Abstract
In adult stroke models, 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP), a sigma receptor agonist, attenuates activity of neuronal nitric oxide synthase (nNOS), blunts ischemia-induced nitric oxide production, and provides neuroprotection. Here, we tested the hypothesis that PPBP attenuates neuronal damage in a model of global hypoxia-ischemia (H–I) in newborn piglets. Piglets subjected to hypoxia followed by asphyxic cardiac arrest were treated with saline or two dosing regimens of PPBP after resuscitation. Sigma-1 receptors were found in striatal neurons. PPBP dose-dependently protected neurons in putamen at 4 days of recovery from H–I. Immunoblots of putamen extracts at 3 h of recovery showed that PPBP decreased H–I-induced recruitment of nNOS in the membrane fraction and reduced the association of nNOS with NMDA receptor NR2 subunit. The latter effect was associated with changes in the coupling of nNOS to postsynaptic density-95 (PSD-95), but not NR2-PSD-95 interactions. Moreover, PPBP suppressed NOS activity in the membrane fraction and reduced H–I–induced nitrative and oxidative damage to proteins and nucleic acids. These findings indicate that PPBP protects striatal neurons in a large animal model of neonatal H–I and that the protection is associated with decreased coupling of nNOS to PSD-95.
Keywords: cardiac arrest, global cerebral ischemia, hypoxia-ischemia, neonate, NMDA receptor, PSD-95
Introduction
Neonatal hypoxic-ischemic encephalopathy, resulting from difficulties during labor and delivery or cardiorespiratory arrest after birth, causes significant infant mortality and morbidity (Sunshine, 1997). N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity is considered to play an important role in this pathophysiological process (Ferriero, 2004; Johnston, 2005). However, blocking NMDA receptors has proven to be an unsuccessful treatment strategy in large-animal models (LeBlanc et al., 1991), possibly because the positive effects of NMDA receptors are also suppressed (Ikonomidou et al., 1999). Therefore, alternative strategies for treating neonatal hypoxic-ischemic brain damage by modulating NMDA receptor function or interrupting the excitotoxic signaling cascade may hold greater promise.
Sigma-1 receptors, mainly localized on endoplasmic reticulum (ER), are widely distributed in neurons, astrocytes, and oligodendrocytes of brain (Hayashi and Su, 2001; Hayashi and Su, 2008). They can modulate a variety of intracellular signal transduction pathways through protein-protein interactions. In the resting state, sigma-1 receptors on the ER associate with inositol 1,4,5-triphosphate receptors and regulate Ca2+ efflux from the ER (Hayashi and Su, 2001; Hayashi and Su, 2007). After stimulation by its ligands, sigma-1 receptors translocate to the plasma membrane, where they regulate membrane-associated ion channels, such as NMDA receptors (Monnet et al., 1990).
Studies have shown the potential usefulness of sigma receptor ligands in treating adult brain ischemia. For example, systemic administration of 4-phenyl-1-(4-phenylbutyl)-piperidine (PPBP), a sigma receptor ligand, prevented early brain injury in animal models of transient focal ischemia (Takahashi et al., 1995; Takahashi et al., 1996). In addition, the high-affinity sigma-1 receptor agonist 1, 3-di-o-tolyl-guanidine reduced infarct volume, even when given 24 h after stroke (Ajmo et al., 2006). The neuroprotective effect of sigma receptor ligands involves the prevention of ischemia-induced intracellular Ca2+ dysregulation (Katnik et al., 2006). PPBP in particular has been shown to attenuate neuronal nitric oxide synthase (nNOS) activity and ischemia-evoked nitric oxide (NO) production (Bhardwaj et al., 1998; Goyagi et al., 2001) and to increase phosphorylation of CREB and bcl-2 expression in neurons after oxygen-glucose deprivation (Yang et al., 2009; Yang et al., 2007a). Therefore, sigma receptor ligands might regulate NMDA receptor-mediated excitotoxicity at post-receptor levels of the cell death cascade, although the details of this modulation remain unclear.
Whereas data on the neuroprotective action of sigma receptor ligands in adult cerebral ischemia has been published widely, the effect of sigma receptor ligands in neonatal hypoxia-ischemia (H–I) remains unexplored. To evaluate their role in neonatal H–I, the sigma ligand PPBP was selected because of its ability to provide robust neuroprotection in adult animals when administered systemically (Takahashi 1995; Takahashi 1996). We investigated whether PPBP could attenuate hypoxic-ischemic neuronal damage in newborn piglet brain and how PPBP might modulate NMDA receptor-mediated neurotoxicity after global H–I. We focused on putamen because this region is the most vulnerable to H–I in this model (Martin et al., 1997). We found that PPBP protects striatal neurons from ischemic damage through mechanisms that involve occlusion of nNOS-PSD95 coupling and supression of NOS activity and oxidative/nitrative cellular injury.
Materials and Methods
Experimental protocol
Procedures carried out on piglets were approved by the Animal Care and Use Committee of the Johns Hopkins University and have been described previously (Yang et al., 2007b). In brief, 4- to 7-day-old male piglets (2.5 to 3 kg) were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal) and intubated. Catheters were placed into a femoral artery and vein under aseptic conditions. To induce H–I, inspired O2 was decreased to 10.0 ± 0.2% for 40 mins, followed by ventilation with 21% O2 for 5 mins and airway occlusion for 7 mins to produce cardiac arrest. The 5-min period of 21% O2 ventilation was required for cardiac resuscitation. Piglets were resuscitated by ventilation with 50% O2, manual chest compressions, and/or intravenous injection of epinephrine until return of spontaneous circulation. After resuscitation, inspired O2 was gradually reduced to 30% to maintain arterial O2 saturation. Arterial blood pressure, blood gases, and glucose were monitored until the animal regained consciousness. Sham-operated animals were subjected to catheterization but not hypoxia and asphyxia.
