Abstract
Simultaneous and sequential transmission of multiple parasites, and their resultant overlapping chronic infections, are facts of life in many underdeveloped rural areas. These represent significant but often poorly-measured health and economic burdens for affected populations. For example, the chronic inflammatory process associated with long-term schistosomiasis contributes to anaemia and undernutrition, which, in turn, can lead to growth stunting, poor school performance, poor work productivity, and continued poverty. To date, most national and international programs aimed at parasite control have not considered the varied economic and ecological factors underlying multi-parasite transmission, but some are beginning to provide a coordinated approach to control. In addition, interest is emerging in new studies for the re-evaluation and recalibration of the health burden of helminthic parasite infection. Their results should highlight the strong potential of integrated parasite control in efforts for poverty reduction.
Keywords: Schistosomiasis, complications, cost of illness, poverty, economics, prevention and control
INTRODUCTION
The problem of helminthic parasitism has not gone away. Fortunately, the issue is gaining renewed prominence in the discussions public health policy, a phenomenon that is based on several factors: First, there is increasing appreciation of the health and social burden of long-term chronic infections (Guerrant et al., 2002, Reidpath et al., 2003, King et al., 2005); second, newer, more sensitive diagnostics indicate that concurrent polyparasitism is much more common than we previously thought (Kasehagen et al., 2006, Raso et al., 2006b); and third, new, inexpensive approaches are making parasite treatment and transmission control increasingly more accessible (Molyneux et al., 2005, Fenwick, 2006, Ottesen, 2006).
Until recently, the conventional wisdom on helminth infections was that `light worm burdens remain asymptomatic'—implying (erroneously) that they do not provoke disease nor do they specifically require medical care (Warren, 1982, Gryseels, 1989). However, recent studies on the immunopathology of parasite infection and its chronic disease formation (Wamachi et al., 2004, Coutinho et al., 2005, Leenstra et al., 2006, Coutinho et al., 2007), indicate that it is the presence, as well as the intensity of infection, that drives morbidity due to chronic parasites such as Schistosoma spp. (King et al., 2006). Under-recognized, `subtle' morbidities such as caloric malnutrition, growth stunting,, anaemia, and poor school performance are all significant correlates of both helminthic and protozoan parasitic infections (Guyatt, 2000, Coutinho et al., 2005, Ezeamama et al., 2005, King et al., 2005, Fernando et al., 2006, Leenstra et al., 2006).
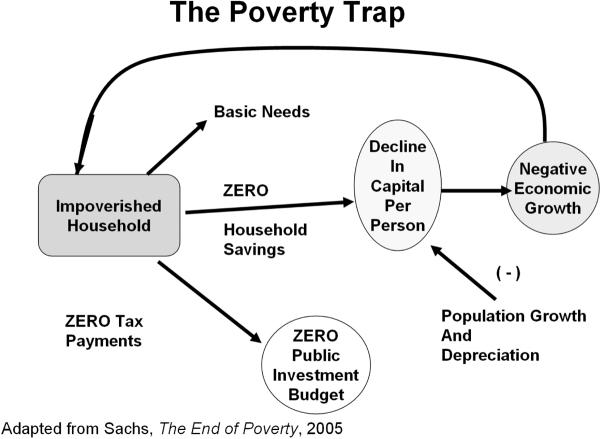
Internationally, concern is developing that combined health effects of multiple concurrent parasite infections are the source, as well as the effect, of poverty (Fig.1) (Sachs, 2005, Engels and Savioli, 2006). Moreover, recent immunology research indicates that chronic parasitic infections can impair protective responses against many unrelated acute bacterial and viral infections, including impaired responses to childhood vaccines (Malhotra et al., 1999, Labeaud et al., 2009) and increased risk of mother-to-child HIV transmission (Gallagher et al., 2005, Secor, 2006). Thus, the beneficial effects of the existing major international programs for HIV prevention and for expanded vaccination coverage are jeopardized by the existence of all-pervasive parasite burden in targeted areas. In sum, it is time to critically re-evaluate the aggregate health impact of chronic helminthic infections, and, in particular, their link to poverty formation.
Figure 1.
Proposed vicious cycle of schistosomiasis in the presence of poverty. Left arrow-- Poverty reduces water use options and increases risk of infection, while also influencing personal adaptation and coping for disease syndromes caused by infection. Right arrow-- Increased disability, related to the impact of chronic and recurrent infection, reduces productivity and perpetuates poverty.
THE POLICY CHALLENGE: DECISIONS BASED ON INACCURATE OR INCOMPLETE INFORMATION ON PREVALENCE
Although most experts are proponents of evidence-based decision-making, until recently, our policies regarding helminth control have often been based on flawed information about infection prevalence, particularly in countries where resources for gathering vital statistics are severely limited (Brooker et al., 2000, van der Werf et al., 2003, Steinmann et al., 2006). Because of misclassification, there has also been continuing debate about how much morbidity can be attributed to any single parasite infection, given the complex reality that parasitic infections and their related disease syndromes frequently overlap in the most seriously affected areas (Murray and Lopez, 1996, Chan, 1997, Guyatt, 2000). The very common syndrome of combined schistosomiasis and soil-transmitted helminth (STH) infection has only begun to be studied in a detailed fashion for its potential synergistic impact (Ezeamama et al., 2008).
Many of our `gold-standard' field diagnostic tests are too insensitive for such research. That is, the standard tests routinely misdiagnose as `uninfected' those individuals who actually carry light infections (Wilson et al., 2006). In the case of schistosomiasis, the Kato-Katz stool test used to detect and quantify intensity of intestinal schistosome infections is only 40% – 60% sensitive when performed on a single stool specimen (de Vlas et al., 1993, Carabin et al., 2005). While this microscopic exam may variously detect eggs of hookworm, Ascaris, Trichuris, and other intestinal worms, it is not particularly sensitive for detection of hookworm, and will often miss the other STH species if their infection intensities happen to be light. As a result, this standard field screening approach will significantly misclassify (by underdiagnosis) the clinical burden of single and mixed parasite infection.
Inadequate testing for Schistosoma or STH results in misclassification bias that substantially reduces our power to detect numerically small, but clinically relevant infection-related differences in health outcomes, including anaemia (reductions in haemoglobin level), or stunting and chronic undernutrition (age-related height and weight deficits). Misdiagnosis, when combined with co-morbid conditions that are competing causes of disease (e.g., hookworm or malaria), effectively limits our appreciation of both the individual and the combined health impacts of common human parasitic infections.
For example, in 2007, the World Health Organization estimated 235 million cases of schistosomiasis worldwide, with 732 million people at risk for infection in known transmission areas (WHO, 2009). These often-quoted statistics are based on our standard but admittedly insensitive methods of field testing (Savioli et al., 1990, de Vlas et al., 1993, Brooker et al., 2000, Chitsulo et al., 2000, Carabin et al., 2005), and therefore can only represent floor estimates for active and potential cases. If these WHO values are adjusted for the probable 40–60% of missed diagnoses (de Vlas et al., 1993, Carabin et al., 2005, Wilson et al., 2006), the true number of active Schistosoma infections in 2007 was more likely between 391 and 587 million people worldwide.
