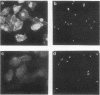
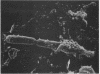
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaoui A., Mounier J., Prévost M. C., Sansonetti P. J., Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992 Jun;6(12):1605–1616. doi: 10.1111/j.1365-2958.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry B., Maurelli A. T., Clerc P., Sadoff J. C., Sansonetti P. J. Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol. 1987 Dec;133(12):3403–3413. doi: 10.1099/00221287-133-12-3403. [DOI] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse J. M., Stover C. K., Oaks E. V., Venkatesan M., Kopecko D. J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987 Jun;169(6):2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORMAL S. B., KUNDEL D., SCHNEIDER H., KUNEVN, SPRINZ H. Studies with Vibrio cholerae in the ligated loop of the rabbit intestine. Br J Exp Pathol. 1961 Oct;42:504–510. [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990 Nov;162(5):1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- Goldberg M. B., Bârzu O., Parsot C., Sansonetti P. J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993 Apr;175(8):2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High N., Mounier J., Prévost M. C., Sansonetti P. J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992 May;11(5):1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Rohde M., Wehland J., Timmis K. N. Intercellular spread of Shigella flexneri through a monolayer mediated by membranous protrusions and associated with reorganization of the cytoskeletal protein vinculin. Infect Immun. 1991 Oct;59(10):3463–3471. doi: 10.1128/iai.59.10.3463-3471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992 Oct;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991 Mar;16(3):87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Vasselon T., Hellio R., Lesourd M., Sansonetti P. J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992 Jan;60(1):237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Nakamura A., Nakaya R. Cinemicrographic study of tissue cell cultures infected with Shigella flexneri. Jpn J Med Sci Biol. 1968 Aug;21(4):259–273. doi: 10.7883/yoken1952.21.259. [DOI] [PubMed] [Google Scholar]
- Polotskii YuE, Snigirevskaya E. S., Dragunskaya E. M. Electron-microscopic data on the method of penetration of shigellas into the intestinal epithelial cells. Bull Exp Biol Med. 1974 Aug;77(2):202–205. doi: 10.1007/BF00809636. [DOI] [PubMed] [Google Scholar]
- Prévost M. C., Lesourd M., Arpin M., Vernel F., Mounier J., Hellio R., Sansonetti P. J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992 Oct;60(10):4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Newland J. W., Tall B. D., Formal S. B., Hale T. L. Intracellular spread of Shigella flexneri associated with the kcpA locus and a 140-kilodalton protein. Infect Immun. 1989 Feb;57(2):477–486. doi: 10.1128/iai.57.2.477-486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., d'Hauteville H., Ecobichon C., Pourcel C. Molecular comparison of virulence plasmids in Shigella and enteroinvasive Escherichia coli. Ann Microbiol (Paris) 1983 May-Jun;134A(3):295–318. [PubMed] [Google Scholar]
- Sasakawa C., Adler B., Tobe T., Okada N., Nagai S., Komatsu K., Yoshikawa M. Functional organization and nucleotide sequence of virulence Region-2 on the large virulence plasmid in Shigella flexneri 2a. Mol Microbiol. 1989 Sep;3(9):1191–1201. doi: 10.1111/j.1365-2958.1989.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Makino S., Yamada M., Okada N., Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988 Jun;170(6):2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O. I., Hartwig J. H., Brotschi E. A., Stossel T. P. Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol. 1980 Feb;84(2):215–224. doi: 10.1083/jcb.84.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Formal S. B., Sprinz H. Exerimental acute colitis in the Rhesus monkey following peroral infection with Shigella flexneri. An electron microscope study. Am J Pathol. 1968 Mar;52(3):503–529. [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A. Bacterial pathogens caught in the actin. Curr Biol. 1992 Dec;2(12):649–651. doi: 10.1016/0960-9822(92)90115-q. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Hellio R., Sansonetti P. J. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992 Mar;60(3):1031–1040. doi: 10.1128/iai.60.3.1031-1040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Prevost M. C., Hellio R., Sansonetti P. J. Stress fiber-based movement of Shigella flexneri within cells. Infect Immun. 1991 May;59(5):1723–1732. doi: 10.1128/iai.59.5.1723-1732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef J. S., Keren D. F., Mailloux J. L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989 Mar;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Hauteville H., Sansonetti P. J. Phosphorylation of IcsA by cAMP-dependent protein kinase and its effect on intracellular spread of Shigella flexneri. Mol Microbiol. 1992 Apr;6(7):833–841. doi: 10.1111/j.1365-2958.1992.tb01534.x. [DOI] [PubMed] [Google Scholar]