For the low-dose PPBP treatment, hypoxic-ischemic piglets received continuous intravenous infusion of 1 µmol/kg/h of PPBP (Tocris Bioscience; Ellisville, MO) from 5 mins to 6 h of recovery. This dose was effective in decreasing infarct volume from transient focal ischemia in adult rats and cats (Takahashi 1995; Takahashi 1996). To determine if increasing the dose provided greater protection in piglets, sham-operated or hypoxic-ischemic piglets were administered a high-dose regimen consisting of 3 µmol/kg of PPBP injected intravenously at 5 mins of recovery and then a maintenance infusion of 2 µmol/kg/h for the first 3 h and 1 µmol/kg/h for the next 3 h. Although doses as high as 10 µmol/kg/h can provide long-term protection in rats (Harakuni 2000), this dose was found to produce substantial hypotension in the piglet and thus was not used. To compare the neuroprotective effects of PPBP with those of a well-established NMDA receptor antagonist, hypoxic-ischemic piglets were injected intravenously with 1 mg/kg MK-801 (Ascent Scientific; Princeton, NJ; 20 min before hypoxia). Control animals received the 0.9% saline vehicle (containing 0.5% ethanol).
Because neuronal cell death progresses between 3 and 24 h after reoxygenation in this region, we made biochemical measurements at 3 h before the loss of neurons would affect the measurements. To allow for a possible delay in the maturation of histological injury with PPBP treatment, an extra 3 days was added to the recovery time beyond the normal 1 day required for full expression of medium spiny neuronal cell loss (Martin et al., 2000).
Neurobehavioral Assessment
A neurologic deficit score (Agnew et al., 2003; Brambrink et al., 1999; Yang et al., 2007b) (NDS; 0 = best outcome, 154 = worst outcome) was used to quantify neurologic function in piglets based on seven different components: 1) level of consciousness (maximum score 15); 2) brain stem function (respiration, light reflex, corneal reflex, and swallow reflex; maximum score 22); 3) sensory responses (olfaction, vision, audition, algesia, and tactile localization; maximum score 20); 4) motor function (muscle tone in trunk and limbs, postural reflexes, and mobility; maximum score 46); 5) other activities (appetite, vocalization, psychomotor activity, and social interactivity; maximum score 16); 6) spatial orientation (during locomotion, with sniffing, and toward depth; maximum score 20); 7) excitability (startle myoclonus, seizures; maximum score 15).
Immunohistochemistry
The anesthetized piglets were perfused transcardially with ice-cold phosphate-buffered saline and 4% paraformaldehyde (PFA) at 3 h or 4 days of recovery. Brains were removed, bisected midsagittally and cut into 1-cm slabs. The left forebrain was paraffin embedded for histology with hematoxylin and eosin (H&E) staining and neuropathological assessment of damaged neurons. To show that the 4-to-7-day piglet striatum is cytoarchitectonically and chemoarchitectonically distinct from the adult pig striatum, an adult pig was perfusion-fixed with 4% PFA. The right forebrains from all animals were cryoprotected, frozen, and cut into serial 40 µm coronal sections through the putamen. In H–I and control piglets mmunohistochemical assessments were made of sigma-1 receptor; 8-nitroguanosine, a marker of DNA nitration (Akaike et al., 2003); and 8-hydroxyguanosine (OHG) or 8-hydroxy-2-deoxyguanosine (OHdG), markers of oxidative damage to DNA and RNA (Martin et al., 2000). Free-floating sections were blocked in 10% normal horse serum and incubated with, mouse anti-DNA and RNA oxidative damage markers (1:1500, QED Bioscience. San Diego, CA), or mouse anti-nitroguanosine (1:2000, Dojindo, Japan) overnight at 4°C. For comparisons between newborn and adult pigs (supplemental Figure 1), sections were stained with cresyl violet and immunohistochemically using antibodies to microtubule associated protein-2 (MAP-2) (Roche, Mannheim, Germany) and calbindin D28 (Sigma-Aldrich, St. Louis MO). Antibody binding was visualized by incubating sections with biotinylated anti-mouse or anti-rabbit IgG (1:200, Vector Laboratories, Burlingame, CA) and VECTASTAIN Elite ABC reagent (Vector). Immunostaining was developed with diaminobenzidine (Vector) as a chromogen. Negative controls were treated without primary antibodies and showed no positive signals.
Immuno-reactivity was quantified using ImageJ software (version 1.42q, NIH), the optical density (OD) was integrated over the area of individual cells. Four to five positive stained cells per section were randomly selected in anterior, median, and posterior putamen sections (bregma level: +15 mm, +9 mm, and +5 mm) (Salinas-Zeballos et al., 1986). The area of stained cells was measured and the OD per area was quantified after subtracting the background OD in unstained tissue. Comparisons between groups were based on measurements from 85 to 150 stained cells. To confirm the expression of sigma-1 receptor in putamen, sections were incubated with rabbit anti-sigma-1 receptor (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-NeuN (1:500, Chemicon, Temecula, CA), followed by incubation with anti-rabbit Cy3 (1:500, Jackson ImmunoResearch, West Grove, PA) and anti-mouse Alexa Fluor 488 (1:500, Invitrogen, Eugene, OR).
