Abstract
The effects of cAMP in cell are predominantly mediated by the cAMP-dependent protein kinase (PKA), which is composed of two genetically distinct subunits, catalytic (C) and regulatory (R), forming a tetrameric holoenzyme R2C2. The only known function for the R subunit is that of inhibiting the activity of the C subunit kinase. It has been shown that overexpression of RIα, but not the C subunit kinase, is associated with neoplastic transformation. In addition, it has also been demonstrated that mutation in the RIα, but not the C subunit is associated with increased resistance to the DNA-damaging anticancer drug cisplatin, thus suggesting that the RIα subunit of PKA may have functions independent of the kinase. We show here that the RIα subunit interacts with a BTB/POZ domain zinc finger transcription factor, PATZ1 (ZNF278), and co-expression with RIα results in its sequestration in the cytoplasm. The cytoplasmic/nuclear translocation is inducible by cAMP. C-terminus deletion abolishes PATZ1 interaction with RIα and results in its localization in the nucleus. PATZ1 transactivates the cMyc promoter and the presence of cAMP and co-expression with RIα modulates its transactivation. Moreover, PATZ1 is aberrantly expressed in cancer. Taken together, our results showed a potentially novel mechanism of cAMP signaling mediated through the interaction of RIα with PATZ1 that is independent of the kinase activity of PKA, and the aberrant expression of PATZ1 in cancer point to its role in cell growth regulation.
Keywords: Regulatory subunit, BTB-POZ, cAMP-dependent protein kinase, PRKAR1A, PATZ1, ZNF278
Introduction
Phosphorylation mediated by the cAMP signal transduction pathway is involved in the regulation of metabolism, cell growth and differentiation, apoptosis, and gene expression [1, 2]. In mammalian cells, PKA is a tetrameric holoenzyme of two catalytic (C) and two regulatory (R) subunits (R2C2), encoded by four genetically distinct R subunit isoforms designated as RIα and RIβ, and RIIα and RIIβ, and three isoforms of the C subunit, Cα, Cβ, and Cγ [1, 3–5].
The principal mechanism of cAMP signaling involves the binding of cAMP to the R subunit, which causes dissociation of the holoenzyme and the activation of the C subunit kinase. However, recent discoveries have shown that there are other receptors for cAMP in addition to the R subunit, as well as alternative signaling pathways activatable by the C subunit including the direct activation of a class of cyclic nucleotide-gated ion channels by cAMP in the central nervous system [6–8]. It was also demonstrated that the C subunit could be activated in a ternary complex of NFκB-IκB-C subunit, independent of cAMP and the R subunit, and degradation of IκB following the exposure to inducers of NFκB activates the C subunit, which then phosphorylates NFκB [9]. It was further shown that a family of novel cAMP-binding guanine nucleotide exchange factors can selectively activate the Ras superfamily of guanine nucleotide binding protein, Rap1, in a cAMP-dependent but PKA-independent manner [10, 11]. Moreover, it has also been reported that a cAMP-bound RII subunit complex inhibits phosphatase activity [12].
Defects in cAMP response and differential expression of RI and RII has been correlated with cell differentiation and neoplastic transformation, with RI preferentially expressed in transformed cells, and increased RII expression in terminally differentiated tissues, which cannot be attributed to the kinase activity of PKA [3]. That the R subunit may have function independent of the C subunit kinase activity is further implicated in the report that, PKA genetic mutants derived from CHO and the mouse Y1 adrenocortical carcinoma cells having mutations in the RIα but not the C subunit, exhibit increased resistance to cisplatin, a DNA-damaging anticancer drug [13, 14]. Subsequently, it was shown that the RIα subunit interacts with the subunit Vb of cytochrome c oxidase (CoxVb) that is regulated by cAMP, and elevation of cAMP levels inhibits cytochrome c oxidase activity in CHO cells [15]. More notably, the RIα subunit is a genetic target for alteration in the inherited autosomal dominant Carney complex (CNC) disorder, a multiple neoplasia syndrome, suggesting its role as a tumor suppressor [16–18]. These results together suggest that the cAMP/PKA signaling mechanism is more complex and diverse that previously appreciated and this signaling network warrants further exploration.
