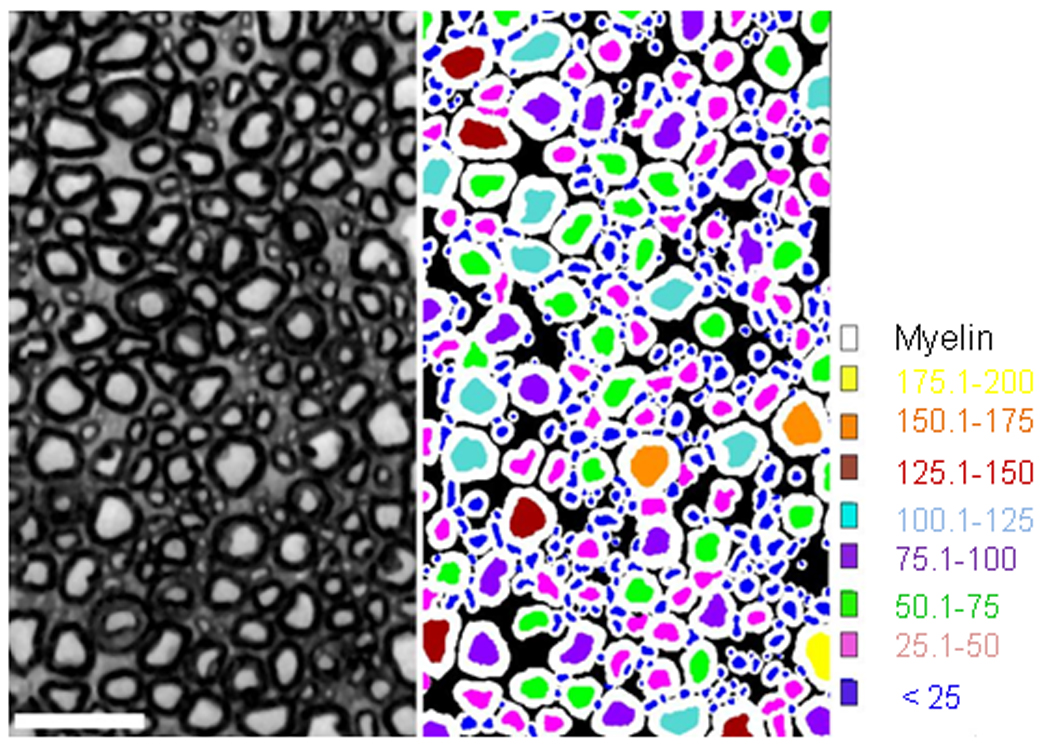
Representation of sensory axon cross sectional area as computed by Myelinated Axon Segmentation Algorithm (MASA). The left side is a representative cross section of the dorsal root. MASA identifies axons by their shape and presence of a myelin sheath. Axons were binned by size, indicated in the right side of the figure by color.
2.6 EMG data analysis
Latency and peak amplitude of the first main component of each M and H response were measured. Averages of the last 11 M and H responses were then calculated for both stimulus frequency trains. The change in H-reflex response at 10 Hz was calculated as a percentage of the response at 0.3Hz in order to determine the stimulation depression of the H-reflex (=H10Hz/H0.3Hz ×100), a method of analysis proposed by Skinner and colleagues (Reese, et al., 2006, Skinner, et al., 1996). The ratio of the maximal H-wave amplitude to the maximal M wave amplitude (Hmax/Mmax) was also calculated using the single pulse measurements. By providing an index of the proportion of motoneurons recruited via a monosynaptic reflex relative to the total motoneuron pool (Magladery and Mc, 1950, Magladery, et al., 1951), this ratio shows possible changes in H-reflex amplitudes among the groups and reflects the motoneurons or central synaptic excitability.
One-way ANOVAs or t-tests were used to compare the threshold, M and H latencies, Hmax/Mmax ratio and H10Hz/H0.3Hz amplitude between animal groups. Then, Tukey HSD posthoc tests were used to determine significant effect between groups with the ANOVAs. Significance was set at p<0.05. All values are mean ± standard deviation (SD).
3 Results
3.1 Anatomical results
The efficacy of our pyridoxine treatment was confirmed by measuring the cross sectional areas of DRG neurons and dorsal root axons and calculating the percentages of large and small neurons and axons in vehicle and pyridoxine treated groups. Since pyridoxine is especially toxic to large sensory neurons, treatment should result in a shift towards a higher percentage of small neurons and axons (Perry, et al., 2004). Bin sizes for grouping of neurons were chosen to distinguish between A and B cells (large and light versus small and dark staining DRG neurons respectively), with 1000 µm2 representing the upper bounds of B cells and A cells reaching an upper limit of ~2500 µm2 (Harper and Lawson, 1985, Tandrup, 2004). A cross sectional area of 100 µm2 (~11 µm diameter) was chosen as the lower bound of group I axons and larger caliber axons reached over 200 µm2 (~16 µm diameter).