Co-immunoprecipitation and Western Blotting
Brain tissues obtained from hypoxic-ischemic piglets at 3 h of recovery and time-matched sham-operated animals were homogenized and fractionated into membrane-enriched and cytosol-enriched fractions, as described previously (Guerguerian et al., 2002; Yang et al., 2007b).
For co-immunoprecipitation (co-IP), membrane-enriched homogenates (500 µg of protein) were pre-incubated for 1 h with 40 µl protein A/G-Sepharose (GE Healthcare, Sweden) and then centrifuged at 12,000 × g to remove any nonspecific binding to protein A/G. The supernatant was incubated with 2 µg anti-postsynaptic density-95 (PSD-95) antibody (Santa Cruz) or anti-NR2A/B antibody (Chemicon) overnight at 4°C and then with protein A/G-Sepharose (40 µl) overnight at 4°C. Samples were centrifuged at 12,000 × g, and the pellets were washed five times with homogenization buffer (Yang et al., 2007b). Bound proteins were eluted by adding sample buffer (20 µl) and boiling at 100°C for 5 mins. Samples were finally centrifuged and supernatants were analyzed by Western blotting.
For Western blotting, 20 µg of total protein (boiled) or immunoprecipitated protein were separated by 4 – 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The membranes were probed with the following primary antibodies: anti-NR1 (1:2000, BD Pharmingen, San Jose, CA), anti-phospho-NR1 Ser896, anti-phospho-NR1 Ser897 (1:2000, Upstate, Lake Placid, NY), anti-synaptophysin (1:20,000, Chemicon), anti-actin (1:2000, Santa Cruz), anti-sigma-1 receptor, anti-nNOS (1:1000, Santa Cruz), anti-NR2 (1:200, Chemicon), anti-PSD-95 (1:500, Santa Cruz), anti-phospho-nNOS Ser847 (1:2000, Abcam, Cambridge, MA), or anti-nitrotyrosine (1:40,000, Upstate). Synaptophysin and actin were used as protein loading standards for membrane- and cytosol-enriched fractions, respectively. After being immunoblotted, the bands were scanned and analyzed with Quantityone software (Bio-Rad, Hercules, CA). Values of optical density (OD) were normalized to the value of a sham-operated, saline-treated animal on each gel. Each experiment was performed in quadruplicate using tissue from four to six piglets per group.
NOS Activity Assay
Membrane-enriched homogenates (50 µg of protein) were used to measure NOS activity by the conversion of [14C] arginine to [14C] citrulline with a NOS activity assay kit (Cayman Chemical. Ann Arbor, MI). Briefly, homogenates were incubated with 40 µl reaction buffer containing 1.25 mM NADPH, 0.75 mM CaCl2, and 1.25 µCi/ml [14C] arginine for 30 min; the reactions were terminated with a stop buffer. Remaining [14C] arginine was removed from the solution with equilibrated resin. Finally, the radioactivity was quantified by liquid scintillation spectroscopy and expressed as a percentage of the value of a sham-operated, saline-treated animal.
Data and Statistical Analysis
Profile counting was used to estimate ischemic neuronal damage in mid-coronal sections of putamen on H&E-stained paraffin sections. In sections that were matched for level, the number of ischemic and non-ischemic neuronal profiles was counted in an observer-blinded fashion in seven non-overlapping microscopic fields at 1000× power in anterior, median, and posterior putamen sections (bregma level: +15 mm, +9 mm, and +5 mm). The values were averaged to obtain a single value of viable neurons per mm2 for each piglet to be used in the statistical analysis.
All values are expressed as mean ± s.d. The neurological deficit score was analyzed by two-way analysis of variance (ANOVA) with repeated measures. Other measurements were analyzed with one-way ANOVA followed by the Student-Newman-Keuls multiple range test. P < 0.05 was considered statistically different.
Results
PPBP reduced neuronal damage in putamen after H–I
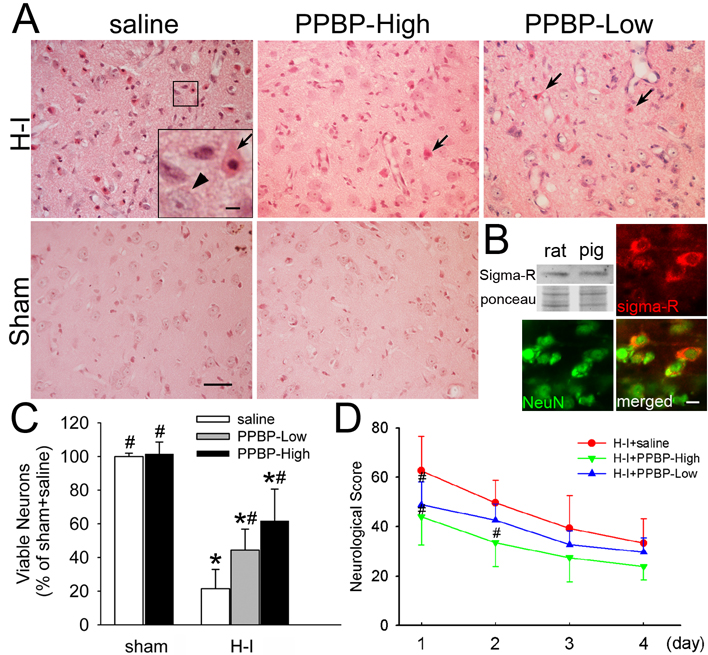
Previous studies have shown that brain damage in this model is selectively distributed in basal ganglia and somatosensory cortex, with a consistent neuronal death in central putamen at 4 days of recovery (Martin et al., 1997; Martin et al., 2000). In the present study, we observed normal cellular morphology and cytoarchitecture in the striatum and sensorimotor cortex (Figure 1A) of sham-operated animals treated with saline or high-dose PPBP. Neuronal density in the putamen of sham-operated animals treated with PPBP was 101.5 ± 7.1% (n = 4) of those treated with saline (n = 4).