We show here that a BTB-POZ domain zinc-finger transcription factor, PAZT1 (ZNF278) interacts with the RIα subunit, and translocation of PATZ1 from the cytoplasm into the nucleus is regulated by cAMP. We showed further that PATZ1 is a transcription activator and is aberrantly expressed in cancer. Our results suggest that in addition to inhibiting the C subunit kinase activity, the RIα subunit may have novel function by interacting with and sequestering PATZ1 in the cytoplasm, thereby, regulating its transcriptional activity and function in cell growth control in response to cAMP.
Materials and methods
RIα interactions by the yeast two-hybrid cloning assay
Yeast two-hybrid interaction cloning experiments with RIα were conducted as described before [15, 19]. Full-length coding sequence of RIα fused to the Gal4 DNA-binding domain in plasmid pAS2-1 (pAS2- RIα) was used as bait to screen a human liver cDNA library fused to the Gal4 activation domain in the pGAD-GH vector. Positive clones were detected by β-galactosidase assay followed by confirmation with the mating assay using pAS2- RIα cells.
GST fusion, RIα deletion, and pull down assay
Expression of the glutathione S-transferase (GST)-RIα fusion protein and its partial purification with glutathione beads were conducted as previously described [15]. In brief, RIα cloned into pGEX-4T-1 vector was expressed in the Escherichia coli DH5α cells with IPTG, lysed by sonication, and the lysates were incubated with glutathione resin to immobilize the GST fusion proteins. GST-RIα beads were then incubated with either yeast lysates overexpressing clone, which harbors the RIα interacting protein domain, or in vitro translated PATZ1. The RIα amino-terminal (GST-RIα(Δ1-76) and the carboxyl-terminal (GST-RIα(Δ77-380) deletion mutants were constructed as described previously [15]. Deletion mutants were expressed and bound to glutathione resin and then incubated with yeast lysates containing clone pACT-A14, comprising of PATZ1, separated on SDS-PAGE and immunoblotted with anti-GAD antibody.
Cell cultures and green fluorescent protein analysis
Cells were obtained from American Type Culture Collection (Manassas, VA) and grown in 100 mm petri dishes at 37°C, in 5% CO2, in the appropriate media supplemented with 10% or 15% serum. Cells were then washed and replenished in Opti-MEM (Invitrogen, Carlsbad, CA) for transfection using Lipofectamine (Invitrogen) with various plasmid constructs as indicated for approximately 4 hr according to manufacturer’s specifications. Transfected cells were analyzed 36 hr later and subcellular localization of GFP/PATZ1 was imaged using a Zeiss Axioskop fluorescence microscope. Transfected cells were also treated with 8-bromo-cAMP (100 μM) and the GFP/PATZ1 subcellular localization was analyzed as above.
RNA blot analysis
Normal human tissue RNA blots (BD Biosciences, Palo Alto, CA) were probed with the 1.5 kb insert encompassing the 3′-end of PATZ1, derived from clone pACT-A14. RNAs from normal human breast tissue and breast cancer cell lines were prepared using the Qiagen RNeasy kit according to manufacturer’s specification, fractionated on denaturing formaldehyde agarose gel, and then transferred to nitrocellulose for probing with the 1.5 kb PATZ1 cDNA fragment as above.
Construction of PATZ1
Full-length PATZ1 cDNA isolated from the human spleen cDNA library was cloned into the pBlueScript vector (Stratagene, La Jolla, CA) to yield pBPATZ1. The plasmid was further digested with Hind III and SgrA 1 to remove 5 upstream ATGs in the 5′ leader sequence, and then religated to yield •PATZ1. A 1.9 kb Sal I/Hinc II fragment of PATZ1, containing the coding sequence, was excised from •PATZ1 and ligated in frame into pLEGFP-C1 (BD Biosciences) digested with Sal I and Sma I. The same fragment was also ligated in frame into the pLEGFP-N2 vector. C-terminus deletion mutant of PATZ1 was made by PCR using the forward primer 5′-AAATAAGCTTCCATGGAGCGG-GTAAAC and the reverse primer 5′-GCGGTCTCTTCACTCAGCTGATT and cloned as a Hind III/Sal I fragment, in frame into pLEGFP-C1. The forward and reverse primers included the linkers sequence.
