Abstract
OBJECTIVE
To determine the human endometrial transcriptome during embryonic implantation.
DESIGN
Case report
SETTING
Tertiary fertility center
PATIENT(S)
A 24-year-old woman who inadvertently became pregnant during an endometrial biopsy procedure
INTERVENTION(S)
Endometrial biopsy was performed with a Pipelle device during the mid-luteal phase (days 19–21) of the cycle; blood samples for hormonal assessments were collected and a transvaginal ultrasound was performed.
MAIN OUTCOME MEASURE(S)
Gene expression analysis of the endometrium during the window of implantation (during the implantation of an embryo) in a natural cycle. Localization of selected genes in endometrial tissue with immunohistochemistry.
RESULT(S)
394 probe sets were differentially expressed in the pregnant sample when compared to mid-secretory phase non-pregnant endometrial samples. Different gene networks were involved, and selected genes from these signaling pathways were confirmed at the protein level.
CONCLUSION(S)
Endometrial gene expression of a pregnant patient in a natural cycle is significantly different from non-pregnant patients during the mid-secretory phase.
Keywords: Gene expression, implantation, pregnancy, endometrium, natural cycle
Introduction
In earlier studies by Armstrong et al. (1973) (1) and Gordon et al. (1975) (2), cyclic uterine changes in the secretory phase have been described in detail. However, most observations on endometrial morphology were, and still are, performed in non-conception cycles. Indeed, reported series of endometrial biopsies in conception cycles are very limited. While no adverse effect on implantation has been reported due to the endometrial biopsy procedure (3–5), an endometrial biopsy holds no morphological predictive information suggestive of ultimate pregnancy outcome (5,6). Therefore, the aim of the current study was to define the endometrial transcriptome during the process of embryonic implantation.
Case report
Materials and Methods
This study was approved by the Ethics Committee of the University Hospital of the Vrije Universiteit Brussel.
As part of the assessment of the natural cycle, prior to a stimulated cycle in a controlled trial protocol, an endometrial biopsy (n=1) was performed. The 24-year old patient had a regular menstrual cycle and previously conceived twice in a natural cycle (G2P0A2). She did not suffer from endometriosis, PCOS or any other endometrial pathology. IVF was indicated due to male infertility. The endometrial biopsy was performed with a Pipelle device 6 days (± 1 day) from the LH surge observed by urinary LH kits provided to the patient at the screening visit (first visit). At the day of the biopsy procedure, a blood sample was collected for hormonal assessments (progesterone, mid-luteal phase) and a transvaginal ultrasound examination of the ovaries and uterus was performed. The endometrial biopsy was divided into two parts: one part for histological analysis, with haematoxylin and eosin staining, and the other part for RNA isolation and further gene expression analysis. The endometrial biopsy was evaluated with conventional histological Noyes criteria (7) of secretory transformation by a specialized pathologist, unaware of treatment conditions.
Gene expression was analyzed with the Affymetrix HGU133 Plus 2.0 microarray. The sample was used in duplicate as a ‘technical replicate’, to reduce interassay variability and enhance the robustness of the microarray data, and hybridized using identical methods by two different laboratories (Gene Expression Unit, KU Leuven, Belgium and UCSF, Gladstone Genomics Core, San Francisco) (8). The RNA sample was reverse-transcribed with the SuperScript Choice System (Invitrogen, Carlsbad, CA, USA) with oligo-dT primers containing a T7 RNA polymerase promotor site. The resulting cDNA was in vitro transcribed and labeled with biotin using the IVT labeling kit (Affymetrix, Santa Clara, CA, USA), followed by fragmentation of the biotinylated cRNA. Next, the quality of this cRNA was assessed with the Agilent 2100 Bioanalyzer. The fragmented cRNA was hybridized overnight to the Affymetrix Human Genome (HG) U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA, USA), containing more than 54000 probe sets. Subsequently, the arrays were washed and stained according to the protocol (Affymetrix Expression Analyses Technical Manual) and scanned on a 2-μm Affymetrix 3000 GeneScanner. Data were analyzed with GeneSpring GX 7.3 (Agilent Technologies, Palo Alto, CA, USA). The ‘pregnant’ sample (in duplicate) was compared with non-pregnant mid-luteal endometria (n=8) from natural cycles. The probe level intensities data were preprocessed using the RMA algorithm (robust multiarray average, GeneSpring) for background adjustment, normalization, and log-transformation of the perfect match values (9). Additionally, the data were normalized per gene to the median. Data were then further analyzed with Ingenuity Pathways Analysis (Ingenuity Systems, www.ingenuity.com, Redwood City, CA, USA) for network and pathway analysis, as previously described (10). Selected genes were confirmed with immunohistochemistry on the pregnant patient sample (n=1) and on control mid-luteal non-pregnant patient samples (n=7). Commercially available antibodies were used (Abcam plc, Cambridge, UK), together with appropriate positive and negative controls (11–16). Antigen retrieval was performed by heating in citrate buffer (pH 6.0; ScyTek, Logan, Utah, USA) for 10 min. in a water bath. Slides were incubated overnight with the appropriate primary antibodies at 4°C in a humidified chamber, treated with a horse radish peroxidase-conjugated secondary antibody (EnVision+ Dual Link System-HRP, Dako, Glostrup, Denmark), treated with diaminobenzidine (DAB, 10min, Dako), and counterstained with haematoxylin. Staining intensity was scored semiquantitatively from 0 (negative) to 3 (intense) on the whole tissue by a single observer.