Figure 1.
Effects of PPBP on neuronal damage and neurologic deficits in piglets subjected to hypoxia-ischemia (H–I). Piglets exposed to H–I or sham surgery were infused intravenously with saline, low-dose PPBP (PPBP-Low), or high-dose PPBP (PPBP-High). (A) Representative photographs of H&E-stained sections show that low and high doses of PPBP alleviate ischemic neuronal damage in putamen at 4 days of recovery. Arrows point to representative ischemia-damaged neurons and arrow head shows the normal neurons. Scale bar=40 µm and scale bar in insert = 8 µm. (B) Western blot and double-immunofluorescent results show that sigma-1 receptors exist in pig striatum and are localized mainly in neurons. Ponceau S was used as a loading control. Scale bar = 8 µm. (C) Quantitative results for viable putamen neurons expressed as a percent of the mean value of the sham+saline group. (D) Neurologic scores during the 4-day recovery. Data represent means ± s.d. (n = 4 to 10 per group). *P < 0.05 versus sham+saline group; #P < 0.05 versus H–I+saline group; ANOVA followed by the Student-Newman-Keuls test.
During the 40 mins of hypoxia, mean arterial blood pressure (MABP) was maintained near the baseline values and did not differ among groups (Table 1). Severe bradycardia and hypotension occurred during the 7-min period of asphyxia. Successful resuscitation was achieved in 88% of piglets. The amount of epinephrine used in piglets successfully resuscitated did not differ among those subsequently treated with saline (62 ± 111 µg/kg), low-dose PPBP (34 ± 57 µg/kg), and high-dose PPBP (46 ± 73 µg/kg). Most physiological parameters were not different among the H–I groups. However, piglets that received the high-dose of PPBP after H–I had lower MABP during the infusion period than did those that received saline. Sham-operated piglets that received the high-dose PPBP infusion also exhibited lower MABP (57 ± 6 vs 70 ± 8 mmHg) at 3 h of recovery. Subsequent mortality during the 4-day recovery period was 3 of 13 in the saline group, 1 of 8 in the low-dose PPBP group, and 1 of 8 in the high-dose PPBP group. Mortality included those euthanized because of severe generalized seizures (2, 1, and 0 in saline, low-dose PPBP, and high-dose PPBP groups, respectively).
Table 1.
Physiologic parameters during and after hypoxia-ischemia
| Baseline | Hypoxia | 21% O2 | Asphyxia | Post-resuscitation |
|||
|---|---|---|---|---|---|---|---|
| 37 min | 5 min | 1 hr | 2 hr | 3 hr | |||
| MABP (mmHg) | |||||||
| Saline | 75 ± 5 | 79 ± 25 | 99 ± 13 | 23 ± 12 | 76 ± 8 | 69 ± 4 | 70 ± 4 |
| PPBP-Low | 75 ± 6 | 73 ± 20 | 84 ± 5 | 26 ± 11 | 65 ± 5 | 66 ± 5 | 68 ± 4 |
| PPBP-High | 69 ± 10 | 67 ± 8 | 88 ± 15 | 27 ± 11 | 54 ± 5# | 53 ± 7# | 54 ± 8# |
| Arterial PO2 (mmHg) | |||||||
| Saline | 135 ± 18 | 23 ± 3 | 67 ± 15 | 11 ± 8 | 117 ± 10 | 126 ± 17 | 134 ± 22 |
| PPBP-Low | 134 ± 20 | 21 ± 3 | 70 ± 8 | 7 ± 2 | 131 ± 24 | 132 ± 21 | 132 ± 20 |
| PPBP-High | 127 ± 21 | 22 ± 4 | 76 ± 17 | 11 ± 5 | 115 ± 17 | 118 ± 17 | 120 ± 12 |
| Arterial PCO2 (mmHg) | |||||||
| Saline | 39 ± 3 | 38 ± 5 | 37 ± 10 | 84 ± 15 | 38 ± 5 | 40 ± 5 | 37 ± 5 |
| PPBP-Low | 37 ± 2 | 40 ± 5 | 38 ± 5 | 79 ± 12 | 41 ± 7 | 37 ± 6 | 37 ± 4 |
| PPBP-High | 40 ± 4 | 43 ± 5 | 40 ± 7 | 84 ± 11 | 41 ± 4 | 40 ± 5 | 41 ± 3 |
| Arterial pH | |||||||