At the same time, there has been a significant underestimation of the disability caused by schistosomiasis (King et al., 2005). Schistosomiasis causes both acute granulomatous injury as well as cumulative, long-lasting fibrotic injury to the human host's organs (Smith and Christie, 1986). The net effect is that even after active infection is over, significant disease and disability still remain (Giboda and Bergquist, 1999). It is appropriate, therefore, to consider all past and present Schistosoma infections as part of the ongoing disease burden of schistosomiasis (Jia et al., 2007). If we consider the likelihood that 80–90% of people at risk (i.e., those residing in Schistosoma transmission areas) will experience infection during childhood or early adulthood, then 80–90% of the 732 million at risk, or 586 – 659 million people are currently living with some form of the disease schistosomiasis. In effect, there are 2 to 3 times more people living with disease and disability caused by schistosomiasis than accounted for in previous disease burden estimates.
INACCURATE ESTIMATES OF SCHISTOSOMIASIS-RELATED DISABILITY
Past efforts to quantify the global burden of disease caused by schistosomiasis (Murray and Lopez, 1996, Michaud et al., 2004) have focused or on official estimates of incident or prevalent cases of active infection, and have neglected the life-time duration of chronic sequelae of infection (Giboda and Bergquist, 1999). In many respects, Schistosoma infection can be seen as an acute communicable disease that transitions into a chronic, non-communicable disease in later life. Thus, Schistotoma infection could rightly be viewed as two health risks—as the immediate and concurrent cause of disease due to acute inflammation, and the dominant risk factor for non-communicable morbidity and disability in later adult life.
Unfortunately, previous efforts at quantifying the Schistosoma-related global burden of disease have focused primarily on schistosomiasis-specific mortality during active infection, and the more severe, but `pathognomonic' end-stage outcomes of Schistosoma infection in later life (Murray and Lopez, 1996, van der Werf et al., 2003, Michaud et al., 2004). Furthermore, because of difficulties in determining attributable risk, they have intentionally ignored other recognizable (but more clinically subtle) sequelae of infection (King et al., 2005).
The currently employed schistosomiasis disability-adjusted life-year (DALY) estimates of the WHO/World Bank Global Burden of Disease (GBD) program were developed using a panel-based Person-Trade-Off method to assign a token 0.5% disability for `average' schistosomiasis. Subsequent calculations based on estimated prevalence and duration of disease resulted in an estimated 1.7 million DALYs lost due to Schistosoma infection in 2004 (WHO Disease and injury regional estimates, http://www.who.int/healthinfo/global_burden_disease/estimates_regional/en/index.htm)
Today, using more realistic estimates of i) prevalence (see above section), ii) disability due to schistosomiasis (e.g., 2–15% (King et al., 2005), 10–19% (Finkelstein et al., 2008), 9–25% (Jia et al., 2007), or up to 2.4% (Michaud et al., 2004)), and iii) new knowledge about the early onset of infection (median age = 3 to 4 yr, (Stothard and Gabrielli, 2007) and life-time persistence of irreversible components of Schistosoma-related disability (growth stunting, cognitive impairment, lost years of schooling (King et al., 2005, Giboda and Bergquist, 1999), the schistosomiasis-related disease burden can be recalibrated (using calculations based on the standard GBD DALY formulae, including age-weighting and 3% time discounting (Murray and Lopez, 1996)). Not surprisingly, a recalculation (using a conservative estimate of disability weight (2%), with an estimated 623–701 million 2004 cases affected from age 4 (median age of onset) throughout an expected 55 year developing country lifespan), yields a significantly much larger, but probably more realistic floor estimate of 13 to 15 million DALYs lost due to schistosomiasis worldwide in 2004. If the average schistosomiasis disability weight is taken as 5%, the DALY values are 25–28 million.
Much of disease due to schistosomiasis occurs several decades after the first incidence of Schistosoma infection in early childhood. If we remove time-preference discounting of future events from the standard DALY calculations, the present day values for current schistosomiasis burden are 24–29 million DALYs using a 2% average disability, and 49–56 million DALYs for a higher 5% estimate of disability. Although these values may seem remarkably high compared to previous DALY valuations for schistosomiasis (cf. the GBD estimates of 35 million DALYs lost to malaria, a more frequently lethal condition), these recalculated values for schistosomiasis primarily reflect inclusion of the more realistic assessment of infection-associated disability, and the enduring impact of childhood Schistosoma infection throughout later adult life. This noteworthy increase in DALY estimates also denotes the inadequate job done by the 1996 GBD program in assessing the impact of parasitic diseases on individual health, and in gauging the magnitude and duration of infection-related chronic disease on a global scale (Bustinduy and King, 2009).
As Gwatkin, et al., (1999) have pointed out, disability due to communicable diseases remains the major disease burden in less-developed countries, and furthermore, this infection-related burden is fully preventable, and so continues to deserve priority in the global health agenda. In this regard, the recent policy trend to prioritize `non-communicable' over communicable diseases in developing countries (Jamison et al., 2006) is clearly mistaken—given the very large unacknowledged burden of infection-related chronic disease, it is premature to focus on what, in essence, are diseases of the developed world, while discounting and ignoring the existing major health burdens of developing countries. The shift in focus to non-communicable diseases, inspired by the plainly flawed DALY estimates for parasitic infections in the 1990s GBD program, appears to be a direct result of the GBD program's serious underestimation of perennial disability caused by non-lethal infectious diseases in poorer countries (King and Bertino, 2008).
IMPLICATIONS FOR THE CURRENT RESEARCH AGENDA
Although subjectively, it is difficult to believe that any parasitic infection can be consonant with good health (Guyatt, 2000), in past field surveys it has been difficult to clearly demonstrate objective findings of parasite-associated morbidity, disease, or economic impact (Gryseels, 1989, Tanner, 1989, Guyatt, 2000). In fact, many the previous field surveys that are cited in policymaking were statistically underpowered in terms of sample size for the purpose of measuring numerically small, but clinically significant effects in nutrition, work performance, and anaemia outcomes. Nonetheless, the failure to show a statistically significant effect is not the same as the absence of a significant effect.
What disease associations might we expect to see in large-scale studies? In a recent meta-analysis of 135 available research reports on disability-related outcomes in schistosomiasis it was observed that, summing across all studies, schistosomiasis is significantly associated with symptoms of diarrhea, pain, and fatigue, as well as the objective findings of hemoglobin deficit, undernutrition, and reduced exercise tolerance (King et al., 2005). Such findings, along with ongoing health burden re-assessments for hookworm and filariasis (Engels and Savioli, 2006), indicate a need to carefully re-examine the impact of single and multiple parasitic infections on all human spheres of performance.
As an example of chronic parasitic worm infection, current evidence suggests that schistosomiasis, although rarely lethal, has significant impact on multiple dimensions of human performance both during childhood and later adult life (Jia et al., 2007, King and Dangerfield-Cha, 2008). Studies of physical and intellectual function indicate significant schistosomiasis-associated reductions in physical fitness and spontaneous activity among children (Kvalsvig, 1981, Latham et al., 1990), and reduced `bonus' earnings from manual labor among adults (Fenwick and Figenschou, 1972, Parker, 1992). Linear growth and nutritional is impaired, resulting in stunting and underweight status among infected children (Stephenson et al., 1989, Parraga et al., 1996, Assis et al., 2004, Coutinho et al., 2006). Short stature, in particular, has been repeatedly associated with lower earning capacity in manual and other labor, even after adjusting for school attainment (Strauss and Thomas, 1998, Florencio et al., 2008). School attendance has been affected in areas of high Schistosoma transmission (de Clercq et al., 1998), and poor performance on standardized intelligence and achievement tests has also been associated with schistosomiasis, even after adjustment for other concurrent parasitic infections (Nazel et al., 1999, Nokes et al., 1999, Jukes et al., 2002, Ezeamama et al., 2005). Symptoms of chronic schistosomiasis persist into adulthood, and include pain, diarrhea, dypareunia, and dysuria. The most severe, late consequences of Schistosoma infection are rare (< 1%), but include significant recurrent GI bleeding, renal dysfunction, bladder outlet obstruction, bladder cancer, painful intercourse and secondary infertility or loss of fecundity (Ndhlovu et al., 2007, Kjetland et al., 2008).
