Abstract
Addition of H+ to a synthetic (µ-1,2-peroxo)diiron(III) model complex results in protonation of a carboxylate rather than the peroxo ligand. This conclusion is based on spectroscopic evidence from UV-vis, 57Fe Mössbauer, resonance Raman, infrared, and 1H/19F NMR studies. These results suggest a similar role for protons in the dioxygen activation reactions in soluble methane monooxygenase and related carboxylate-bridged diiron enzymes.
Dioxygen activation by carboxylate-bridged diiron enzymes is involved in essential biological processes1,2 ranging from DNA synthesis and hydrocarbon metabolism to cell proliferation.3 The carboxylate-bridged diiron superfamily of proteins includes ribonucleotide reductase (RNR),4 Δ9 desaturase,5 bacterial multicomponent monooxygenases (BMMs),6,7 and most recently human deoxyhypusine hydroxylase (hDOHH).3 In all of these systems, the O2 reduction step proceeds through a (peroxo)diiron(III) intermediate in which the resulting peroxo ligand is proposed to bridge two iron atoms in a µ-1,2 or µ-η2 η2 coordination mode.8–10 Extensive studies of soluble methane monooxygenase (sMMO), a BMM family member that oxidizes methane to methanol, reveal that the generation and activation of Fe2O2 units requires protons.11,12 Given the complexity of protein environments, identifying the sites involved in such proton translocation processes and their effect on O2 activation is not a trivial undertaking.
To shed light on the possible role of protons in the dioxygen activation chemistry at carboxylate-bridged diiron enzyme active sites, we investigated the reaction of H+ with a well-characterized synthetic (µ-peroxo)(µ-carboxylato)diiron(III) complex, [Fe2(µ-O2)(N-EtHPTB)(µ-PhCO2)]2+ (1a·O2).13,14 The dinucleating N-EtHPTB ligand provides kinetic stabilization of the Fe2O2 core and the benzoate group serves as a good mimic of the Asp and Glu carboxylate side chains in the protein diiron centers. By application of several spectroscopic methods, we show that the reaction of H+ with 1a·O2 results in protonation at the carboxylate unit rather than the peroxo ligand (Scheme 1). This work provides experimental support for recent theoretical studies suggesting that (hydroperoxo)-diiron(III) species of non-heme diiron enzymes are too reactive to be isolable protein intermediates.15
Scheme 1.
Reaction of dioxygen with 1a and H+. A possible structure for [1a·O2]H+ is depicted.
To aid spectral interpretation of results obtained during studies of the parent [Fe2(N-EtHPTB)(µ-PhCO2)]2+ complex (1a), two related diiron(II) precursors were synthesized (Supporting Information). One is [Fe2(N-EtHPTB)-(Ph13CO2)]2+ (1b), which contains a 13C-enriched carboxylate ligand, and the other is [Fe2(N-EtHPTB)(C6F5CO2)]2+ (2), in which the benzoate ring is fluorinated.
Exposure of 1a to O2 in CH3CN at −30 °C generates a deep blue-green solution (1a·O2) with λmax at 590 nm.13 Addition of an acetonitrile solution of [H(OEt2)2][3,5-(CF3)2C6H3)4B] (H[BArF4]) to 1a·O2 red-shifts the peroxo-to-iron(III) charge transfer band to ~600 nm (Fig. 1). This absorption is assigned to the formation of a new [1a·O2]H+ species that maximizes with addition of ~1.5 equiv of H[BArF4]. The spectrum of 1a·O2 is restored upon addition of 2.0 equiv of NEt3 (Fig. 1, inset), indicating that protonation does not lead to irreversible decomposition of the 1a·O2 unit. Reaction of 2 with O2 affords [Fe2(µ-O2)(N-EtHPTB)(µ-C6F5CO2)]2+ (2·O2), which exhibits a broad absorption feature centered at ~600 nm. When H[BArF4] was titrated into a solution of 2·O2, a small bathochromic shift to ~610 nm occurs (Fig. S1). Unlike 1a·O2, 2·O2 requires ~3.0 equiv of H[BArF4]. to fully generate the protonated species [2·O2]H+. Given that pentafluorobenzoate, for which the acid has a pKa of 1.2, is more electron deficient than benzoate (acid pKa = 4.6), the greater amount of H+ necessary to produce [2·O2]H+ from 2·O2 compared to [1a·O2]H+ from 1a·O2 suggests either that the carboxylate ligand influences the basicity of the protonation site or that it is itself the proton acceptor.