| Saline | 7.40 ± 0.04 | 7.16 ± 0.16 | 7.09 ± 0.19 | 6.83 ± 0.10 | 7.33 ± 0.07 | 7.44 ± 0.06 | 7.48 ± 0.04 |
| PPBP-Low | 7.44 ± 0.04 | 7.25 ± 0.05 | 7.21 ± 0.10 | 6.80 ± 0.08 | 7.38 ± 0.07 | 7.46 ± 0.05 | 7.49 ± 0.03 |
| PPBP-High | 7.42 ± 0.03 | 7.22 ± 0.11 | 7.12 ± 0.14 | 6.80 ± 0.08 | 7.26 ± 0.15 | 7.40 ± 0.06 | 7.44 ± 0.07 |
| Arterial glucose (mmol/L) | |||||||
| Saline | 3.3 ± 0.9 | 7.9 ± 3.8 | 9.5 ± 5.2 | 14.1 ± 8.0 | 5.6 ± 4.1 | 4.1 ± 2.2 | 3.7 ± 1.3 |
| PPBP-Low | 3.5 ± 1.2 | 10.1 ± 2.2 | 10.8 ± 3.7 | 12.0 ± 4.5 | 7.3 ± 4.7 | 4.7 ± 1.8 | 3.9 ± 1.7 |
| PPBP-High | 2.7 ± 1.1 | 10.0 ± 5.4 | 8.9 ± 5.0 | 13.3 ± 7.2 | 6.2 ± 2.8 | 4.3 ± 2.2 | 3.4 ± 1.4 |
| Rectal Temperature (°C) | |||||||
| Saline | 38.4 ± 0.2 | 38.7 ± 0.4 | 38.8 ± 0.5 | 38.9 ± 0.5 | 38.8 ± 0.4 | 38.7 ± 0.2 | 38.7 ± 0.5 |
| PPBP-Low | 38.6 ± 0.5 | 38.6 ± 0.2 | 38.7 ± 0.2 | 38.8 ± 0.2 | 39.1 ± 0.3 | 38.4 ± 0.3 | 38.5 ± 0.5 |
| PPBP-High | 38.4 ± 0.5 | 38.8 ± 0.4 | 39.0 ± 0.4 | 39.0 ± 0.3 | 38.6 ± 0.4 | 38.8 ± 0.7 | 38.6 ± 0.3 |
P<0.05 vs H–I+saline
Western blots of piglet striatum confirmed the presence of sigma-1 receptors with a molecular weight similar to that in mature rat striatum (Figure 1B). Immunohistochemistry showed that sigma-1 receptors were localized specifically in the perinuclear region of neurons in newborn piglet striatum (Figure 1B). Four days after H–I, most putaminal neurons of saline-treated animals exhibited ischemic morphology that consisted of cytoplasmic microvacuolation, eosinophilia, and nuclear pyknosis, or no longer possessed distinct structure based on H&E-stained sections (Figure 1A). At this time point, the number of viable neurons that could be found in the putamen was 21.5 ± 11.4% (n = 10) that of sham-operated animals. The density of viable neurons was significantly increased by post-ischemic treatment with low-dose PPBP (44.4 ± 12.5% of sham, saline group, n = 7) and high-dose PPBP (61.7 ± 19% of sham, saline group, n = 7). Protection with the low dose of PPBP was equivalent to that seen with the NMDA antagonist MK-801 (44.5 ± 17.5% of sham, saline group, n = 7).
Consistent with previous work (Martin et al., 1997; Martin et al., 2000; Yang et al., 2007b), neuronal injury was less severe in the caudate nucleus, with the density of viable neurons equal to 66.8 ± 32.0%, 67.5 ± 15.6%, and 74.3 ± 26.4% of the sham saline value in the H–I saline, low-dose PPBP, and high-dose PPBP groups, respectively. No significant differences were found among H–I groups.
Neurobehavioral deficits were the most severe on the first day of recovery from H–I (Figure 1D). Most H–I piglets treated with saline showed impaired consciousness, no light and/or no auditory reflexes, low muscle tone, and sometimes no response to pain stimulation. Two-way repeated measures ANOVA indicated an overall effect of treatment (P < 0.001) and time (P < 0.001). High-dose PPBP treatment significantly improved neurobehavioral recovery of H–I piglets at 24 and 48 h of recovery, and low-dose PPBP treatment reduced deficits at 24 h of recovery. Sham-operated animals treated with saline or high-dose PPBP did not show neurologic deficit after recovery from anesthesia.
Based on our histological and neurologic deficit results, high-dose PPBP treatment was used for the biochemical experiments.