While these latter, more directly-related, `classic' forms of disease may seem rare and consequently of negligible public health impact, it is the less well-defined, negative impact of Schistosoma infection on physical and intellectual performance that probably has the most telling effect on the human capital of endemic communities. Indirect effects (`externalities') of infection, resulting in poor school performance and limited job performance, doubtless hurt the overall development of local economies, and reversing these effects through mass treatment may provide the greatest utility in terms of economic impact in affected areas (Miguel and Kremer, 2004).
In subsistence economies, it is not true that persons without cash income are merely `surplus labor'. In such resource-limited settings, every family member has to work in the non-cash sector in order to continue to survive (King and Bertino, 2008). Unfortunately, there may be a trade-off between such non-cash `earning' versus schooling that is not readily captured in standard economic analyses (Jacoby and Skoufias, 1997). Similarly, losses in productivity, reflected in reduced food production, are not readily captured in the regional and national balance sheets if the goods do not flow through the market economy. As a result the 2–24% disability that is associated with schistosomiasis (as taken from the patient's perspective) is easily missed in routine economic assessments.
For this reason, evaluation of loss of human capital due to parasitic diseases may be the more appropriate metric of disability in poverty settings. In addition, the human capital approach is more consonant with the WHO definition of health as given in the Declaration of Human Rights1 (WHO, 1946). The developmental transition from subsistence to cash economies requires both intellectual and physical skills that favor higher productivity (Collier, 2007). If these are impaired by chronic infection, it is unlikely that the individual, his or her family, or the local community will be able to jump this divide. The impact of poor health on individual-level and community-level poverty and on individual and household cash and non-cash earnings are most fitting topics for the current research agenda, and should have priority among developmental and health policy agencies. In particular, the potentially synergistic effects of combined anti-parasite control (e.g., Preventive Chemotherapy (WHO, 2006)) need to be fully evaluated.
Based on the analysis of currently available evidence, newer studies will need to involve larger cohorts, more sophisticated testing, and multi-level outcomes analysis (Raso et al., 2006a, Brooker, 2007). New, more sensitive diagnostic techniques will need to refine estimates of infection prevalence (McNamara et al., 2006, Wilson et al., 2006), which will, in turn, significantly alter our measures of attributable pathology due to chronic parasitic infection. This new evidence will undoubtedly lead to a re-evaluation in thinking about the utility of preventive vs. curative strategies for parasitic diseases. It is encouraging to see that, in recent years, studies of sufficient size and complexity are now examining the interacting roles of individual and household socioeconomic status, nutritional intake, and co-existing parasitic infections in risk for such disability-related health outcomes (Parraga et al., 1996, Assis et al., 1998, Bethony et al., 2001).
THE CHALLENGE OF POLYPARASITISM AND POVERTY
Although the empiric phenomenon of the `wormy village' has long been recognized (Ashford et al., 1993), advances in diagnostic technology are now revealing that in endemic areas overlapping chronic parasitic infection with Schistosoma spp. (Wilson et al., 2006), Plasmodia spp. (McNamara et al., 2006), and filaria (Michael et al., 2001) is substantially more common than previously thought.
Where multiple causative pathogens co-exist within the same person, how do we attribute the morbidity-related outcomes? This is a difficult problem. One operational approach is to approach polyparasitism as a clinical syndrome per se, screen communities for all types of helminths, and provide combination treatment for all individuals, assuming that chances of exposure and infection are inevitably high for all local residents (WHO, 2006). In the near future, we expect there will be a better scientific appreciation of the attributable morbidity due to each co-infecting pathogen, and the possible synergistic interaction of multiple infections (Ezeamama et al., 2008), such that control efforts may be most appropriately linked and then retargeted as the individual parasites' prevalences decline over time.
REASSESS THE TOTAL BURDEN OF CHRONIC PARASITIC INFECTIONS
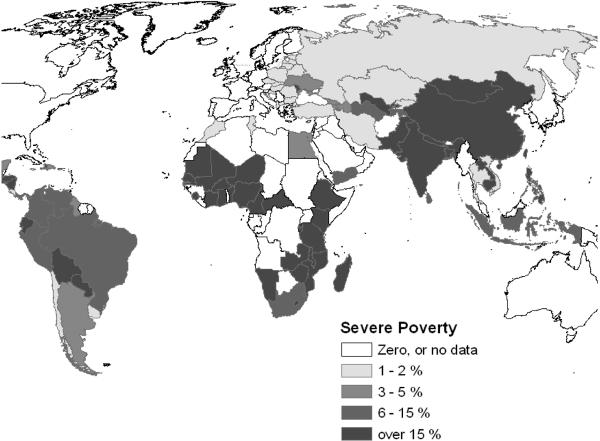
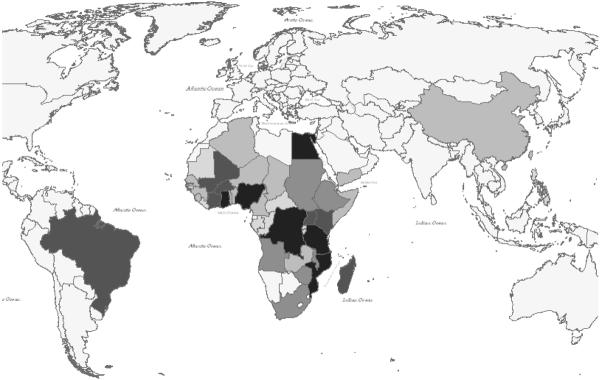
Why should we pay attention to chronic parasitic infections, if they are mostly `minimally' disabling and non-lethal? The answer comes from the typical context of infection, i.e., that of severe rural poverty. Although our Global Burden of Disease assessments have gone to great lengths to remove location or social context from the disability estimates for all diseases (Murray and Lopez, 1996), it is an inescapable fact that small deficits in performance status have a strong, asymmetric leverage on household productivity in the face of severe poverty. In the setting of the `poverty trap' (Fig. 2) where the household, on average, must invest its entire human and physical capital each year simply to break even and survive (Sachs, 2005), a 2–5% disability can have a telling effect on income and food production. This non-linear effect is not unique to developing countries. It was eloquently described among impoverished rural tenant farmers in the U.S. over 70 years ago (Agee and Evans, 1941). This aspect of disability means that standard economic models of disease burden do not readily apply in the settings where most parasitic disease transmission occurs. As has been previously demonstrated for malaria (Sachs, 2005), there is a strong overlap between the worldwide distribution of extreme poverty and the distribution of schistosomiasis (Fig. 3) and most other chronic/recurrent helminthic diseases.
Figure 2.
Features of the rural poverty trap, in which all of the household's capital must be invested each year in order to survive. On average each year, the family just breaks even. Because of continued depreciation of physical capital (tools, soil quality, etc.) and increasing family numbers, per capita income tends to fall each year.