There was no significant change in the pre-sacrifice weight of pyridoxine treated animals compared to those in saline treated groups, nor was there a significant change in weight after spinal cord transection with or without Ex. There was no difference in DRG neuron cross sectional area between saline treated groups (intact-Sal: 1619.7 ± 189.6 µm2, Tx-Sal: 1632.9 ± 121.1 µm2 and Tx-Sal-Ex: 1607.7 ± 101.8 µm2). Pyridoxine treatment led to a significant decrease in mean size of DRG neurons in all groups when compared to the corresponding saline treated group, i.e. the saline group with the same experimental condition (intact-pyr versus intact-sal, p<0.001; Tx-Pyr versus Tx-Sal, p=0.016; and Tx-Pyr-Ex versus Tx-Sal-Ex, p<0.001).
In intact animals, pyridoxine treatment significantly shifted the distribution of DRG neurons from a near equal representation of cells within each of the bins between 500 and 2500 µm2 to most cells being less than 1500 µm2 (Fig. 2A) as determined by chi-square (p<0.005). Further analysis revealed that pyridoxine treatment significantly increased the percentage of small DRG neurons in the less than 1000 µm2 and 1000 to 1500 µm2 bins, (p=0.004 and p=0.003, respectively) and significantly decreased the percentage of DRG neurons in the 1500 to 2000 and greater than 2000 µm2 bins (p=0.003 and p=0.001, respectively). Similar effects of pyridoxine treatment were detected in the size of dorsal root axons (Fig. 3A) where the mean cross-sectional area decreased. The percentage of dorsal root axons in the bin of smallest caliber fibers (<100 µm2) increased significantly with pyridoxine treatment (p=0.004) with concomitant decrease in the bins of larger size axons (100–150 µm2, p=0.002; 150–200 µm2, p=0.01; greater than 200 µm2, p=0.074). When the mean of all dorsal root axons measured from the saline treated group was compared to the mean for all dorsal root axons from the pyridoxine treated groups there was a significant decrease in mean size (51.775 ± 6.566 µm2 vs. 42.647 ± 5.944 µm2, respectively; p=0.030). Results with DRG neuron and dorsal root fiber sizes indicate an overall effect of pyridoxine on size parameters, but the mean number of axons per L5 dorsal root was unaffected by pyridoxine treatment (intact-sal: 10,783 ± 2329 vs. intact-pyr: 10,621 ± 2258, p=0.927). It is unclear whether DRG neurons are lost or merely atrophied by pyridoxine treatment.
Figure 2.
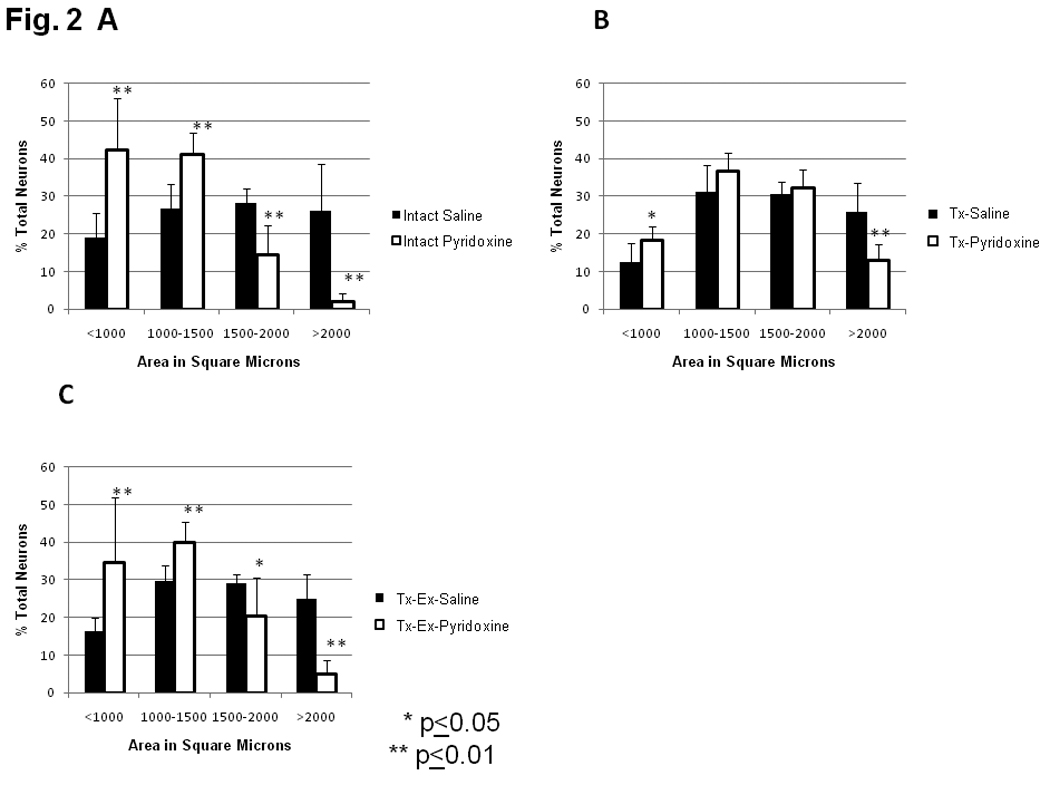
Size distribution of dorsal root ganglion neurons counted on three non-sequential sections. Size bins were chosen to discriminate between large, lightly staining A-cells (greater than 1000 µm2) and small, darkly staining B-cells. Pyridoxine treatment resulted in a decrease in mean DRG neuron size in all groups (intact: p<0.001; Tx: p<0.016; Tx-Ex (p<0.001). A) Intact animals treated for 6 wks with either saline or pyridoxine. Pyridoxine treatment resulted in a significant shift towards decreased size of DRG neurons as determined by chi-square (p<0.005). B) Animals transected at T10 treated for 6 wks with either saline or pyridoxine. Pyridoxine treatment resulted in a slight shift towards smaller DRG neurons, but this effect was not significant as determined by chi-square (p>0.1). C) Animals transected at T10 receiving cycling Ex, treated for 6 wks with either saline or pyridoxine. Pyridoxine treatment resulted in a significant shift towards decreased size of DRG neurons as determined by chi-square (p<0.005).