Transfection and reporter assay
Approximately 1 × 105 cells were plated per well in 12-well cluster dishes for overnight, and then washed, replenished with Opti-MEM and transfected using Lipofectamine as with the human cMyc promoter luciferase reporter plasmid and various plasmid constructs as indicated. The Renilla luciferase expression plasmid pRL-CMV (Promega) (0.5 μg) was included in the cotransfections as internal standard for normalization. Luciferase activity was determined using the Turner Designs Luminometer Model TD-20/20 (Promega) according to manufacturer’s specifications.
Results
Identification of RIα interaction with PATZ1
To further understand the function of RIα that is independent of PKA, we performed yeast two-hybrid interaction experiments using the full-length RIα as bait and screened a human liver two-hybrid cDNA library, and found interaction of RIα with PATZ1 (ZNF278), a member of the superfamily of BTB-POZ domain zinc finger proteins [20, 21]. Yeast mating assay confirmed the interaction of PATZ1 with RIα (Fig. 1A). PATZ1 encodes a BTB-POZ domain zinc finger protein of 641 amino acids with an estimated molecular mass of 69 kDa. Computational analysis of the intron-exon boundaries of the genomic sequence of PATZ1 on chromosome 22 predicted four splice variants, which include PATZ [21], ZSG (Zinc finger Sarcoma Gene) [22], and the variant identified here that interacts with RIα. The splice variant PATZ retains most of the features of PATZ1 except for the three zinc finger motifs and the RIα interacting domain that are absent at the C-terminus of PATZ [21]. In Ewing sarcoma (EWS), all four splice variants are targeted for rearrangement and form a chimera with the EWS gene on 22q12, that is thought to activate EWS in soft tissue sarcoma [22].
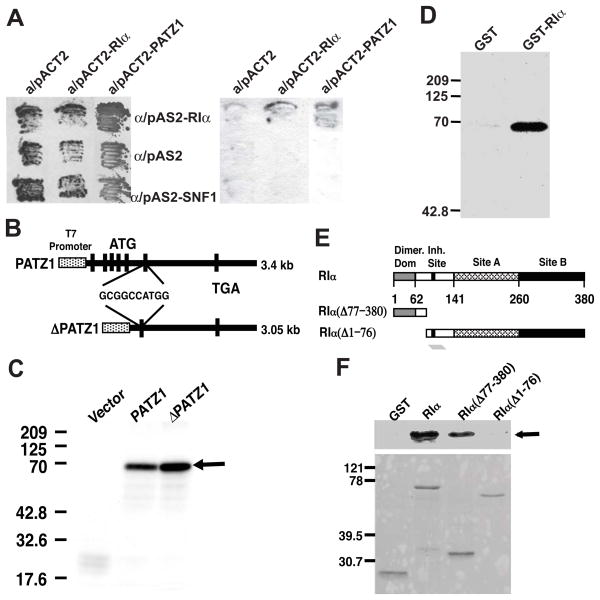
Fig. 1.
Interaction of RIα with PATZ1. (A) Yeast two-hybrid interaction experiment showing the association of RIα with PATZ1. (B) Schematic representation of the full length and the upstream ATGs-deletion mutant of PATZ1. Approximately 350 bp from the 5′ end of PATZ1 cDNA, containing a cluster of upstream ATGs, was deleted. (C) In vitro transcription and translation was performed with the full length PATZ1 or its upstream ATGs-deletion mutant (•PATZ1) compared to the pBluescript II SK(−) vector control. (D) The in vitro-[35S]Methionine-labeled translated PATZ1 was interacted with either GST or GST-RIα immobilized on glutathione resins. (E) Schematic representation of wild-type and deletion mutants of RIα. Dimer. Dom., the N-terminal dimerization domain; Inh. Site, inhibitory site; Sites A and B are the two cAMP binding domains. (F) Top panel, interaction of PATZ1 from yeast extracts with either recombinant GST, GST-RIα, GST-RIα(Δ1-76), and GST-RIα(Δ77-380); bottom panel, bacterial lysates containing the respective expressed proteins GST, GST-RIα; GST-RIα(Δ77-380) and GST-RIα(Δ1-76).