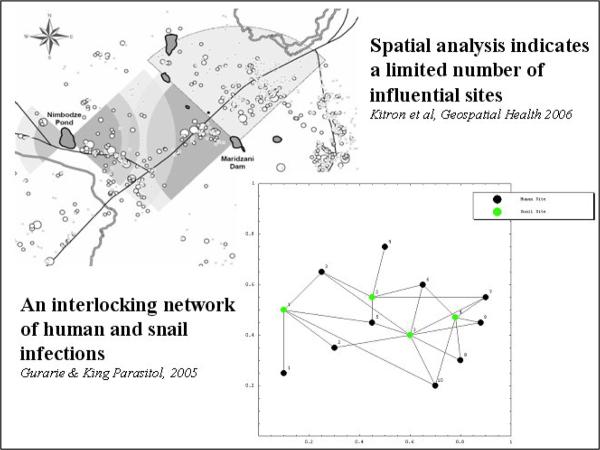
Field experience in Kenya (Satayathum et al., 2006), and recent network simulation studies (Gurarie and King, 2005) indicate that parasite transmission is often unaffected by standard drug-based treatment programs that do not account for embedded village-level factors (heterogeneities in distribution of infection) and intervillage links at shared transmission foci. Both abiotic and biotic landscape features (Kariuki et al., 2004, Clennon et al., 2006a), along with human movement and social factors associated with water use (White et al., 1972, el Kholy et al., 1989), appear to contribute to the evolution of the complex pattern of infection prevalence in these communities (Fig.4). We have also examined the impact of individual and household wealth factors on exposure to S. haematobium and among rural villagers (Van Dyke, 2006). Results indicate that low SES may be associated with contact with fewer transmission sites, but a significantly higher age-adjusted risk of acquiring infection because of restrictions in choice among water sources.
Figure 4.
Environmental features and human behaviour strongly tie Schistosoma transmission to certain locales, and embed the disease caused by infection into the pattern of rural life.
IMPLICATIONS FOR CONTROL-A NEW FOCUS ON TRANSMISSION
Although current large-scale drug treatment can significantly reduce schistosome infectious burden (measured as egg output) in participating communities, (King et al., 1988), parasite transmission may, nevertheless, continue unaffected because of several different factors (Muchiri et al., 1996, Satayathum et al., 2006). Therefore, alternative and complementary strategies (Table1) need to be considered.
Table 1.
Pros and Cons of Different Schistosomiasis Control Strategies
| Intervention | Advantages | Disadvantages | Relative Cost | Impact on Disease |
|---|---|---|---|---|
| Drug treatment: | ||||
| Mass drug delivery | High initial uptake. Wider advocacy and community mobilization. Rapid impact | Reduced adherence over time. Incomplete cure of heavy infections. Transmission continues unabated in worst areas. Risk of drug resistance over time. | +++ | Partial control of early and late morbidities. Indefinite need for re-treatment. |
| Age-targeted drug delivery | Structured framework for delivery where community census data are limited. Rapid impact | Often poor coverage for children who are not in school. Incomplete cures. Transmission continues. Risk of drug resistance over time. | ++ | Partial control of early and late morbidities. Indefinite need for re-treatment. |
| Symptomatic treatment | Limited cost. Focus on controlling or preventing late morbidity | Symptom driven. In practice, limited access and inadequate diagnostics mean that treatment is rarely given. | + | Late outcomes improved but not prevented. These are often irreversible. |
| Health Education: | Important advocacy for control, shift in behavioural risk. Collateral benefits for other disease prevention | Time and teacher dependent. Cultural context may determine acceptance. | + | Health education frequently has limited impact when used alone due to lack of choices in water use. |
| Snail Control: | ||||
| Areawide | Limited need for community mobilization | Cost. Cross-species toxicity. Requires high tech personnel input. | +++++ | Gradual reductions in human prevalence and disease |
| Focal | Lower cost and side-effects than areawide | May miss important transmission points. | +++ | Gradual reductions in human prevalence and disease |
| Habitat modification | Greater initial costs. Need for land management planning and community mobilization | Indirect effects of local changes in ecology | ++++ | Single interventions may have only minimal impact on area transmission |
| Water supply and Sanitation: | ||||
| Latrines/sanitation | Impact on multiple infections | Requires significant changes in human behaviour | +++ | Performed alone, may have only limited impact on schistosomiasis due its to leveraged, networked transmission |
| Basic well or piped water | Impact on multiple infections | Technology dependent, uptake not universal | +++ | Can reduce risk of transmission and slowly reduce morbidity in some areas |
| Water plus sanitation | Impact on multiple infections | (As above) | ++++ | Can reduce risk of transmission and slowly reduce morbidity in most areas |
| Water/Sanitation plus alternative laundry, bathing, recreation | Impact on multiple infections. | Requires futher significant changes in many daily activities and human behaviour | +++++ | Can eliminate transmission and slowly eliminate morbidity in most areas. The definitive solution to transmission if no zoonotic reservoir exists. |
Schistosome transmission is often a periodic, inhomogeneous process in which any single untreated individual can contaminate a water site and convert it to a high risk transmission zone for a period of several months. In practice, drug-based control programs experience a high rate of absenteeism, (Hussein et al., 1996) and any persistent, untreated infectious burden in the community-at-large can maintain local contamination of water bodies and ensure perpetuation of transmission (King et al., 1991, Talaat et al., 1999). In effect, the process of local transmission is often saturated, and egg contamination must be significantly reduced before it declines (Wang et al., 2009). A single, brief exposure to cercariae-infested water is sufficient to effect transmission (CDC, 1983, Vercruysse et al., 1994), even where the number of shedding snails is low (Mubila and Rollinson, 2002). Additionally, networked usage of water sites across villages serves to entrench local transmission risk when control implementation is staggered by location (i.e., where there are lags in treatment over time and space (Woolhouse et al., 1998, Gurarie and King, 2005, Liang et al., 2006, Gurarie and Seto, 2009)). Under the pressure of mass treatment, 30–50% of the snails in community water contact sites still remain infected with S. haematobium, as determined in PCR studies (Hamburger et al., 2004). Spatial analysis (Clennon et al., 2004, Clennon et al., 2006b) shows there is a strong relation between proximity to high-risk water bodies (those that support large infected snail populations) and high household-level infection risk.
These data, combined with the experience of other programs (Crossland, 1963, Jordan, 1985, Liang et al., 2006, Steinmann et al., 2006, Gurarie and Seto, 2009), suggest the importance of water-site factors in maintaining transmission risk. They also indicate the potential disease-preventing leverage of transmission control measures when implemented at specific, high-risk locations (Woolhouse et al., 1998). There is no universal prescription for snail treatment or other forms of transmission control, as patterns of exposure may vary significantly from region to region, and among different ecosystems in Africa, Asia, and the Americas. Health education is an extremely important part of advocacy in any program, and will be an essential part of any successful elimination program—in essence, there must be a significant societal change in patterns of water use and sanitation, and these, combined with provision of alternative safe water sites could have a significantly leveraged effect on transmission and have a long-term cumulative impact on local disease burden. However, health education given alone may have only a minimal impact on transmission, due to remaining limitations in choices for safe water use (White et al., 1972, Bruun and Aagaard-Hansen, 2008).