Figure 3.
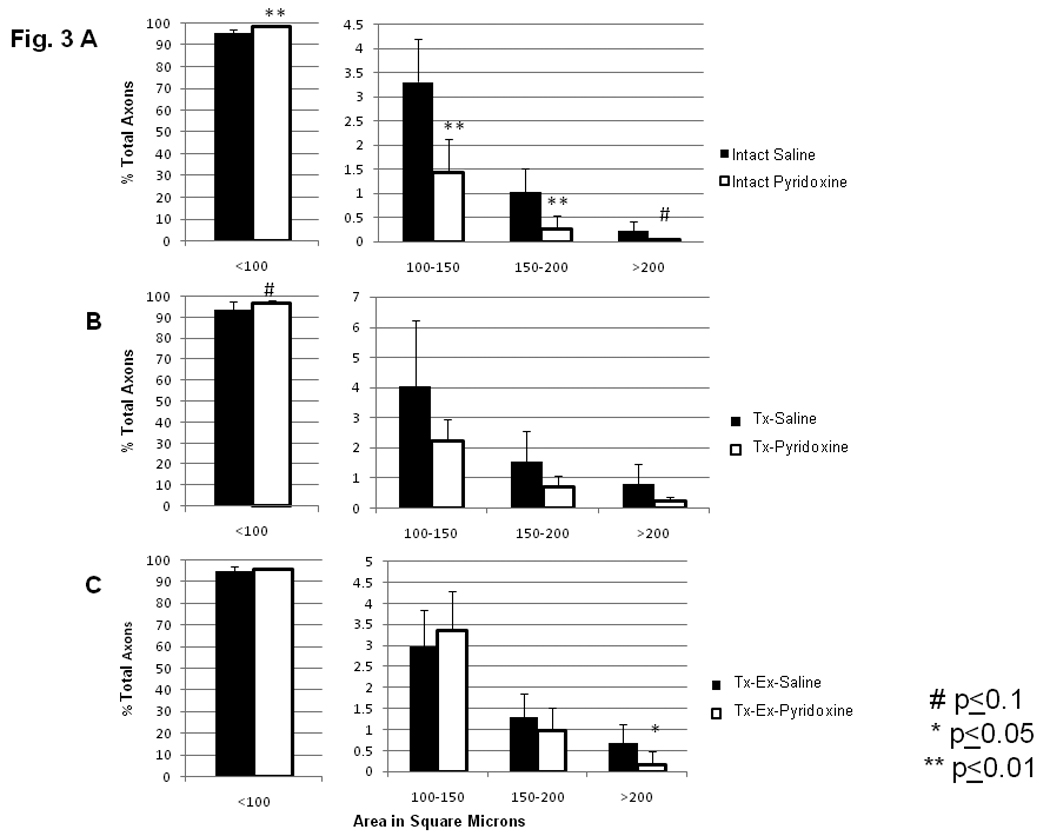
Size distribution of dorsal root axons counted by MASA. Pyridoxine treatment resulted in a decreased mean fiber size when compared to saline treated animals (Sal treated groups: 51.775 ± 6.566 vs. Pyridoxine treated groups: 42.647 ± 5.944; p<0.03). For all graphs, A–C, the scale for the <100 µm2 size bin is from 0–100%, while the scale for the other three size bins is much smaller. The same axis label still applies and numbers represent the percentage of total axons within that size range. A) Intact animals treated for 6 wks with either saline or pyridoxine. B) Animals transected at T10 treated for 6 wks with either saline or pyridoxine. C) Animals transected at T10 receiving cycling Ex, treated for 6 wks with either saline or pyridoxine.
Animals undergoing T10 transection and pyridoxine treatment followed a similar trend as intact animals, with an apparent shift towards decreased DRG neuron size yet this was not significant when analyzed by chi square (p>0.10). However, ANOVA analysis of DRG neurons’ sizes did reveal significant differences between these groups. Percentages of DRG neurons of the Tx-Pyr group in the less than 1000 µm2 size bin increased, while percentages in the greater than 2000 µm2 bin decreased (p=0.04 and p=0.006, respectively, Fig. 2B). The percentage of DRG neurons in intermediate size bins of 1000–1500 µm2 and 1500–2000 µm2 were not significantly changed by pyridoxine treatment following transection (Tx-Sal versus Tx-Pyr, Fig. 2B). The size distribution of L5 dorsal root axons from transected animals (Sal/Pyr) was similar to those in intact animals (Fig. 3A, 3B). Dorsal root axons of transected animals treated with pyridoxine were compared to dorsal root axons of transected animals treated with saline. In pyridoxine treated animals, there was an increased percentage of dorsal root axons in the less than 100 µm2 bin, although this did not reach significance, (p=0.086, Fig. 3B). Again, pyridoxine treatment had no effect on the number of axons per L5 dorsal root (Tx-Sal: 7,912 ± 3429 vs. Tx-Pyr: 10,764 ± 1973, p=0.111).