In vitro translation and interaction of PATZ1 with RIα
To further characterize the above interaction, PATZ1 was translated and labeled in vitro with [35S]methionine. Gel electrophoresis showed a product with molecular size of approximately 67–70 kDa (Fig. 1C), corresponding closely to the calculated molecular weight from the open reading frame of PATZ1. Deleting a cluster of upstream ATGs in the 5′ end of the PATZ1 cDNA (Fig. 1B) seemed to enhance translation from the 3′-most ATG start codon (Fig. 1C). In-vitro translated PATZ1 interacted with GST-RIα but not GST (Fig. 1D).
Coimmunoprecipitation using GST-RIα showed interaction with PATZ1 from yeast lysates (Fig. 1F). Deleting RIα C-terminus, GST-RIα(• 77-380) (Fig. 1E), which includes the autoinhibitory region and the two cAMP binding sites, did not affect its association with PATZ1 (Fig. 1F), while the deletion of RIα N-terminus, GST-RIα(•1-76) (Fig. 1E), containing the dimerization as well as the A-kinase anchoring protein (AKAP) binding domains abolished its interaction with PATZ1 (Fig. 1F).
Subcellular distribution of PATZ1 and its interaction with RIα
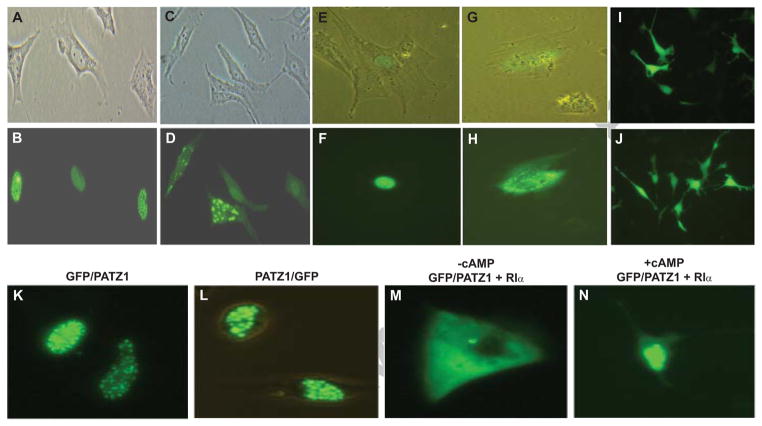
Type I PKA is predominantly localized in the cytoplasm [23] and RI subunit has been shown to bind tightly to the plasma membrane [24]. We examined whether interaction of PATZ1 with RIα might influence its localization. PATZ1 was fused to the green fluorescence protein (GFP) at either the N- (GFP/PATZ1) or the C-terminus (PATZ1/GFP) and a speckled/punctate distribution in the nucleus was observed in either PC3M prostate carcinoma cells (Fig. 2B), or the normal fibroblasts, GM38A (Fig. 2F). Fusion of GFP to the N- or the C-terminus of PATZ1 did not alter its nuclear distribution (Figs. 2K and L). Cotransfection of GFP/PATZ1 with RIα in these cells resulted in the redistribution of PATZ1 in both the cytoplasm and the nucleus (Figs. 2D, H and M); suggesting that RIα may sequester PATZ1 in the cytoplasm. RIα did not alter GFP localization (Figs. 2I and J). Addition of cAMP caused the translocation of PATZ1 from the cytoplasm to the nucleus (Fig. 3N).
Fig. 2.
Subcellular localization of RIAZ. A, C, E, and G, light micrograph of PC3M cells (A, C) or normal fibroblasts (E, G) either transfected with GFP/PATZ1 (A, B, E, F) or cotransfected with GFP/PATZ1 and RIα (C, D, G, H). B, D, F, H, are fluorescence image of GFP/PATZ1 localization. I, fluorescence image of PC3M cells transfected with GFP alone; and J, cotransfected with GFP and RIα. K, L, localization of either GFP/PATZ1 or PATZ1/GFP in HTB-46 cells; L, transfected with RIAZ/GFP; M, cotransfected with GFP/RIAZ and RIα in the absence of 8-Br-cAMP; N, cotransfected with GFP/RIAZ and RIα in the presence of 1 mM 8-Br-cAMP. Subcellular localization of GFP/RIAZ was visualized using a Zeiss Axioskop fluorescence microscope.