Figure 1.
UV-vis absorption spectra of [1a·O2](BF4)2 (112 µM in CH3CN, −30°C, downward arrow) when up to 2.0 equiv of H[BArF4] were added. Inset: restoration of initial spectrum (orange trace, upward arrow) upon treatment with 2.0 equiv of NEt3.
To determine whether a (hydroperoxo)diiron(III) species may form upon addition of H+ to 1a·O2 or 2·O2, 57Fe Mössbauer and resonance Raman (RR) spectra were recorded to examine possible changes in the Fe2O2 core. In the absence of H+, the Mössbauer spectrum of a frozen solution of 1a·O2 in CH3CN could be fit to a single iron site, with δ = 0.53(2) mm/s and ΔEQ = 0.71(2) mm/s (Fig. S2A). Addition of H[BArF4]. to 1a·O2 gives [1a·O2]H+ having the same isomer shift (δ = 0.53(2) mm/s) and a slightly larger quadrupole splitting parameter (ΔEQ = 0.80(2) mm/s) (Fig. S2B). For comparison, the Mössbauer spectra of 2·O2 and [2·O2]H+ were also recorded. The (peroxo)diiron(III) complex of 2 has δ = 0.53(2) mm/s and ΔEQ = 0.77(2) mm/s (Fig. S2C), whereas the protonated [2·O2]H+ form exhibits parameters of δ = 0.54(2) mm/s and ΔEQ = 0.84(2) mm/s (Fig. S2D). The similar isomer shifts obtained for 1a·O2, [1a·O2]H+, 2·O2, and [2·O2]H+ are indicative of iron(III) centers and the small increase in ΔEQ values for the protonated forms implies that only minor changes occur in the coordination environment.
To investigate more directly the nature of the peroxo moiety, Fe–O and O–O vibrations were measured by RR spectroscopy for species generated with both 16O2 and 18O2. As previously reported,13 1a·O2 exhibits Fe–O and O–O stretching vibrations with Fermi splitting (hereafter “/”) centered at 470 and 897 cm−1, respectively (Fig. S3). Also observed are weaker bands at 513/532 cm−1 that downshift to 500 cm−1 with 18O2, which we therefore assign to the asymmetric Fe–O stretch of the Fe2O2 core. Addition of H+ to 1a·O2 only marginally affects its RR spectrum, with small upshift in Fe–O and downshift in O–O vibrations (Fig. S3, Table 1). These shifts in RR frequencies upon H+ addition may reflect subtle changes in Fe–O–O–Fe angles,16 but are too small to support the conclusion that a (µ-1,2-peroxo)diiron(III) unit has been converted to a (hydroperoxo)diiron(III) species. The RR spectrum of 2·O2 is practically identical to that of 1a·O2, with symmetric and asymmetric Fe–O modes at 466/475 and 513/532 cm−1, respectively, and Fermi-coupled O–O stretches centered at 897 cm−1 (Fig. 2). Addition of up to 2.0 equiv of H[BArF4] to generate [2·O2]H+ primarily affects the symmetric Fe–O stretch, which upshifts only a few wavenumbers compared to the spectrum of 2·O2 (Table 1). From the RR data and Mössbauer parameters for 1a·O2, [1a·O2]H+, 2·O2, and [2·O2]H+, we conclude that protonation does not lead to formation of a (hydroperoxo)diiron(III) species.