Results
Hormonal assessment nine days after the endometrial biopsy procedure showed a β-hCG (human chorionic gonadotrophin) value of 2151 IU/L. Seven days later the β-hCG value had risen to 20525 IU/L. The patient had an ongoing pregnancy and delivered a healthy baby. It was calculated afterwards from hCG values that implantation had occurred on the day of the endometrial biopsy procedure. The endometrial morphology was dated as day 6 of the luteal phase.
Out of 54.675 probe sets, 394 were significantly differentially expressed between pregnant (n=2, technical replicate) and non-pregnant (n=8) mid-luteal endometrium in natural cycles (Volcano plot with Bonferroni correction; fold change ≥ 2.0, p < 0.05). Three hundred and ten probe sets were upregulated in the pregnant patient and 84 probe sets were downregulated.
In total, 30 networks were derived from the list of 394 differentially expressed probe sets. The top networks (according to their relevance to the list of genes) (10) were involved in cancer, reproductive system disease, post-translational modification, cell-to-cell signaling, cellular movement, and hematological system development and function. Oxidative phosphorylation, ephrin receptor signaling, neurotrophin/TRK signaling, purine metabolism and PPAR signaling were the most significant canonical pathways involved in the endometrium of the pregnant patient.
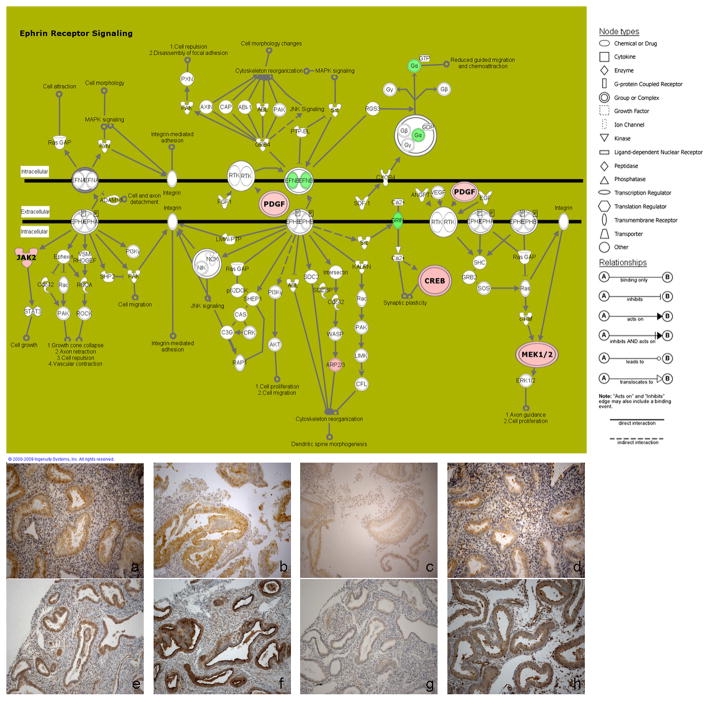
Four different genes, which are involved in these pathways, were selected to confirm tissue localization at the protein level. Immunohistochemistry showed cytoplasmic positive staining, predominantly in the glandular compartment, in the endometrial biopsy from the pregnant patient and in mid-luteal biopsies from control non-pregnant patients (Figure 1) for JAK2 (Janus Activating Kinase 2), PDGFA (platelet-derived growth factor-A), CREB3 (cAMP response element-binding protein 3) and MEK1 (mitogen activated protein kinase kinase 1). The staining pattern in glands and stroma was comparable between the pregnant sample and non-pregnant samples, with stronger intensity in non-pregnant tissues for PDGFA (glands scored as 3 in non-pregnant sample, scored as 2 in pregnant sample) and MEK1 (glands scored as 3 in non-pregnant and 2 in pregnant).