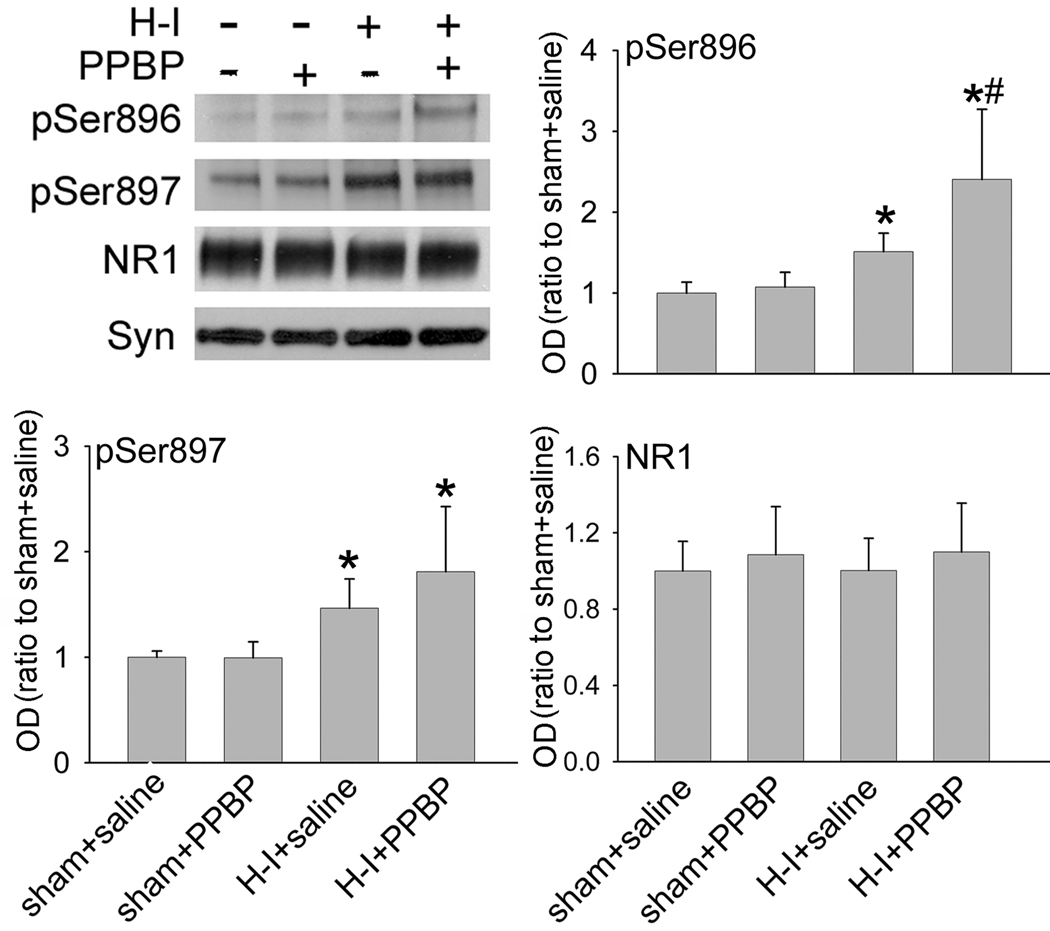
PPBP altered ischemia-induced phosphorylation of NR1 in a site-specific manner
NR1 phosphorylation occurs at Ser897 by protein kinase A (PKA) and at Ser896 by protein kinase C (PKC) (Tingley et al., 1997). Because H–I produces an increase in NR1 phosphorylation soon after resuscitation in piglet putamen (Guerguerian et al., 2002; Mueller-Burke et al., 2008; Yang et al., 2007b), we tested the possibility that PPBP altered the level of phosphorylated NR1 after H–I. Western blot results (Figure 2) showed that PPBP did not change the basal level of phosphorylated NR1 Ser896 or Ser897 in sham-operated animals. H–I increased phosphorylation of NR1 at Ser897 and Ser896 in putamen at 3 h of recovery. The increase in Ser897 phosphorylation was not inhibited by PPBP. Interestingly, PPBP augmented the H–I–induced level of Ser896 phosphorylation. The levels of total NR1 protein remained unchanged after H–I and PPBP treatment.
Figure 2.
Western blot analysis showing effects of PPBP on levels of Ser896- and Ser897-phosphorylated NR1 and total NR1 in membrane-enriched fraction of putamen at 3 h of recovery (n = 4 to 6 per group). Synaptophysin (Syn) was used as a loading control. Data (means ± s.d.) were normalized to the sham+saline value. *P < 0.05 versus sham+saline groups; #P < 0.05 versus H–I+saline group; one-way ANOVA followed by the Student-Newman-Keuls test.