Exercise therapy following transection did not alter the effects of pyridoxine on neuron or axon size distribution. Percentages of DRG neurons for animals in the Tx-Pyr-Ex group showed a shift towards reduced size when compared to Tx-Sal-Ex, as determined by chi square (p<0.005, Fig. 2C). The percentage of DRG neurons in the bins of less than 1000 µm2 and 1000–1500 µm2 increased significantly with pyridoxine treatment (p=0.005 and p=0.001, respectively). Pyridoxine treatment also significantly decreased the percentages of DRG neurons in the largest size bins, 1500–2000 µm2 and greater than 2000 µm2 (p=0.021 and p<0.001, respectively). There was no significant difference in the distribution of dorsal root axons’ cross sectional area with pyridoxine treatment following injury and exercise (Fig. 3C) except for those greater than 200 µm2, which were decreased (p=0.041). Unlike the other conditions, treatment with pyridoxine in transected and exercised animals decreased the number of fibers per section (Tx-Sal-Ex: 10,709 ± 1876 vs. Tx-Pyr-Ex: 8643 ± 947, p=0.042).
3.2 Electrophysiological results
Measurement of H-reflex was attempted in all 50 animals. All presented a reproducible H-reflex response except for 5/5 animals in the Uninjured-Pyr group, 2/7 in the Uninjured-Sal group, 4/9 in the Tx-Sal-Ex group and 2/18 in the Tx-Sal group. Rats with erratic H-reflex responses (no consistent H wave to single pulse stimulation) were excluded from the analysis, except for those receiving the pyridoxine treatment since the administration of this drug has been shown to eliminate the H wave (Helgren, et al., 1997, Perry, et al., 2004).
Thresholds were not statistically different between any of the groups (Table 1), illustrating good procedural consistency in our experiments (ANOVA, p=0.109). All of the thresholds provided correspond to the H-reflex threshold except for the Pyr group. Since there was no H-reflex, the average for the Pyr group corresponds to the M-wave threshold and is consequently higher than in the other groups.
Table 1.
Threshold and latencies of the M-wave and H-reflex for each group (Mean ± SD)
| Sal (n=5) |
Pyr (n=5) |
Tx-Sal (n=16) |
Tx-Pyr (n=5) |
Tx-Ex-Sal (n=5) |
Tx-Ex-Pyr (n=6) |
|
|---|---|---|---|---|---|---|
| Threshold (mA) | 0.04 ± 0.02 | 0.11 ± 0.05 | 0.05 ± 0.05 | 0.03 ± 0.00 | 0.05 ± 0.04 | 0.08 ± 0.04 |
| M latency (ms) | 3.90 ± 0.34 | 3.34 ± 0.30 | 3.05 ± 0.33 | 2.82 ± 0.18 | 2.96 ± 0.13 | 3.03 ± 0.46 |
| H latency (ms) | 10.88 ± 0.81 | - | 9.29 ± 0.57 | 9.06 ± 0.17 | 9.18 ± 0.32 | 9.21 ± 0.66 |
Examples of the M-wave and H-reflex recorded in representative animals from each group are shown in Fig. 4A. The mean latencies of the M-wave and H-reflex were significantly higher (Tukey HSD posthoc, p<0.0001 for all comparisons) in control animals compared to transected animals (3.62 ± 0.42 ms vs. 2.99 ± 0.31 ms respectively for the M; 10.88 ± 0.81 ms vs. 9.22 ± 0.50 ms respectively for the H). There were no differences in latencies between transected groups (Table 1).
Figure 4.