Table 1.
UV-Vis, Mössbauer, RR, and FTIR data for 1a·O2, [1a·O2]H+, 1b·O2, [1b·O2]H+, 2·O2, and [2·O2]H+.
| Complex | λmax, nm (ε, M−1 cm−1) |
δ, mm/s |
ΔEQ, mm/s |
ν (Fe–O), cm−1 (Δ18O) |
ν (O–O), cm−1 (Δ18O) |
νas(COO−) cm−1 (Δ13C) |
νs(COO−) cm−1 (Δ13C) |
Δνas-s cm−1 |
|---|---|---|---|---|---|---|---|---|
| 1a·O2 | 590 (3100) | 0.53(2) | 0.71(2) | 466/474 (−18) | 897 (−50) | 1607/1572 (>−22) | 1358 (−29) | >200 |
| [1a·O2]H+ | 600 (2360) | 0.53(2) | 0.80(2) | 467/478 (−20) | 896 (−53) | 1553 (−32) | - | - |
| 2·O2 | 600 (3300) | 0.53(2) | 0.77(2) | 466/475 (−18) | 897 (−50) | - | - | - |
| [2·O2]H+ | 610 (2700) | 0.54(2) | 0.84(2) | 478 (−19) | 897 (−50) | - | - | - |
Figure 2.
RR spectra of 2·16O2 (black) and 2·18O2 (red) after addition of 0, 0.5, 1.0, 1.5, and 2.0 equiv of H[BArF4]. Each spectrum was normalized based on the solvent CH3CN bands at 392, 400, and 920 cm−1.
Since the benzimidazole and propoxy groups of N-EtHPTB are kinetically inaccessible due to the multidentate nature of the ligand, we assign the carboxylate unit as the site of protonation. To test this hypothesis, we examined the carboxylate stretches of 1a and 1b, and their peroxo complexes by FTIR spectroscopy. The assignment of frequencies in terms of coordination geometry are complicated by mixing of the COO− symmetric stretch with the O–C–O bend and C–C stretch.17 Nevertheless, if the asymmetric and symmetric COO− stretches can be identified, the binding geometry of the carboxylate ligand can be derived from the difference in the two, Δνas-s.18−20 Specifically, Δνas-s should be close to that of the free ionic form, 150 cm−1 for PhCOO−, for carboxylates bridging two metal ions, larger in unidentate coordination geometry, and smaller in bidentate mononuclear complexes. As expected, 1a and 1b exhibit Δνas-s values of 149 and 166 cm−1, respectively, consistent with µ-1,3 bridging carboxylate groups (Fig. S4B). In 1a·O2, νas and νs are at 1572/1607 and 1358 cm−1, respectively, and in 1b·O2 are at 1550 and 1329 cm−1 (Fig. 3A). These frequencies give Δνas-s > 200 cm−1 and suggests a switch from bridging to unidentate coordination for the carboxylate groups in 1a·O2 and 1b·O2. A bridging carboxylate geometry was demonstrated by X-ray crystallography in a similar (µ-peroxo)diiron(III) species,21 but facile conversion in carboxylate geometry might occur in solution. Generation of [1a·O2]H+ and [1b·O2]H+ is associated with downshift of the νas modes by at least 20 cm−1, whereas νs modes are not observed, possibly shifting below 1300 cm−1 (Fig. 3B). Most importantly, the FTIR spectra show no evidence of free carboxylic acid vibrations, i.e. νC=O > 1700 cm−1.
Figure 3.
Solution FTIR spectra of 1a·O2 (black) and 1b·O2 (red) before (A, top) and after (B, bottom) the addition of 1.5 equiv of H[BArF4]. The spectra were acquired in CH2Cl2 at approx. −30 °C with a diiron concentration of ~55 mM. The intense peaks at 1356 and 1420 cm−1 are due to the [BArF4]− anion and solvent, respectively.