A variety of snail control measures have been proven and are available to implement, but at present, these are rarely used in schistosomiasis control programs due to cost. The approaches to snail control including habitat modification, modification of human water-use, and water-related infrastructure development to provide clean water for all activities (Jordan, 1985). The best studied among these interventions is snail control by mollusciciding. Typically, this technique uses wettable niclosamide powder (Bayluscide), which is a safe and effective, US EPA-approved molluscicide. Experience from the 1950s–1970s in St. Lucia (Jordan, 1985), Japan (Ohmae et al., 2003), China (Utzinger et al., 2005), the Philippines (Ohmae et al., 2003), and Iraq (Baquir, 1974) indicates that schistosomiasis transmission can indeed be interrupted in high-risk communities by implementing significant changes in snail abundance and/or water use. Although the cost of mollusciciding material is presently high due to its limited use, ecological modelling indicates that it can be given in a highly focused manner, taking advantage of the known heterogeneities of transmission (Woolhouse et al., 1997, Woolhouse et al., 1998). Given strongly leveraged effects and economies of scale, as well as a greater appreciation of the health burden of schistosomiasis, snail control, integrated with drug-treatment should prove to be most efficacious in preventing and controlling schistosomiasis infection and disease. Additional, coordinated integration with sanitation efforts and anti-parasite treatment for other parasites will substantially strengthen the local health impact of these efforts.
OUTLOOK FOR THE NEXT DECADE
The link between schistosomiasis and poverty now appears evident, though causation is likely to be bidirectional (Fig. 1). Schistosomiasis caused a disabling state of chronic ill-health that impairs human capital, while local poverty fosters schistosomiasis transmission by enforcing exposure to contaminated water, limiting access to health care resources, and reducing resources to decrease transmission. While some may argue that structural social and governmental factors may be the proximate cause of both schistosomiasis and local poverty, the body of evidence, cited above, points to a direct interaction of parasites and poverty. That said, the complexity of the problem may require a more complex medical, governmental, and eco-social combined intervention (Craig et al., 2008) in order to permanently alter the status quo.
Single interventions, such as annual drug delivery for morbidity control, will not likely alter transmission (Table 1), and will thus be unable to gain the full direct and indirect benefits of schistosomiasis elimination (Miguel and Kremer, 2004). Additional steps taken toward schistosomiasis elimination (water supply, sanitation, health education) will have significant incremental costs, but will likely yield indirect beneficial `side effects' (beyond increases in human capital) that can provide the impetus for a virtuous cycle of local development. A broader consideration of community benefits (including the possible local elimination of schistosomiasis) should make more aggressive levels of intervention economically attractive.
`Integrated pest management' (IPM) is already a mainstay of control for agricultural pathogens and is becoming part of our approach to malaria control; based on new environmental knowledge and analytic systems, IPM lessons can be applied to provide economical human parasite control, even in limited-resource settings. Current large-scale, population-based schistosomiasis treatment programs are a first step to reducing the global burden of Schistosoma-related disease, yet, as we have noted, such programs may not significantly alter parasite transmission in high-risk areas. Consequently, the sustainability of these programs' initial benefits remains in doubt, as recurring low-level reinfection can be associated with persistent morbidity such as anaemia, undernutrition, and diminished performance status (King et al., 2006), and treatment may need to continue for many decades to maintain disease control.
From a public health standpoint, the significant benefits of comprehensive helminth control, including transmission control, need to be reconsidered, and new thought given to more aggressive (and ultimately more affordable) parasite eradication strategies (Sachs, 2005, King et al., 2006). Novel molecular tools for rapid, sensitive transmission monitoring will enable much more informative surveillance of schistosome propagation over extended areas. With this knowledge, effective integration of transmission control can be used to optimize the design of the next generation of schistosomiasis morbidity control and prevention programs, and tailor intervention strategies to specific locales.
In a real sense, the ongoing presence of schistosomiasis in developing communities represents a silent `disability tax' on every local inhabitant. The low-level but persistent daily disability associated with Schistosoma infection means that those who are affected may never reach their full potential for healthy development or productivity (King et al., 2005). In endemic areas, nearly 100% of local residents will become infected at some point during their lives, and will be at risk for schistosomiasis-associated disease. Schistosomiasis is likely to be both a cause and an effect of continuing rural poverty in these areas. Truly effective control will require broad-reaching, multi-decade commitment towards reduction and prevention of Schistosoma transmission (Utzinger et al., 2003). In consonance with the Millennium Development Goals, we see the need for adequate and appropriately managed investment in snail control, safe water supply, sanitation, and health education in order to effectively reduce (and then eliminate) the very significant global burden of disease due to schistosomiasis and other helminthic parasites.
Figure 3a.
Global distribution of severe poverty, measured as proportion of national population earning less that 1 US Dollar per day. Data are from the World Bank for 2000, or the most recent values prior to 2000.
Figure 3b.
Distribution of human schistosomiasis; shading intensity indicates the number of cases per country in 2000, ranging from less than 200,000 (unshaded) to over 10 million cases per country (darkest shading), based on WHO estimates (Chitsulo et al., 2000)
ACKNOWLEDGMENTS
This project was supported in part by National Institutes of Health Research Grant R01 TW008067 funded by the Fogarty International Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
“A state of complete physical, mental and social well-being-- and not merely the absence of disease or infirmity.”
REFERENCES
- Agee J, Evans W. Let Us Now Praise Famous Men: three tenant families. Houghton Mifflin Company; Boston: 1941. [Google Scholar]
- Ashford RW, Craig PS, Oppenheimer SJ. Polyparasitism on the Kenya coast. 2. Spatial heterogeneity in parasite distributions. Ann Trop Med Parasitol. 1993;87:283–93. doi: 10.1080/00034983.1993.11812768. [DOI] [PubMed] [Google Scholar]
- Assis AM, Barreto ML, Prado MS, Reis MG, Parraga IM, Blanton RE. Schistosoma mansoni infection and nutritional status in schoolchildren: a randomized, double-blind trial in northeastern Brazil. Am J Clin Nutr. 1998;68:1247–53. doi: 10.1093/ajcn/68.6.1247. [DOI] [PubMed] [Google Scholar]