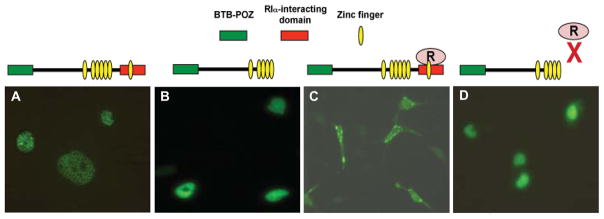
Fig. 3.
Interaction with RIα with PATZ1 deletion mutant. PC3M cells were transfected with (A) GFP/PATZ1; (B) GFP/PATZ1 and RIα; (C) GFP fusion of C-terminus deletion mutant of PATZ1 (GFP/Δ PATZ1); or (D) GFP/ΔPATZ1 and RIα. Subcellular localization of GFP/PATZ1 was visualized 24 hr after transfection.
Effects of PATZ1 C-terminus deletion on its interaction with RIα
As shown above, the dimerization and the AKAP binding domains of RIα is required for interaction with PATZ1 (Fig. 1F). We found further that deleting the C-terminus of PATZ1 (•461-641) did not affect its nuclear localization compared to wild-type (Fig. 3A and B). Cotransfection with RIα led to a redistribution of PATZ1 to both the cytoplasm and the nucleus (Fig. 3C). In contrast, cotransfecting RIα with PATZ1(•461-641) showed only nuclear distribution (Fig. 3D); thus suggesting that PATZ1 C-terminus deletion abolishes its interaction with RIα and sequestration in the cytoplasm.
Expression of RIAZ in normal tissues and breast cancer
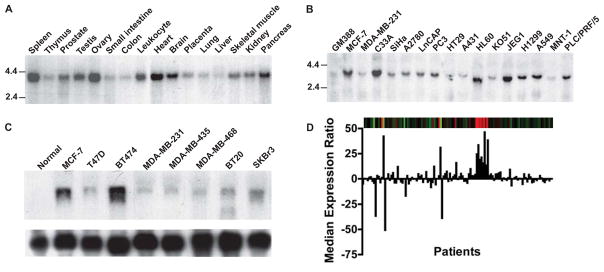
Previous studies suggest that the various splice variants of PATZ1 might be targeted for genetic alterations in cancer and have potential roles in growth control [22, 25, 26]. To gain insight into its pathological role in cancer, we determined PATZ1 expression by Northern blot analysis in normal tissues and found its ubiquitous expression in many human tissues with the highest abundance in the spleen, ovary, and heart, and significant levels in the brain, prostate, testis, pancreas, and leukocyte (Fig. 4A). Compared to the normal human fibroblasts, GM388, aberrant PATZ1 expression was found in some cancer cells of the breast (MCF-7) and cervix (C33A), as well as the Jurkat lymphoma, JEG1 cells (Fig. 4B). PATZ1 overexpression was also observed in a panel of breast tumor cell lines including MCF-7, BT474 and SKBr3, compared to normal breast tissue. Notably, we further observed approximately 16% (22 of 119 cases) of breast cancer specimens from patients in a previous microarray study [27] exhibited elevated levels of PATZ1 expression.
Fig. 4.
Expression of PATZ1 in normal human tissue, cancer cell lines, and clinical cancer specimens. (A) RNA blot analysis of PATZ1 in normal human tissues. Northern blot analysis of PATZ1 in various (C) human cancer cell lines, and a panel of (C) breast cancer cells. (D) PATZ1 expression in breast cancer specimens from 119 patients. Red bars in dendrogram showed increased PATZ1 levels, and green bar, decreased levels compared with reference to mean.
Discussion
The cAMP signal transduction system is one of the best-characterized biochemical pathways in mammalian cells. Until recently, the R subunit has been the only known receptor for cAMP in cells and cAMP binding to the holoenzyme has been the accepted mechanism that regulates PKA activity. The only known function for the R subunit is that of inhibiting the C subunit kinase activity in the holoenzyme complex. Previous work from our laboratory using mammalian genetic mutant cell lines implicates potential functions for the R subunit that is independent of the C subunit kinase (17,28,29). In this report, we showed by interaction cloning experiment the association of the RIα subunit of PKA with a BTB-POZ domain zinc finger transcription factor PATZ1, and regulation by cAMP (Fig. 1 and 2). We further showed that PATZ1 is aberrantly expressed in about 16% of breast cancer cases (Fig. 4).