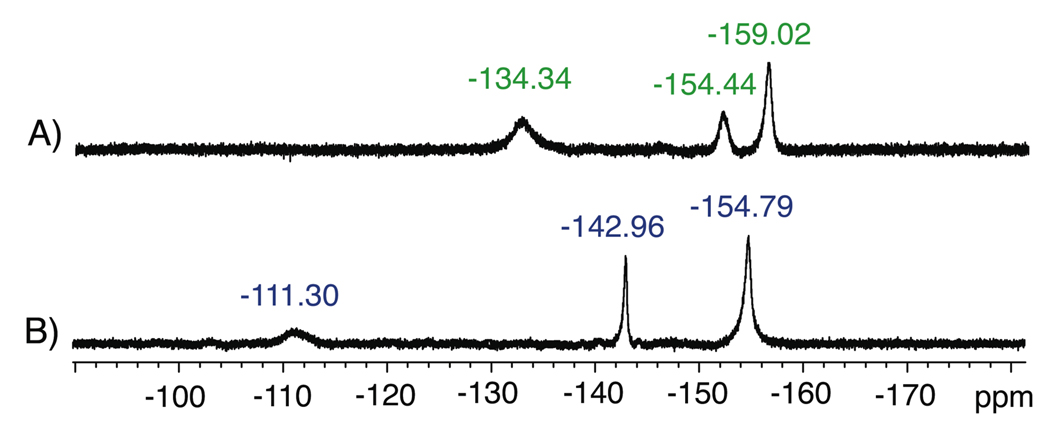
A comparison of the 1H NMR spectra of the benzoate and pentafluorobenzoate diiron complexes allows the phenyl ring protons of the former to be identified in 1a·O2 as paramagnetically broadened peaks at 7.0, 8.7, and 11.4 ppm (Fig. S5). Upon addition of H[BArF4], these resonances shift to 7.5, 8.0, and 9.8 ppm (Fig. S9), in support of the protonation of the benzoate ligand. This conclusion is confirmed by analysis of the 19F NMR spectra of 2·O2 and [2·O2]H+. The fluorine resonances of the pentafluorobenzoate ring in 2·O2 occur at −134.4, −154.4, and −159.0 ppm (Fig. 4A) and shift to −111.3, −143.0, and −154.8 ppm upon addition of 3 equiv of H[BArF4] (Fig. 4B). These results demonstrate that the C6F5CO2H ligand is bound to iron, since the resonances of free pentafluorobenzoic acid appear at −139.5, −150.3, and −162.0 ppm.
Figure 4.
19F NMR spectra (470 MHz, CD2Cl2, −30°C) of [2·O2](OSO2CF3)2 (A); and [2·O2](OSO2CF3)2/ H[BArF4] (1:3) (B).
In conclusion, the spectroscopic evidence (Table 1) clearly indicate that the carboxylate is preferred over the peroxo ligand as the site of protonation in these (peroxo)diiron(III) model complexes, a possible structure for which is depicted in Scheme 1. Our results suggest that, during the O2 activation steps in the catalytic cycle of sMMO and related enzymes, protons might generate and/or transform the (peroxo)diiron(III) core by inducing a carboxylate shift,22,23 possibly increasing the electrophilicity of the diiron unit and facilitating substrate access to the active site. Future work with synthetic analogs will address these important questions.
Supplementary Material
Acknowledgement
This work was supported by grants GM032134 (S.J.L.) and GM74785 (P.M.-L.) from the National Institute of General Medical Sciences.