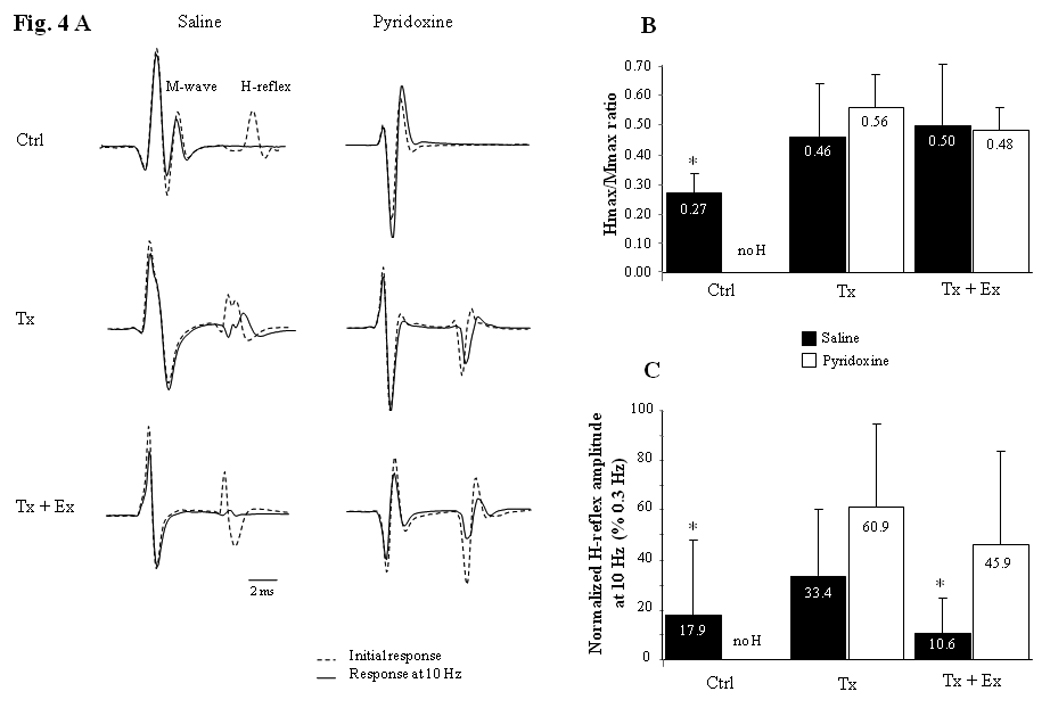
H-reflex responses recorded in plantar muscle following tibial nerve stimulation. A) Initial M wave and H-reflex responses (dotted line) and averages of the last 11 consecutive responses (6th to 16th) at a 10 Hz stimulation frequency (solid line) for a representative animal in each group. Note the absence of H-reflex in the control animal treated with pyridoxine (top right). The H-reflexes at 10 Hz stimulation are decreased due to frequency-dependent depression. However, H-reflex at 10 Hz in Tx-Sal, Tx-Pyr and Tx-Ex-Pyr animals (middle left and right + bottom right) did not decrease as much compared to H-reflex in Sal and Tx-Ex-Sal animals (top left and bottom left). Calibration bar: 2 ms for all records. B) Hmax/Mmax ratio for each group (Mean ± SD). H-reflex in control group is significantly smaller than in all transected groups combined suggesting an increase in motoneuron excitability after SCI (t-test, p=0.032). C) Mean and SD of the H-reflex at 10 Hz stimulation frequency as a percent of the response observed at 0.3 Hz stimulation frequency. The frequency-dependent depression of the H-reflex is statistically more important in saline-treated Ctrl and saline-treated Tx-Ex groups combined compared to the three other groups combined (t-test, p=0.033). Ctrl = control animals, Tx = transected animals, Tx + Ex = transected + exercise animals.
The Hmax/Mmax ratio results, which reflect the central synaptic excitability for each group, are presented in Fig. 4B. H-reflex in all transected animals combined was significantly larger (t-test, p=0.032) than in the Sal (uninjured control) group (averages = 0.49 ± 0.16 vs. 0.27 ± 0.07 respectively), but the Hmax/Mmax ratio was not significantly different between transected animals.
The H-reflex obtained when stimulating with a 0.3 Hz frequency stimulus train was consistent in every group (96.3 ± 16.1 % in average for the last 11/16 responses compared to initial values), showing no depression or habituation with repeated stimulation at that frequency. In contrast, the H-reflex decreased in amplitude when stimulating with a 10 Hz frequency stimulus train, but the decrease was significantly smaller after pyridoxine administration. In order to determine the frequency-dependent depression of the H-reflex, the change in H-reflex response at 10 Hz was calculated as a percentage of the response at 0.3Hz (=H10Hz/H0.3Hz ×100) (Fig. 4C). Our results showed a large depression of the H-reflex (with 10 Hz stimulus train) which was statistically greater (t-test, p=0.033) for the Sal and Tx-Sal-Ex groups combined compared to the Tx-Pyr-Ex, Tx-Sal and Tx-Pyr groups combined (amplitude averages = 14.2 ± 21.8 vs. 40.5 ± 30.4 % respectively).
4 Discussion
Results show that pyridoxine treatment significantly reduced the percentage of large neurons in the lumbar DRGs, reduced the mean size of dorsal root axons and completely eliminated the H-reflex in intact animals. These results provide compelling evidence that pyridoxine treatment was effective in targeting a significant proportion of large (Group I and some Group II) proprioceptive fibers. Even though the H-reflex was eliminated in intact animals treated with pyridoxine, it returned in injured-pyridoxine treated animals. As expected, the low frequency-dependent depression of the H-reflex (obtained when stimulating at a 10 Hz frequency) in transected-trained animals treated with saline was similar to that seen in controls, which was markedly increased compared to most of the untrained animals. In contrast, the depression of the H-reflex at 10 Hz was much lower in transected animals treated with pyridoxine and was not affected by the training regimen. These results indicate that a near normal large fiber afferent input is critical to achieve exercise-based reversal of the H-reflex hyper-reflexia after SCI.
4.1 Effect of a complete spinal transection on the H-reflex
The H-reflex represents a reliable measure of signal transmission between muscle afferents and motoneurons. The H-wave amplitude is proportional to the number of motoneurons activated (Magladery and Mc, 1950, Magladery, et al., 1951) via stimulation of the large afferent fibers, mostly type Ia but not exclusively (Knikou, 2008). In uninjured control animals, this reflex habituated (in 4 animals out of 5) when a 10Hz stimulation frequency train was used, likely due to presynaptic inhibition from descending projections of the large afferent fiber synapses onto motoneurons. Transection of the spinal cord resulted in reduced depression of the H-reflex with repeated stimulation in half of our animals after 6 weeks. Two subgroups of H-reflex depression responses with 10 Hz stimulation frequency were actually noticed for the Tx-Sal group: 7/16 animals showed a moderate depression of the H-reflex (58.9 ± 14.1 %) while the others (9/16) showed a depression equal to that of exercised animals (13.6 ± 12.8 %). We found no obvious reason to explain this variability, but it limits the interpretation of our results. Six weeks was perhaps too short of a post injury period for a stable response; however Reese et al. (Reese, et al., 2006) did observe a difference between trained and non-trained transected animals after the same period.