The role of cAMP in cell growth has been intensely investigated [28]. In a large number of human cancer as well as cancer cell lines, only type I PKA or the RI subunit is detected. Moreover, overexpression of RIα in CHO cells confers growth advantage in monolayer and soft agar conditions, but overexpression of the C subunit does not [29]. It was further observed that overexpression of RIα, but not the C subunit, in MCF-10A cells confers the ability to grow in serum and growth factor free conditions [30]. It is intriguing that the cAMP-mediated cell growth is independent of the kinase activity, thus raising the possibility that the R subunit may have hitherto unknown function that contribute to cell growth. Such a possibility is supported by the finding that the RIα subunit gene is targeted for genetic alterations in the inherited autosomal dominant Carney complex (CNC) disorder, which is a multiple neoplasia syndrome characterized by spotty skin pigmentation, cardiac and other myxomas, endocrine tumors and psammomatous melanotic schwannomas [16, 17, 31]. The germline mutations in RIα suggest that it is a tumor suppressor gene and have potential role in cell growth regulation. This and the aberrant expression of PATZ1 in cancer (Fig. 4) and its abnormal translocation in Ewing sarcoma, suggest an important synergy between the interaction of RIα with PATZ1 in tumorigenesis. Moreover, the splice variant PATZ has been shown to transcriptionally repress the c-myc, CDC6 and galectin-1 promoters [21]. In addition, MAZR, the mouse homologue of PATZ1, exhibit transactivation potential from the SV40 and the c-myc promoter [32], thus suggesting that PATZ1 may have transcriptional potential.
The precise mechanism by which RIα regulates cell growth that is independent of PKA, remains unclear. Our observation here suggest a novel signaling mechanism in which the sequestration of PATZ1 in the cytoplasm through its interaction with RIα is regulated by cAMP, thus enabling PATZ1 to translocate into nucleus and transactivate its target genes upon activation of the cAMP pathway. Therefore, further understanding of the interaction of RIα with RIAZ and their effects in growth regulation may offer insights on the mechanisms of cAMP mediated growth control.
Acknowledgments
This research was supported in part by National Institutes of Health grant R01 CA102204.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chin KV, Yang WL, Ravatn R, Kita T, Reitman E, Vettori D, et al. Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann N Y Acad Sci. 2002;968:49–64. doi: 10.1111/j.1749-6632.2002.tb04326.x. [DOI] [PubMed] [Google Scholar]
- 2.Kirschner LS, Yin Z, Jones GN, Mahoney E. Mouse models of altered protein kinase A signaling. Endocr Relat Cancer. 2009;16:773–93. doi: 10.1677/ERC-09-0068. [DOI] [PubMed] [Google Scholar]
- 3.Cho-Chung YS. cAMP signaling in cancer genesis and treatment. Cancer Treat Res. 2003;115:123–43. doi: 10.1007/0-306-48158-8_5. [DOI] [PubMed] [Google Scholar]
- 4.Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- 5.Edelman AM, Blumenthal DK, Krebs EG. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- 6.Liu FC, Takahashi H, McKay RD, Graybiel AM. Dopaminergic regulation of transcription factor expression in organotypic cultures of developing striatum. J Neurosci. 1995;15:2367–84. doi: 10.1523/JNEUROSCI.15-03-02367.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, et al. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–29. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 8.Zufall F, Shepherd GM, Barnstable CJ. Cyclic nucleotide gated channels as regulators of CNS development and plasticity. Curr Opin Neurobiol. 1997;7:404–12. doi: 10.1016/s0959-4388(97)80070-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–24. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–9. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 11.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 12.Khatra BS, Printz R, Cobb CE, Corbin JD. Regulatory subunit of cAMP-dependent protein kinase inhibits phosphoprotein phosphatase. Biochem Biophys Res Commun. 1985;130:567–73. doi: 10.1016/0006-291x(85)90454-1. [DOI] [PubMed] [Google Scholar]