Footnotes
Supporting Information Available. Synthesis and characterization of diiron(II) complexes, experimental procedures, and spectroscopic data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Feig AL, Lippard SJ. Chem. Rev. 1994;94:759. [Google Scholar]
- 2.Wallar BJ, Lipscomb JD. Chem. Rev. 1996;96:2625. doi: 10.1021/cr9500489. [DOI] [PubMed] [Google Scholar]
- 3.Vu VV, Emerson JP, Martinho M, Kim YS, Münck E, Park MH, Que L., Jr Proc. Natl. Acad. Sci. U.S.A. 2009;106:14814. doi: 10.1073/pnas.0904553106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordlund P, Reichard P. Annu. Rev. Biochem. 2006;75:681. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 5.Fox BG, Lyle KS, Rogge CE. Acc. Chem. Res. 2004;37:421. doi: 10.1021/ar030186h. [DOI] [PubMed] [Google Scholar]
- 6.Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ. Angew. Chem., Int. Ed. Engl. 2001;40:2782. doi: 10.1002/1521-3773(20010803)40:15<2782::AID-ANIE2782>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Leahy JG, Batchelor PJ, Morcomb SM. FEMS Microbiol. Rev. 2003;27:449. doi: 10.1016/S0168-6445(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 8.Liu KE, Wang D, Huynh BH, Edmondson DE, Salifoglou A, Lippard SJ. J. Am. Chem. Soc. 1994;116:7465. [Google Scholar]
- 9.Moënne-Loccoz P, Baldwin J, Ley BA, Loehr TM, Bollinger JM., Jr Biochemistry. 1998;37:14659. doi: 10.1021/bi981838q. [DOI] [PubMed] [Google Scholar]
- 10.Murray LJ, Naik SG, Ortillo DO, García-Serres R, Lee JK, Huynh BH, Lippard SJ. J. Am. Chem. Soc. 2007;129:14500. doi: 10.1021/ja076121h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S-K, Lipscomb JD. Biochemistry. 1999;38:4423. doi: 10.1021/bi982712w. [DOI] [PubMed] [Google Scholar]
- 12.Tinberg CE, Lippard SJ. Biochemistry. 2009 In press. [Google Scholar]
- 13.Dong Y, Ménage S, Brennan BA, Elgren TE, Jang HG, Pearce LL, Que L., Jr J. Am. Chem. Soc. 1993;115:1851. [Google Scholar]
- 14.Dong Y, Yan S, Young VG, Jr, Que L., Jr Angew. Chem., Int. Ed. Engl. 1996;35:618. [Google Scholar]
- 15.Jensen KP, Bell CB, III, Clay MD, Solomon EI. J. Am. Chem. Soc. 2009;131:12155. doi: 10.1021/ja809983g. [DOI] [PubMed] [Google Scholar]
- 16.Brunold TC, Tamura N, Kitajima N, Moro-oka Y, Solomon EI. J. Am. Chem. Soc. 1998;120:5674. [Google Scholar]
- 17.Nara M, Torii H, Tasumi M. J. Phys. Chem. 1996;100:19812. [Google Scholar]
- 18.Deacon GB, Phillips RJ. Coord. Chem. Rev. 1980;33:227. [Google Scholar]
- 19.Nakamoto K. Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry. 5th ed. New York: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 20.Costas M, Cady CW, Kryatov SV, Ray M, Ryan MJ, Rybak-Akimova EV, Que L., Jr Inorg. Chem. 2003;42:7519. doi: 10.1021/ic034359a. [DOI] [PubMed] [Google Scholar]
- 21.Ookubo T, Sugimoto H, Nagayama T, Masuda H, Sato T, Tanaka K, Maeda Y, Ōkawa H, Hayashi Y, Uehara A, Suzuki M. J. Am. Chem. Soc. 1996;118:701. [Google Scholar]
- 22.Dunietz BD, Beachy MD, Cao Y, Whittington DA, Lippard SJ, Friesner RA. J. Am. Chem. Soc. 2000;122:2828. [Google Scholar]
- 23.Rinaldo D, Philipp DM, Lippard SJ, Friesner RA. J. Am. Chem. Soc. 2007;129:3135. doi: 10.1021/ja0654074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.