Figure 1.
Top: Ephrin receptor signaling pathway with downregulated (in green) and upregulated (in red) genes in the endometrium from the pregnant patient. Node (gene) and line (gene relationships) symbols are described in the right part of the figure.
Bottom: Immunohistochemistry of the endometrial biopsy from the pregnant patient for JAK2 (a), PDGFA (b), CREB3 (c) and MEK1 (d) and from control non-pregnant patients for JAK2 (e), PDGFA (f), CREB3 (g) and MEK1 (h) showed cytoplasmic staining, predominantly in the glandular epithelium.
Discussion
This is the first report to compare the gene expression of conceptional mid-luteal endometrium to non-conceptional mid-luteal endometrium. Gene expression showed a differential expression of 394 probe sets, in which 30 networks were involved. Four upregulated genes, belonging to the ephrin signaling pathway (Figure 1), were selected to confirm protein expression and tissue localization. These were differentially regulated in conception mid-luteal endometrium compared to non-conception mid-luteal endometrium on the RNA level (Figure 1). Immunohistochemistry also showed the presence of the selected genes on protein level in the pregnant patient, as well as in non-pregnant patients. However, this technique is neither quantitative nor sensitive enough to differentiate between the two groups (17). Confirmation with a more quantitative technique, like Western blot, would be useful. But, there was not enough sample material available to perform these experiments.
In the mouse, the Eph-ephrin A system is involved in regulating embryo-endometrial cross-talk during the implantation period and thus functions in cell-to-cell communication. Ephrins are membrane-bound ligands for the Eph tyrosine kinase receptor family. Together, they can induce repulsive forces to control cell position and cell movement (18,19). JAK2, a tyrosine kinase of the non-receptor type (20), is also involved in the ephrin receptor signaling pathway, as well as PDGFA, MEK1 (or MAP2K1) and CREB3 (Figure 1). CREB3 is a member of the leucine zipper family of DNA binding proteins (10) and MEK1 is a member of the MAPK pathway involved in growth and survival of cancer cells (12,13). PDGFA is well known as a growth factor playing an important role in renal disease, as well as in wound healing and different pathophysiologic events, like cell proliferation (15,16). These four selected genes were also part of other significantly regulated pathways in the pregnant patient. MEK1 and PDGFA belong to the PPAR signaling pathway as well. Peroxisome proliferator-activated receptors (PPARs) are known regulators for implantation and decidualization as part of the COX2-PG-PPARδ signaling axis in the uterus (21). In the neurotrophin/TRK signaling pathway, MEK1 and CREB3 were upregulated. Neurotrophins and their Trk tyrosine kinase receptors, originally discovered as factors required for the development of the nervous system, are also involved in early ovarian development (22) and are expressed in non-conception endometrium (Tatsumi and Giudice, unpublished observations; 23).
These discovered networks and canonical pathways are part of the molecular mechanism already known to be involved in embryo-endometrial cross-talk and implantation of the embryo in mouse models and less so in humans. In the current study, while the physical distance of the endometrial sample from the implantation process is unknown, the observed differences are intriguing and raise the question whether they are due to paracrine and/or endocrine effects of an implanting conceptus. This question remains unresolved, but is important to the understanding of embryo-endometrial interactions and uterine receptivity, and successful implantation. While the findings of this study are limited by the fact that only one pregnant sample was available for analysis, the clinical relevance of these (and other) molecules warrants further investigation with other (non-invasive) methods, including blood measurements and intra-uterine flushings, of key markers of the decidual reaction to an implanting conceptus.
Acknowledgments
We would like to thank Novo Nordisk for their permission to use the sample for research purposes.