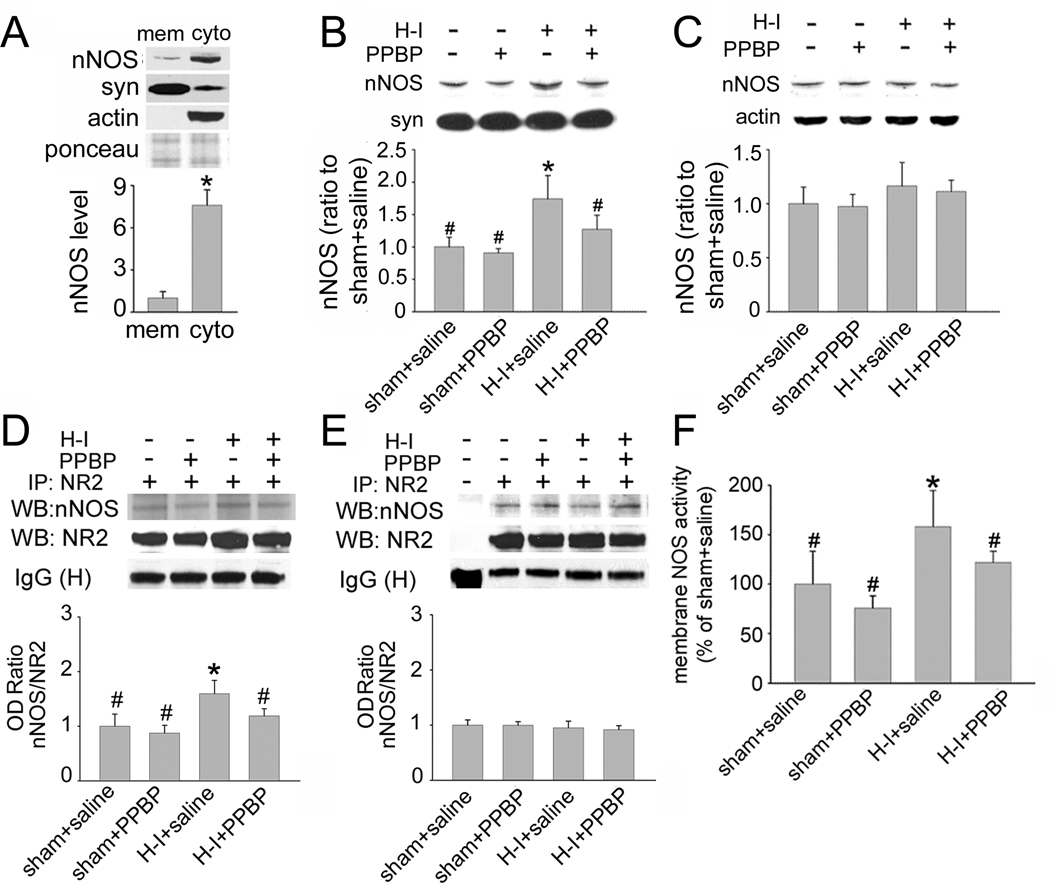
PPBP reduced the formation of nNOS-PSD-95 complex in membrane after H–I
PPBP has been shown to attenuate nNOS activity and to reduce ischemia-evoked NO production in an adult focal ischemia model (Goyagi et al., 2001). To study how PPBP might affect nNOS function after H–I, Western blot analysis was used to examine the expression level of nNOS in the membrane-enriched fraction in putamen of H–I and sham-operated piglets at 3 h of recovery. We found that in sham-operated piglets, nNOS expression was greater in the cytosol-enriched fraction of putamen than in the membrane-enriched fraction (ratio to nNOS level in membrane-enriched fraction: 7.6 ± 1.1; Figure 3A). PPBP did not change the basal level of nNOS in sham-operated animals. However, the nNOS level in the membrane fraction was significantly increased 3 h after H–I (Figure 3B). This increase was markedly reduced by PPBP treatment. In contrast to that in membrane-enriched fraction, the nNOS level in the cytosol-enriched fraction was unchanged 3 h after H–I or by PPBP treatment (Figure 3C). This lack of a detectable decrease in nNOS in the cytosolic fraction is likely because the expression of nNOS protein in the cytosol-enriched fraction was already substantially greater than that in the membrane-enriched fraction.
Figure 3.
Effects of PPBP treatment on nNOS expression and activity in putamen of sham-operated or H–I piglets at 3 h of recovery (n = 4 to 6 per group). (A) nNOS expression was greater in the cytosol (cyto)-enriched fraction of putamen than in the membrane (mem)-enriched fraction. Ponceau S was used as a loading control. H–I increased the nNOS level in the membrane-enriched fraction (B), but not in the cytosol-enriched fraction (C). PPBP alleviated H–I–potentiated nNOS expression in the membrane-enriched fraction. Immunoprecipitation with antibody against NR2A/2B resulted in the co-precipitation of nNOS in the membrane-enriched putamen fraction (D) and prefrontal cortex (E). However, H–I increased the interaction between nNOS and NR2 only in the putamen [as seen by an increase in optical density (OD) ratio] (D). The increase was restored to control levels by PPBP treatment. (F) PPBP treatment alleviated H–I–induced NOS activity in membrane enriched fraction of putamen. All data are shown as means ± s.d., normalized to the sham+saline value. *P < 0.05 versus sham+saline group; #P < 0.05 versus HI+ saline group; one-way ANOVA followed by the Student-Newman-Keuls test.
nNOS activity can be regulated by its coupling to the NMDA receptor NR2 subunit via PSD-95 (Aarts et al., 2002; Sattler et al., 1999). Considering the abundant expression of NR2A and NR2B in newborn piglet striatum (Guerguerian et al., 2002), we used co-IP to investigate whether PPBP changes nNOS level in the membrane-enriched fraction through an altered association of nNOS and NR2A/2B. Immunoprecipitation with NR2A/2B antibody resulted in co-precipitation of nNOS (Figure 3D). H–I significantly increased the interaction of NR2A/2B with nNOS in the putamen at 3 h, but this increase was attenuated by PPBP treatment (ratio to sham+saline: 1.19 ± 0.13, P < 0.05). PPBP did not change the basal level of nNOS/NR2 association. To elucidate if the association of nNOS and NR2A/B after H–I and PPBP treatment is specific for regions destined for neuronal death, the level of NR2A/2B and nNOS association was examined in the prefrontal cortex, which is spared from neuronal death. No change of association was detected there after H–I or with PPBP treatment (Figure 3E).
These results were also consistent with NOS catalytic activity in the membrane-enriched fraction of putamen (Figure 3F). NOS activity was increased at 3 h after H–I. PPBP administration had no significant effect on activity in tissue from sham-operated animals but reduced activity in tissue from H–I animals.