- Assis AM, Prado MS, Barreto ML, Reis MG, Conceicao Pinheiro SM, Parraga IM, Blanton RE. Childhood stunting in Northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. Eur J Clin Nutr. 2004;58:1022–9. doi: 10.1038/sj.ejcn.1601926. [DOI] [PubMed] [Google Scholar]
- Baquir H. Letter: Present status of Hor Rajab bilharziasis control project Iraq 15, WHO-TA. Trans R Soc Trop Med Hyg. 1974;68:345. doi: 10.1016/0035-9203(74)90053-4. [DOI] [PubMed] [Google Scholar]
- Bethony J, Williams JT, Kloos H, Blangero J, Alves-Fraga L, Buck G, Michalek A, Williams-Blangero S, Loverde PT, Correa-Oliveira R, Gazzinelli A. Exposure to Schistosoma mansoni infection in a rural area in Brazil. II: household risk factors. Trop Med Int Health. 2001;6:136–45. doi: 10.1046/j.1365-3156.2001.00685.x. [DOI] [PubMed] [Google Scholar]
- Brooker S. Spatial epidemiology of human schistosomiasis in Africa: risk models, transmission dynamics and control. Trans R Soc Trop Med Hyg. 2007;101:1–8. doi: 10.1016/j.trstmh.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Donnelly CA, Guyatt HL. Estimating the number of helminthic infections in the Republic of Cameroon from data on infection prevalence in schoolchildren. Bull World Health Organ. 2000;78:1456–65. [PMC free article] [PubMed] [Google Scholar]
- Bruun B, Aagaard-Hansen J. The Social Context of Schistosomiasis and Its Control. World Health Organization; Geneva: 2008. [Google Scholar]
- Bustinduy AL, King CH. Parasitic Helminths. In: Fratamico Smith, Brogden, editors. Post-Infectious Sequelae and Long-Term Consequences of Infectious Diseases. American Society for Microbiology Press; Washington: 2009. pp. 291–329. [Google Scholar]
- Carabin H, Marshall CM, Joseph L, Riley S, Olveda R, McGarvey ST. Estimating the intensity of infection with Schistosoma japonicum in villagers of Leyte, Philippines. Part I: A Bayesian cumulative logit model. The Schistosomiasis Transmission & Ecology Project (STEP) Am J Trop Med Hyg. 2005;72:745–753. [PubMed] [Google Scholar]
- Centers for Disease Control Schistosomiasis among river rafters -- Ethiopia. MMWR. 1983;32:585–6. [PubMed] [Google Scholar]
- Chan MS. The global burden of intestinal nematode infections: 50 years on. Parasitology Today. 1997;13:438–43. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clennon JA, King CH, Muchiri EM, Kariuki HC, Ouma JH, Mungai P, Kitron U. Spatial patterns of urinary schistosomiasis infection in a highly-endemic area of coastal Kenya. Am J Trop Med Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- Clennon JA, King CH, Muchiri EM, Kitron U. Hydrological modelling of snail dispersal patterns in Msambweni, Kenya and potential resurgence of Schistosoma haematobium transmission. Parasitology. 2006a:1–11. doi: 10.1017/S0031182006001594. [DOI] [PubMed] [Google Scholar]
- Clennon JA, Mungai PL, Muchiri EM, King CH, Kitron U. Spatial and temporal variations in local transmission of Schistosoma haematobium in Msambweni, Kenya. Am J Trop Med Hyg. 2006b;75:1034–41. [PubMed] [Google Scholar]
- Collier P. The Bottom Billion: Why the Poorest Countries Are Failing and What Can Be Done About It. Oxford University Press; New York: 2007. [Google Scholar]
- Coutinho HM, Acosta LP, McGarvey ST, Jarilla B, Jiz M, Pablo A, Su L, Manalo DL, Olveda RM, Kurtis JD, Friedman JF. Nutritional status improves after treatment of Schistosoma japonicum-infected children and adolescents. J Nutr. 2006;136:183–8. doi: 10.1093/jn/136.1.183. [DOI] [PubMed] [Google Scholar]
- Coutinho HM, Acosta LP, Wu HW, McGarvey ST, Su L, Langdon GC, Jiz MA, Jarilla B, Olveda RM, Friedman JF, Kurtis JD. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection. J Infect Dis. 2007;195:288–95. doi: 10.1086/510313. [DOI] [PubMed] [Google Scholar]
- Coutinho HM, McGarvey ST, Acosta LP, Manalo DL, Langdon GC, Leenstra T, Kanzaria HK, Solomon J, Wu H, Olveda RM, Kurtis JD, Friedman JF. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis. 2005;192:528–36. doi: 10.1086/430929. [DOI] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland NO. A large-scale experiment in the control of aquatic snails by the use of molluscicides on a sugar estate in the northern region of Tanganyika. Bull World Health Organ. 1963;29:515–524. [PMC free article] [PubMed] [Google Scholar]
- de Clercq D, Sacko M, Behnke J, Gilbert F, Vercruysse J. The relationship between Schistosoma haematobium infection and school performance and attendance in Bamako, Mali. Ann Trop Med Parasitol. 1998;92:851–8. doi: 10.1080/00034989858899. [DOI] [PubMed] [Google Scholar]
- de Vlas SJ, Gryseels B, van Oortmarssen GJ, Polderman AM, Habbema JD. A pocket chart to estimate true Schistosoma mansoni prevalences. Parasitol Today. 1993;9:305–7. [PubMed] [Google Scholar]
- el Kholy H, Arap Siongok TK, Koech D, Sturrock RF, Houser H, King CH, Mahmoud AA. Effects of borehole wells on water utilization in Schistosoma haematobium endemic communities in Coast Province, Kenya. Am J Trop Med Hyg. 1989;41:212–219. doi: 10.4269/ajtmh.1989.41.212. [DOI] [PubMed] [Google Scholar]
- Engels D, Savioli L. Reconsidering the underestimated burden caused by neglected tropical diseases. Trends Parasitol. 2006;22:363–6. doi: 10.1016/j.pt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, Olveda RM, Kurtis JD, McGarvey ST. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72:540–548. [PMC free article] [PubMed] [Google Scholar]
- Ezeamama AE, McGarvey ST, Acosta LP, Zierler S, Manalo DL, Wu HW, Kurtis JD, Mor V, Olveda RM, Friedman JF. The synergistic effect of concomitant schistosomiasis, hookworm, and Trichuris infections on children's anemia burden. PLoS Negl Trop Dis. 2008;2:e245. doi: 10.1371/journal.pntd.0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick A. New initiatives against Africa's worms. Trans R Soc Trop Med Hyg. 2006;100:200–7. doi: 10.1016/j.trstmh.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Figenschou BH. The effect of Schistosoma mansoni infection of the productivity of cane cutters on a sugar estate in Tanzania. Bull World Health Organ. 1972;47:567–72. [PMC free article] [PubMed] [Google Scholar]
- Fernando D, de Silva D, Carter R, Mendis KN, Wickremasinghe R. A randomized, double-blind, placebo-controlled, clinical trial of the impact of malaria prevention on the educational attainment of school children. Am J Trop Med Hyg. 2006;74:386–93. [PubMed] [Google Scholar]
- Finkelstein JL, Schleinitz M, Carabin H, McGarvey ST. Decision-model estimation of the age-specific disability weight for schistosomiasis japonica. PLoS Negl Trop Dis. 2008;2:e158. doi: 10.1371/journal.pntd.0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florencio TT, Ferreira HS, Cavalcante JC, Assuncao ML, Sawaya AL. Short stature and food habits as determining factors for the low productivity of sugarcane labourers in the State of Alagoas, north-eastern Brazil. Arch Latinoam Nutr. 2008;58:33–9. [PubMed] [Google Scholar]
- Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, Muchiri E, King CL. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. Aids. 2005;19:1849–55. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- Giboda M, Bergquist NR. Post-transmission schistosomiasis. Parasitol Today. 1999;15:307–8. doi: 10.1016/s0169-4758(99)01487-8. [DOI] [PubMed] [Google Scholar]