This reduction in the depression of the H-reflex with repeated stimulation after transection is likely due to a loss of presynaptic inhibition as proposed by Skinner and colleagues in rats (Reese, et al., 2006, Skinner, et al., 1996) and by others in humans (Calancie, et al., 1993, Schindler-Ivens and Shields, 2000). Furthermore, we also observed an increase in the H-wave amplitude (H/M ratio) after SCI as shown previously (Calancie, et al., 1993, Malmsten, 1983, Phadke, et al., 2007, Taylor, et al., 1984), which reinforces the presynaptic non-inhibition theory. These results (reduced depression and increased H-wave) may also come from collateral sprouting of primary afferents and an increase in the intrinsic excitability of the motoneurons (Calancie, et al., 1993, Li and Bennett, 2003, Little, et al., 1999, Murray and Goldberger, 1974, Taylor, et al., 1984). Furthermore, an increase in the number of excitatory glutamatergic synapses and a reduction in the number of inhibitory synapses related to γ-amino butyric acid below the level of SCI have been reported. After SCI, a reduction in glycine also occurs, adding to the loss of local inhibition (Shapiro, 1997). These synaptic modifications support the idea of facilitation in the synaptic transmission, and thus increased H-reflexes and reduced depression. The shorter latency that we observed in our transected animals for the M and H waves reinforces these suggestions. It may be indicative of increased efficiency (i.e. faster) for both central and peripheral synaptic transmissions.
The hypothesis of better synaptic transmission is favored over faster conduction velocity as the mechanism to explain the shorter latency since the latency difference between groups is twice as long for the H than the M (1.66 vs. 0.63 ms respectively) and corresponds to two synapses for the H versus one (the neuromuscular junction) for the M. Furthermore, a 0.63 ms difference for the M response latencies between spinal intact and transected animals would represent a dramatic change in nerve conduction velocity (around 30 m/s difference) for the approximately 20 mm of nerve separating the stimulating and recording electrodes. Moreover, motor conduction velocities of the tibial nerve are generally normal in spinal cord injured patients (Hunter and Ashby, 1984, Rutz, et al., 2000).
4.2 Effect of a passive exercise after transection on hyper-reflexia
A modest exercise regimen after transection can restore the low frequency depression (10Hz) to levels similar to what is seen in controls and help “normalize” the excitability of the H-reflex (Skinner, et al., 1996). It has been suggested that exercise helps by reorganizing or preserving some local neuronal circuits subserving presynaptic inhibition. This intrasegmental reorganization implies that passive stretching of the muscle in spinalized animals initiates stimulation of the large proprioceptive fibers that can have functional effects on spinal cord reflexes after injury. In our protocol, the bicycle training we used (Skinner, et al., 1996) does not have an effect on the foot muscles since the position of the foot on the pedal is fixed at the ankle. Accordingly, it is interesting to notice that any kind of dynamic exercise has a positive effect on multiple reflex pathways rather than the effects being restricted to the muscles undergoing passive stretch.
4.3 Effect of pyridoxine neurotoxicity
Pyridoxine is known to induce sensory neuropathy (Krinke and Fitzgerald, 1988) by targeting large-diameter A-cells of the dorsal root ganglia leading to atrophy of the large-diameter fibers within the sciatic nerve (Perry, et al., 2004). Neurons in the DRG are usually classified in two types based on their size, appearance, and histochemical reactions, leading to the designation A (large and light) and B (small and dark) cells (Harper and Lawson, 1985, Tandrup, 2004). In our study, pyridoxine treatment significantly reduced the number of large diameter DRG neurons and their centrally projecting fibers. Although this treatment was effective, it failed to completely eliminate all large fibers. Pyridoxine likely affected Group II proprioceptive fibers as well, but it is difficult to know for certain since their size range overlaps that of smaller Group I fibers. In normal conditions, DRG neuron cross-sectional areas are similar for the Group I and Group II sensory fibers (1175 µm2 in average for type I and 1100 µm2 for type II, both types ranging from 300 to 2200 µm2), whereas Groups III and IV are smaller [200–1100 µm2] (Harper and Lawson, 1985). Axon cross-sectional area differs between each sensory fiber type: [30–220 µm2] for type I (also called Aα fibers), and [3–115 µm2] for type II (Aβ fibers) (Vleggeert-Lankamp, et al., 2005). Since pyridoxine treatment induced neuronal and axonal atrophy, large fibers or neurons became smaller and a size-dependent distinction between each type of myelinated afferents (type I and II) became impossible. As anticipated the treatment eliminated the H-reflex in intact animals, which coincided with previous reports (Helgren, et al., 1997, Perry, et al., 2004). The complete absence of an H-reflex in these Pyr rats indicates that the treatment affected enough large afferent neurons to prevent activation of the reflex from stimulation of the afferents. It is unclear whether proprioceptive neurons were lost or merely atrophied by pyridoxine treatment, but their function was clearly decreased. However, it appeared that the neurotoxic effects of pyridoxine were mitigated by spinal cord transection as evidenced by a smaller decrease in neuron and axon sizes and a reduction in the low-frequency habituation of the H-reflex at 10 Hz for the pyridoxine treated transected animals. Neurotoxicity was evident in the exercise group but there was not a comparable effect on size of dorsal root fibers. One explanation is that following transection, active movement of the lower limbs is absent and passive movement is minimal. Animals in either the intact or transected and exercised groups exhibit significantly more hindlimb activity (active and passive), which would activate proprioceptive neurons more frequently. Since it is known that pyridoxine’s effects are mediated through metabolic mechanisms (Krinke and Fitzgerald, 1988), reduced hindlimb activity after transection with no exercise might spare some proprioceptive neurons from metabolic induced lesions.