Financial support: This study was supported by grant AL405 from Research Foundation-Flanders (FWO), Belgium and by NICHD/NIH through a cooperative agreement U54HD055764-02 (LCG) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
Presented at the 25th Annual Meeting of the European Society of Human Reproduction and Embryology, Amsterdam, The Netherlands (28 June - 1 July 2009)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong EM, More IA, McSeveny D, Chatfield WR. Reappraisal of the ultrastructure of the human endometrial glandular cell. J Obstet Gynaecol Br Commonw. 1973;80:446–460. doi: 10.1111/j.1471-0528.1973.tb15961.x. [DOI] [PubMed] [Google Scholar]
- 2.Gordon M. Cyclic changes in the fine structure of the epithelial cells of human endometrium. Int Rev Cytol. 1975;42:127–172. doi: 10.1016/s0074-7696(08)60980-8. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld DL, Garcia CR. Endometrial biopsy in the cycle of conception. Fertil Steril. 1975;26:1088–1093. doi: 10.1016/s0015-0282(16)41475-5. [DOI] [PubMed] [Google Scholar]
- 4.Balasch J, Vanrell JA, Marquez M, Gonzalez-Merlo J. Endometrial biopsy inadvertently taken in the cycle of conception. Int J Gynaecol Obstet. 1984;22:95–99. doi: 10.1016/0020-7292(84)90020-1. [DOI] [PubMed] [Google Scholar]
- 5.Wentz AC, Herbert CM, 3rd, Maxson WS, Hill GA, Pittaway DE. Cycle of conception endometrial biopsy. Fertil Steril. 1986;46:196–199. doi: 10.1016/s0015-0282(16)49510-5. [DOI] [PubMed] [Google Scholar]
- 6.Myers ER, Silva S, Barnhart K, Groben PA, Richardson MS, Robboy SJ, et al. Interobserver and intraobserver variability in the histological dating of the endometrium in fertile and infertile women. Fertil Steril. 2004;82:1278–1282. doi: 10.1016/j.fertnstert.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 7.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 8.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 9.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 10.Van Vaerenbergh I, Van Lommel L, Ghislain V, In’t Veld P, Schuit F, Fatemi HM, et al. In GnRH antagonist/rec-FSH stimulated cycles, advanced endometrial maturation on the day of oocyte retrieval correlates with altered gene expression. Hum Reprod. 2009;24:1085–91. doi: 10.1093/humrep/den501. [DOI] [PubMed] [Google Scholar]
- 11.Labrie C, Lessard J, Ben Aicha S, Savard MP, Pelletier M, Fournier A, et al. Androgen-regulated transcription factor AIbZIP in prostate cancer. J Steroid Biochem Mol Biol. 2008;108:237–244. doi: 10.1016/j.jsbmb.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 13.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KU, Rui H. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia. 2008;13:93–103. doi: 10.1007/s10911-008-9062-z. [DOI] [PubMed] [Google Scholar]
- 15.Alpers CE, Davis CL, Barr D, Marsh CL, Hudkins KL. Identification of platelet-derived growth factor A and B chains in human renal vascular rejection. Am J Pathol. 1996;148:439–451. [PMC free article] [PubMed] [Google Scholar]
- 16.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 17.True L, Feng Z. Immunohistochemical validation of expression microarray results. Journal of Molecular Diagnostics. 2005;7:149–151. doi: 10.1016/S1525-1578(10)60540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara H, Yoshioka S, Tatsumi K, Kosaka K, Satoh Y, Nishioka Y, et al. Human endometrial epithelial cells express ephrin A1: possible interaction between human blastocysts and endometrium via Eph-ephrin system. J Clin Endocrinol Metab. 2002;87:5801–5807. doi: 10.1210/jc.2002-020508. [DOI] [PubMed] [Google Scholar]
- 19.Fujii H, Tatsumi K, Kosaka K, Yoshioka S, Fujiwara H, Fujii S. Eph-ephrin A system regulates murine blastocyst attachment and spreading. Developmental Dynamics. 2006;235:3250–3258. doi: 10.1002/dvdy.20977. [DOI] [PubMed] [Google Scholar]
- 20.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Xie H, Sun X, Tranguch S, Zhang H, Jia X, et al. Stage-specific integration of maternal and embryonic PPARδ signaling is critical to pregnancy success. J Biol Chem. 2007;282:37770–37782. doi: 10.1074/jbc.M706577200. [DOI] [PubMed] [Google Scholar]
- 22.Dissen GA, Romero C, Paredes A, Ojeda SR. Neurotrophic control of ovarian development. Microsc Res Tech. 2002;59:509–515. doi: 10.1002/jemt.10227. [DOI] [PubMed] [Google Scholar]
- 23.Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol. 1996;148:1807–1818. [PMC free article] [PubMed] [Google Scholar]