- Gryseels B. The relevance of schistosomiasis for public health. Trop Med Parasitol. 1989;40:134–42. [PubMed] [Google Scholar]
- Guerrant RL, Kosek M, Lima AA, Lorntz B, Guyatt HL. Updating the DALYs for diarrhoeal disease. Trends Parasitol. 2002;18:191–3. doi: 10.1016/s1471-4922(02)02253-5. [DOI] [PubMed] [Google Scholar]
- Gurarie D, King CH. Heterogeneous model of schistosomiasis transmission and long-term control: the combined influence of spatial variation and age-dependent factors on optimal allocation of drug therapy. Parasitology. 2005;130:49–65. doi: 10.1017/s0031182004006341. [DOI] [PubMed] [Google Scholar]
- Gurarie D, Seto EY. Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities. J R Soc Interface. 2009;6:495–508. doi: 10.1098/rsif.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt H. Do intestinal nematodes affect productivity in adulthood? Parasitol Today. 2000;16:153–8. doi: 10.1016/s0169-4758(99)01634-8. [DOI] [PubMed] [Google Scholar]
- Gwatkin DR, Guillot M, Heuveline P. The burden of disease among the global poor. Lancet. 1999;354:586–9. doi: 10.1016/S0140-6736(99)02108-X. [DOI] [PubMed] [Google Scholar]
- Hamburger J, Hoffman O, Kariuki HC, Muchiri EM, Ouma JH, Koech DK, Sturrock RF, King CH. Large-scale, polymerase chain reaction-based surveillance of Schistosoma haematobium DNA in snails from transmission sites in coastal Kenya: A new tool for studying the dynamics of snail infection. Am J Trop Med Hyg. 2004;1:765–773. [PubMed] [Google Scholar]
- Hussein MH, Talaat M, El-Sayed MK, El-Badawi A, Evans DB. Who misses out with school-based health programmes? a study of schistosomiasis control in Egypt. Trans R Soc Trop Med Hyg. 1996;90:362–65. doi: 10.1016/s0035-9203(96)90506-4. [DOI] [PubMed] [Google Scholar]
- Jacoby HG, Skoufias E. Risk, financial markets, and human capital in a developing country. Review of Economic Studies. 1997;64:311–335. [Google Scholar]
- Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries 2/e. Oxford University Press; New York: 2006. [PubMed] [Google Scholar]
- Jia T-W, Zhou X-N, Wang X-H, Utzinger J, Steinmann P, Wu X-H. Assessment of the age-specific disability weight of chronic schistosomiasis japonica. Bull World Health Organ. 2007;85:458–465. doi: 10.2471/BLT.06.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. Schistosomiasis: The St. Lucia Project. Cambridge University Press; Cambridge: 1985. [Google Scholar]
- Jukes MC, Nokes CA, Alcock KJ, Lambo JK, Kihamia C, Ngorosho N, Mbise A, Lorri W, Yona E, Mwanri L, Baddeley AD, Hall A, Bundy DA. Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–17. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- Kariuki HC, Clennon JA, Brady MS, Kitron U, Sturrock RF, Ouma JH, Ndzovu ST, Mungai P, Hoffman O, Hamburger J, Pellegrini C, Muchiri EM, King CH. Distribution patterns and cercarial shedding of Bulinus nasutus and other snails in the Msambweni area, Coast Province, Kenya. Am J Trop Med Hyg. 2004;70:449–56. [PubMed] [Google Scholar]
- Kasehagen LJ, Mueller I, McNamara DT, Bockarie MJ, Kiniboro B, Rare L, Lorry K, Kastens W, Reeder JC, Kazura JW, Zimmerman PA. Changing patterns of Plasmodium blood-stage infections in the Wosera region of Papua New Guinea monitored by light microscopy and high throughput PCR diagnosis. Am J Trop Med Hyg. 2006;75:588–96. [PMC free article] [PubMed] [Google Scholar]
- King CH, Bertino A-M. Asymmetries of poverty: Why global burden of disease valuations significantly underestimate the burden of neglected tropical diseases. PLoS Negl Trop Dis. 2008;2:e209. doi: 10.1371/journal.pntd.0000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis Chronic Illness. 2008:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, Ouma J, Odiambo O, Bryan PJ, Muruka J, Magak P, Weinert D, Mackay W, Ransohoff D, Houser H, Koech D, Siongok TK, Mahmoud AAF. Chemotherapy-based control of schistosomiasis haematobia. I. Metrifonate versus praziquantel in control of intensity and prevalence of infection. Am J Trop Med Hyg. 1988;39:295–305. doi: 10.4269/ajtmh.1988.39.295. [DOI] [PubMed] [Google Scholar]
- King CH, Muchiri E, Ouma JH, Koech D. Chemotherapy-based control of schistosomiasis haematobia. IV. Impact of repeated annual chemotherapy on prevalence and intensity of Schistosoma haematobium infection in an endemic area of Kenya. Am J Trop Med Hyg. 1991;45:498–508. doi: 10.4269/ajtmh.1991.45.498. [DOI] [PubMed] [Google Scholar]
- King CH, Sturrock RF, Kariuki HC, Hamburger J. Transmission control for schistosomiasis - why it matters now. Trends Parasitol. 2006;22:575–82. doi: 10.1016/j.pt.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kjetland EF, Kurewa EN, Ndhlovu PD, Midzi N, Gwanzura L, Mason PR, Gomo E, Sandvik L, Mduluza T, Friis H, Gundersen SG. Female genital schistosomiasis--a differential diagnosis to sexually transmitted disease: genital itch and vaginal discharge as indicators of genital Schistosoma haematobium morbidity in a cross-sectional study in endemic rural Zimbabwe. Trop Med Int Health. 2008;13:1509–17. doi: 10.1111/j.1365-3156.2008.02161.x. [DOI] [PubMed] [Google Scholar]
- Kvalsvig JD. The effects of schistosomiasis on spontaneous play activity in black schoolchildren in the endemic areas. An ethological study. S Afr Med J. 1981;60:61–4. [PubMed] [Google Scholar]
- Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham MC, Stephenson LS, Kurz KM, Kinoti SN. Metrifonate or praziquantel treatment improves physical fitness and appetite of Kenyan schoolboys with Schistosoma haematobium and hookworm infections. Am J Trop Med Hyg. 1990;43:170–9. doi: 10.4269/ajtmh.1990.43.170. [DOI] [PubMed] [Google Scholar]
- Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines 1. Am J Clin Nutr. 2006;83:371–9. doi: 10.1093/ajcn/83.2.371. [DOI] [PubMed] [Google Scholar]
- Liang S, Yang C, Zhong B, Qiu D. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull World Health Organ. 2006;84:139–44. doi: 10.2471/blt.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, King CL. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–8. [PubMed] [Google Scholar]
- McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg. 2006;74:413–21. [PMC free article] [PubMed] [Google Scholar]
- Michael E, Simonsen PE, Malecela M, Jaoko WG, Pedersen EM, Mukoko D, Rwegoshora RT, Meyrowitsch DW. Transmission intensity and the immunoepidemiology of bancroftian filariasis in East Africa. Parasite Immunol. 2001;23:373–88. doi: 10.1046/j.1365-3024.2001.00398.x. [DOI] [PubMed] [Google Scholar]
- Michaud CM, Gordon WS, Reich MR. The Global Burden of Disease Due to Schistosomiasis. Harvard Center for Population and Development Studies, Harvard School of Public Health; 2004. pp. 1–41. [Google Scholar]
- Miguel E, Kremer M. Worms: Identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72:159–217. [Google Scholar]
- Molyneux DH, Hotez PJ, Fenwick A. “Rapid-impact interventions”: how a policy of integrated control for Africa's neglected tropical diseases could benefit the poor. PLoS Med. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubila L, Rollinson D. Snail-parasite compatibility and prevalence of Schistosoma haematobium on the shores of Lake Kariba, Zambia. Ann Trop Med Parasitol. 2002;96:165–73. doi: 10.1179/000349802125000592. [DOI] [PubMed] [Google Scholar]