Transection paired with pyridoxine treatment resulted in a reduction of the H-reflex depression, especially in animals undergoing passive exercise. The fact that exercise failed to reduce the H-reflex response during 10Hz stimulation in injured animals treated with pyridoxine indicates that substantial large afferent input is necessary to restore inhibition of the H-reflex following loss of descending inputs. The H-reflex was absent in intact Pyr animals but recovered in transected animals treated with pyridoxine. Indeed the loss of descending inhibition following SCI and the fact that fewer fibers were affected by the treatment after transection must be taken into consideration, but does not explain the high Hmax/Mmax ratio. There was no difference in the Hmax/Mmax ratio of pyridoxine treated versus saline treated transected animals (Fig 4B). Although the number of large afferents decreased, about 50 % of the motor units were activated. The remaining unaffected sensory fibers may have compensated by increasing sprouting and synapse formation or by increasing synaptic efficiency. We also noticed that the stimulation necessary to get a maximal H-reflex was systematically very low in pyridoxine-treated transected animals (data not shown). It appears that a small stimulation level can activate a larger number of motoneurons via a smaller number of Group Ia sensory afferent fibers in pyridoxine-treated transected animals. This observation coincides with our observation of a reduced depression of the H-reflex at 10Hz stimulation, a reduction possibly due to a combination of more sprouting and less local inhibition. Pyridoxine must also affect Ib and II proprioceptive fibers which have a significant role in the stretch reflex. In indirect ways, spinal cord interneurons influenced by Group I and II afferents and involved in local inhibition might be affected.
This hypothetical sprouting or increase in synaptic efficiency following pyridoxine treatment in transected rats was not observed in intact animals. It may explain why sensory neuropathy disorders in humans are usually not associated with hyper-reflexia, as only a few cases of hereditary sensory neuropathy with spastic paraplegia have been reported (Kherbaoui-Redouani, et al., 2004, Mostacciuolo, et al., 2000).
In conclusion, pyridoxine-induced sensory neuropathy blocks the effects of exercise in rats with SCI by limiting the recovery in the low frequency depression of the H-reflex. Furthermore, the pyridoxine treatment seems to enhance the transection induced H-reflex hyper-reflexia, as indicated by the elevated Hmax/Mmax ratio (higher than in intact animals in spite of proprioceptive neurophaphy) and a decrease in the depression of this reflex with repeated stimulation. These results suggest a compensatory sprouting of the large sensory fibers after SCI and reduced local inhibition in the spinal cord. The local inhibition involved in the exercise-induced normalization of H-reflexes is dependent on some Group II and/or other large afferents which may also be affected by the pyridoxine treatment. Anatomical changes in the spinal cord should be evaluated with staining and nerve tracing to confirm the significance of a sprouting hypothesis related to changes in exercise dependent plasticity.