- 13.Cvijic ME, Chin KV. Regulation of P-glycoprotein expression in cyclic AMP-dependent protein kinase mutants. Cell Growth Differ. 1997;8:1243–7. [PubMed] [Google Scholar]
- 14.Liu B, Cvijic ME, Jetzt A, Chin KV. Cisplatin resistance and regulation of DNA repair in cAMP-dependent protein kinase mutants. Cell Growth Differ. 1996;7:1105–12. [PubMed] [Google Scholar]
- 15.Yang WL, Iacono L, Tang WM, Chin KV. Novel function of the regulatory subunit of protein kinase A: regulation of cytochrome c oxidase activity and cytochrome c release. Biochemistry. 1998;37:14175–80. doi: 10.1021/bi981402a. [DOI] [PubMed] [Google Scholar]
- 16.Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, Weremowicz S, et al. Mutations in the protein kinase A R1alpha regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest. 2000;106:R31–8. doi: 10.1172/JCI10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 18.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9:3037–46. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 19.Bartel PL, Fields S. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–63. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- 20.Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. 1995;6:1193–8. [PubMed] [Google Scholar]
- 21.Fedele M, Benvenuto G, Pero R, Majello B, Battista S, Lembo F, et al. A novel member of the BTB/POZ family, PATZ, associates with the RNF4 RING finger protein and acts as a transcriptional repressor. J Biol Chem. 2000;275:7894–901. doi: 10.1074/jbc.275.11.7894. [DOI] [PubMed] [Google Scholar]
- 22.Mastrangelo T, Modena P, Tornielli S, Bullrich F, Testi MA, Mezzelani A, et al. A novel zinc finger gene is fused to EWS in small round cell tumor. Oncogene. 2000;19:3799–804. doi: 10.1038/sj.onc.1203762. [DOI] [PubMed] [Google Scholar]
- 23.Deviller P, Vallier P, Bata J, Saez JM. Distribution and characterization of cAMP-dependent protein kinase isoenzymes in bovine adrenal cells. Mol Cell Endocrinol. 1984;38:21–30. doi: 10.1016/0303-7207(84)90141-2. [DOI] [PubMed] [Google Scholar]
- 24.Rubin CS, Erlichman J, Rosen OM. Cyclic adenosine 3′,5′-monophosphate-dependent protein kinase of human erythrocyte membranes. J Biol Chem. 1972;247:6135–9. [PubMed] [Google Scholar]
- 25.Tian X, Sun D, Zhang Y, Zhao S, Xiong H, Fang J. Zinc finger protein 278, a potential oncogene in human colorectal cancer. Acta Biochim Biophys Sin (Shanghai) 2008;40:289–96. doi: 10.1111/j.1745-7270.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- 26.Tritz R, Mueller BM, Hickey MJ, Lin AH, Gomez GG, Hadwiger P, et al. siRNA Down-regulation of the PATZ1 Gene in Human Glioma Cells Increases Their Sensitivity to Apoptotic Stimuli. Cancer Ther. 2008;6:865–76. [PMC free article] [PubMed] [Google Scholar]
- 27.van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 28.Cho-Chung YS, Pepe S, Clair T, Budillon A, Nesterova M. cAMP-dependent protein kinase: role in normal and malignant growth. Crit Rev Oncol Hematol. 1995;21:33–61. doi: 10.1016/1040-8428(94)00166-9. [DOI] [PubMed] [Google Scholar]
- 29.Tortora G, Pepe S, Bianco C, Damiano V, Ruggiero A, Baldassarre G, et al. Differential effects of protein kinase A sub-units on Chinese-hamster-ovary cell cycle and proliferation. Int J Cancer. 1994;59:712–6. doi: 10.1002/ijc.2910590521. [DOI] [PubMed] [Google Scholar]
- 30.Tortora G, Pepe S, Bianco C, Baldassarre G, Budillon A, Clair T, et al. The RI alpha subunit of protein kinase A controls serum dependency and entry into cell cycle of human mammary epithelial cells. Oncogene. 1994;9:3233–40. [PubMed] [Google Scholar]
- 31.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–6. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi A, Yamagiwa H, Hoshino H, Muto A, Sato K, Morita M, et al. A combinatorial code for gene expression generated by transcription factor Bach2 and MAZR (MAZ-related factor) through the BTB/POZ domain. Mol Cell Biol. 2000;20:1733–46. doi: 10.1128/mcb.20.5.1733-1746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]