- Muchiri EM, Ouma JH, King CH. Dynamics and control of Schistosoma haematobium transmission in Kenya: an overview of the Msambweni Project. Am J Trop Med Hyg. 1996;55:127–134. doi: 10.4269/ajtmh.1996.55.127. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD, editors. The Global Burden of Disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard School of Public Health/World Bank; Cambridge MA: 1996. [Google Scholar]
- Nazel MW, el-Morshedy H, Farghaly A, Shatat H, Barakat R. Schistosoma mansoni infection and cognitive functions of primary school children, in Kafr El Sheikh, Egypt. J Egypt Public Health Assoc. 1999;74:97–119. [PubMed] [Google Scholar]
- Ndhlovu PD, Mduluza T, Kjetland EF, Midzi N, Nyanga L, Gundersen SG, Friis H, Gomo E. Prevalence of urinary schistosomiasis and HIV in females living in a rural community of Zimbabwe: does age matter? Trans R Soc Trop Med Hyg. 2007;101:433–8. doi: 10.1016/j.trstmh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy DA, Olds GR. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60:556–65. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- Ohmae H, Iwanaga Y, Nara T, Matsuda H, Yasuraoka K. Biological characteristics and control of intermediate snail host of Schistosoma japonicum. Parasitol Int. 2003;52:409–17. doi: 10.1016/s1383-5769(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Ottesen EA. Lymphatic filariasis: Treatment, control and elimination. Adv Parasitol. 2006;61:395–441. doi: 10.1016/S0065-308X(05)61010-X. [DOI] [PubMed] [Google Scholar]
- Parker M. Re-assessing disability: the impact of schistosomal infection on daily activities among women in Gezira Province, Sudan. Soc Sci Med. 1992;35:877–90. doi: 10.1016/0277-9536(92)90102-v. [DOI] [PubMed] [Google Scholar]
- Parraga IM, Assis AM, Prado MS, Barreto ML, Reis MG, King CH, Blanton RE. Gender differences in growth of school-aged children with schistosomiasis and geohelminth infection. Am J Trop Med Hyg. 1996;55:150–6. doi: 10.4269/ajtmh.1996.55.150. [DOI] [PubMed] [Google Scholar]
- Raso G, Vounatsou P, Gosoniu L, Tanner M, N'Goran EK, Utzinger J. Risk factors and spatial patterns of hookworm infection among schoolchildren in a rural area of western Cote d'Ivoire. Int J Parasitol. 2006a;36:201–10. doi: 10.1016/j.ijpara.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Raso G, Vounatsou P, Singer BH, N'Goran EK, Tanner M, Utzinger J. An integrated approach for risk profiling and spatial prediction of Schistosoma mansoni-hookworm coinfection. Proc Natl Acad Sci U S A. 2006b;103:6934–9. doi: 10.1073/pnas.0601559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidpath DD, Allotey PA, Kouame A, Cummins RA. Measuring health in a vacuum: examining the disability weight of the DALY. Health Policy Plan. 2003;18:351–6. doi: 10.1093/heapol/czg043. [DOI] [PubMed] [Google Scholar]
- Sachs JD. The End of Poverty. Penguin Press; New York: 2005. [Google Scholar]
- Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: Survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. [PMC free article] [PubMed] [Google Scholar]
- Savioli L, Hatz C, Dixon H, Kisumku UM, Mott KE. Control of morbidity due to Schistosoma haematobium on Pemba Island: egg excretion and hematuria as indicators of infection. Am J Trop Med Hyg. 1990;43:289–95. doi: 10.4269/ajtmh.1990.43.289. [DOI] [PubMed] [Google Scholar]
- Secor WE. Interactions between schistosomiasis and infection with HIV-1. Parasite Immunol. 2006;28:597–603. doi: 10.1111/j.1365-3024.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- Smith JH, Christie JD. The pathobiology of Schistosoma haematobium infection in humans. Hum Pathol. 1986;17:333–45. doi: 10.1016/s0046-8177(86)80456-7. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN. Single dose metrifonate or praziquantel treatment in Kenyan children. II. Effects on growth in relation to Schistosoma haematobium and hookworm egg counts. Am J Trop Med Hyg. 1989;41:445–53. doi: 10.4269/ajtmh.1989.41.445. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Gabrielli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol. 2007;23:83–6. doi: 10.1016/j.pt.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Strauss J, Thomas D. Health, nutrition, and economic development. Journal of Economic Literature. 1998;36:766–817. [Google Scholar]
- Talaat M, Omar M, Evans D. Developing strategies to control schistosomiasis morbidity in nonenrolled school-age children: experience from Egypt. Trop Med Int Health. 1999;4:551–6. doi: 10.1046/j.1365-3156.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- Tanner M. Evaluation of public health impact of schistosomiasis. Trop Med Parasitol. 1989;40:143–8. [PubMed] [Google Scholar]
- Utzinger J, Bergquist R, Xiao SH, Singer BH, Tanner M. Sustainable schistosomiasis control--the way forward. Lancet. 2003;362:1932–4. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–39. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Van Dyke MK. PhD thesis. Department of Epidemiology, School of Public Health; Ann Arbor, University of Michigan: 2006. Epidemiology of Urinary Schistosomiasis in Coastal Kenya: Spatial and Social Patterns; p. 115. [Google Scholar]
- Vercruysse J, Southgate VR, Rollinson D, De Clercq D, Sacko M, De Bont J, Mungomba LM. Studies on transmission and schistosome interactions in Senegal, Mali and Zambia. Trop Geogr Med. 1994;46:220–6. [PubMed] [Google Scholar]
- Wamachi AN, Mayadev JS, Mungai PL, Magak PL, Ouma JH, Magambo JK, Muchiri EM, Koech DK, King CH, King CL. Increased ratio of tumor necrosis factor-alpha to interleukin-10 production is associated with Schistosoma haematobium-induced urinary-tract morbidity. J Infect Dis. 2004;190:2020–30. doi: 10.1086/425579. [DOI] [PubMed] [Google Scholar]
- Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G, Hao Y, Chin DP, Zhou XN. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–8. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- Warren KS. Selective primary health care: strategies for control of disease in the developing world. I. Schistosomiasis. Rev Infect Dis. 1982;4:715–26. doi: 10.1093/clinids/4.3.715. [DOI] [PubMed] [Google Scholar]
- White GF, Bradley DJ, White AU. Drawers of Water: Domestic Water Use in East Africa. University of Chicago Press; Chicago, IL: 1972. [PMC free article] [PubMed] [Google Scholar]
- WHO WHO definition of Health. Preamble to the Constitution of the World Health Organization. Official Records of the World Health Organization. 1946 [Google Scholar]
- WHO . Preventive chemotherapy in human helminthiasis: Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. WHO Press; Geneva: 2006. [Google Scholar]
- WHO Preventive Chemotherapy Databank. 2009 available online at: http://www.who.int/neglected_diseases/preventive_chemotherapy/databank/en/i ndex.html.
- Wilson RA, van Dam GJ, Kariuki TM, Farah IO, Deelder AM, Coulson PS. The detection limits for estimates of infection intensity in schistosomiasis mansoni established by a study in non-human primates. Int J Parasitol. 2006;36:1241–4. doi: 10.1016/j.ijpara.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–42. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse ME, Etard JF, Dietz K, Ndhlovu PD, Chandiwana SK. Heterogeneities in schistosome transmission dynamics and control. Parasitology. 1998;117:475–82. doi: 10.1017/s003118209800331x. [DOI] [PubMed] [Google Scholar]