Acknowledgments
This work was supported by National Institute of Health (NIH) grant R01 NS055976. Dr. Ollivier-Lanvin was supported by NINDS Training Grant NIH NS007440. We gratefully acknowledge Kassi N. Miller for postoperative care and bicycle training of animals in this study, Theresa Connors for sectioning of dorsal root material, Dr Jonathon Nissanov for the use and assistance of MASA, Dr Marie-Pascale Côté for comments on the manuscript and Dr Nicholas Au-Yong for technical support in electrophysiological data recording.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- 2.Chin CL, Wehrli FW, Fan Y, Hwang SN, Schwartz ED, Nissanov J, Hackney DB. Assessment of axonal fiber tract architecture in excised rat spinal cord by localized NMR q-space imaging: simulations and experimental studies. Magn Reson Med. 2004;52:733–740. doi: 10.1002/mrm.20223. [DOI] [PubMed] [Google Scholar]
- 3.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helgren ME, Cliffer KD, Torrento K, Cavnor C, Curtis R, DiStefano PS, Wiegand SJ, Lindsay RM. Neurotrophin-3 administration attenuates deficits of pyridoxine-induced large-fiber sensory neuropathy. J Neurosci. 1997;17:372–382. doi: 10.1523/JNEUROSCI.17-01-00372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho SM, Waite PM. Effects of different anesthetics on the paired-pulse depression of the h reflex in adult rat. Exp Neurol. 2002;177:494–502. doi: 10.1006/exnr.2002.8013. [DOI] [PubMed] [Google Scholar]
- 6.Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson CA. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve. 1999;22:846–856. doi: 10.1002/(sici)1097-4598(199907)22:7<846::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Hunter J, Ashby P. Secondary changes in segmental neurons below a spinal cord lesion in man. Arch Phys Med Rehabil. 1984;65:702–705. [PubMed] [Google Scholar]
- 8.Kherbaoui-Redouani L, Ploton D, Abely M, Bednarek N, Stourbe A, Sabouraud P, Motte J. Hereditary sensory neuropathy with spastic paraplegia. Eur J Paediatr Neurol. 2004;8:95–99. doi: 10.1016/j.ejpn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, Pait TG, Garcia-Rill E. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med. 2005;28:241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- 10.Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171:1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Krinke GJ, Fitzgerald RE. The pattern of pyridoxine-induced lesion: difference between the high and the low toxic level. Toxicology. 1988;49:171–178. doi: 10.1016/0300-483x(88)90190-4. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- 13.Little JW, Ditunno JF, Jr, Stiens SA, Harris RM. Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. Arch Phys Med Rehabil. 1999;80:587–599. doi: 10.1016/s0003-9993(99)90204-6. [DOI] [PubMed] [Google Scholar]
- 14.Lodge D, Anis NA. Effects of ketamine and three other anaesthetics on spinal reflexes and inhibitions in the cat. Br J Anaesth. 1984;56:1143–1151. doi: 10.1093/bja/56.10.1143. [DOI] [PubMed] [Google Scholar]
- 15.Magladery JW, Mc DD., Jr Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull Johns Hopkins Hosp. 1950;86:265–290. [PubMed] [Google Scholar]
- 16.Magladery JW, Porter WE, Park AM, Teasdall RD. Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp. 1951;88:499–519. [PubMed] [Google Scholar]
- 17.Malmsten J. Time course of segmental reflex changes after chronic spinal cord hemisection in the rat. Acta Physiol Scand. 1983;119:435–443. doi: 10.1111/j.1748-1716.1983.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 18.Mostacciuolo ML, Rampoldi L, Righetti E, Vazza G, Schiavon F, Angelini C. Hereditary spastic paraplegia associated with peripheral neuropathy: a distinct clinical and genetic entity. Neuromuscul Disord. 2000;10:497–502. doi: 10.1016/s0960-8966(00)00128-0. [DOI] [PubMed] [Google Scholar]
- 19.Murray M, Goldberger ME. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J Comp Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity--from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–180. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 21.Perry TA, Weerasuriya A, Mouton PR, Holloway HW, Greig NH. Pyridoxine-induced toxicity in rats: a stereological quantification of the sensory neuropathy. Exp Neurol. 2004;190:133–144. doi: 10.1016/j.expneurol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Phadke CP, Wu SS, Thompson FJ, Behrman AL. Comparison of soleus H-reflex modulation after incomplete spinal cord injury in 2 walking environments: treadmill with body weight support and overground. Arch Phys Med Rehabil. 2007;88:1606–1613. doi: 10.1016/j.apmr.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- 24.Rutz S, Dietz V, Curt A. Diagnostic and prognostic value of compound motor action potential of lower limbs in acute paraplegic patients. Spinal Cord. 2000;38:203–210. doi: 10.1038/sj.sc.3100979. [DOI] [PubMed] [Google Scholar]
- 25.Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz ED, Cooper ET, Fan Y, Jawad AF, Chin CL, Nissanov J, Hackney DB. MRI diffusion coefficients in spinal cord correlate with axon morphometry. Neuroreport. 2005;16:73–76. doi: 10.1097/00001756-200501190-00017. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro S. Neurotransmission by neurons that use serotonin, noradrenaline, glutamate, glycine, and gamma-aminobutyric acid in the normal and injured spinal cord. Neurosurgery. 1997;40:168–176. doi: 10.1097/00006123-199701000-00037. discussion 177. [DOI] [PubMed] [Google Scholar]
- 28.Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- 29.Tandrup T. Unbiased estimates of number and size of rat dorsal root ganglion cells in studies of structure and cell survival. J Neurocytol. 2004;33:173–192. doi: 10.1023/b:neur.0000030693.91881.53. [DOI] [PubMed] [Google Scholar]
- 30.Tang AH, Schroeder LA. Spinal-cord depressant effects of ketamine and etoxadrol in the cat and the rat. Anesthesiology. 1973;39:37–43. doi: 10.1097/00000542-197307000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Taylor S, Ashby P, Verrier M. Neurophysiological changes following traumatic spinal lesions in man. J Neurol Neurosurg Psychiatry. 1984;47:1102–1108. doi: 10.1136/jnnp.47.10.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68:1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- 33.Vleggeert-Lankamp CL, de Ruiter GC, Wolfs JF, Pego AP, Feirabend HK, Lakke EA, Malessy MJ. Type grouping in skeletal muscles after experimental reinnervation: another explanation. Eur J Neurosci. 2005;21:1249–1256. doi: 10.1111/j.1460-9568.2005.03954.x. [DOI] [PubMed] [Google Scholar]
- 34.Yates C, Charlesworth A, Allen SR, Reese NB, Skinner RD, Garcia-Rill E. The onset of hyperreflexia in the rat following complete spinal cord transection. Spinal Cord. 2008;46:798–803. doi: 10.1038/sc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]