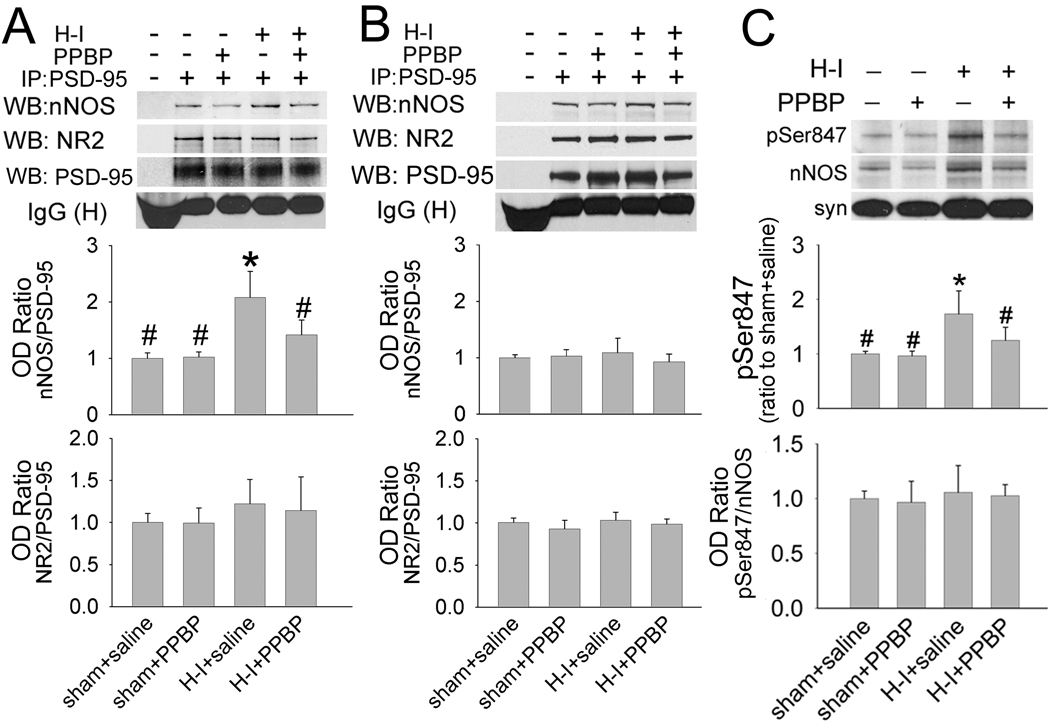
PSD-95 is involved in linking nNOS to NR2 and assembling a ternary complex that efficiently couples Ca2+ influx to NO synthesis (Sattler et al., 1999). To investigate the role of PSD-95 in linking nNOS to NR2 after H–I, we performed co-IP in putamen tissue using PSD-95 antibody at 3 h after H–I. Both NR2A/2B and nNOS could be co-immunoprecipitated with PSD-95. The level of coupling of PSD-95 to nNOS, but not coupling of PSD-95 to NR2A/2B, was significantly increased after H–I. This increase was significantly reduced by PPBP treatment (Figure 4A). The basal level of coupling of PSD-95 to NR2A/2B or nNOS remained unchanged by PPBP treatment in sham-operated animals. In contrast to that in putamen, the level of coupling of PSD-95 to NR2 or nNOS in prefrontal cortex (Figure 4B) was unchanged at 3 h after H–I or by PPBP treatment.
Figure 4.
The impact on association of NR2 with nNOS by H–I and PPBP treatment involves changes in coupling of nNOS to PSD-95, but not PSD-95 to NR2. Immunoprecipitation of membrane-enriched fraction of putamen (A) or prefrontal cortex (B) at 3 h of recovery with antibody against PSD-95 results in the co- precipitation of nNOS and NR2. H–I increased the interaction between nNOS and PSD-95 [as seen by an increase in optical density (OD) ratio] only in putamen. This increase was restored to control level by PPBP treatment. (C) Western blot analysis showed that H–I induced nNOS Ser847 phosphorylation in the membrane-enriched fraction at 3 h of recovery; the induction was prevented by PPBP treatment. The H–I– induced increase in phosphorylated Ser847 was proportional to the increase in total membrane nNOS, resulting in no change in the relative amount of phosphorylated protein. All data are shown as means ± s.d., normalized to the sham+saline value. *P < 0.05 versus sham+saline group; #P < 0.05 versus H–I+saline group; one-way ANOVA followed by the Student-Newman-Keuls test.
Although the precise mechanism that regulates nNOS anchoring to PSD-95 remains unclear, recent studies indicate that phosphorylation of nNOS at Ser 847 may play a role in modulating this coupling (Robison et al., 2005; Zhou et al., 2008). We investigated whether PPBP administration has an effect on the level of phospho-Ser847 in nNOS. Western blot results (Figure 4C) showed that H–I substantially increased the level of Ser847-phosphorylated nNOS in the membrane-enriched fraction of putamen at 3 h of recovery. This increase was attenuated by PPBP treatment. The H–I induction and PPBP reduction of nNOS Ser847 phosphorylation was proportionate to changes of nNOS expression in the membrane-enriched fraction, as indicated by the lack of change in the ratio of phospho-Ser847 to the total nNOS immunoreactivity.
PPBP reduced nitrative and oxidative stress after H–I
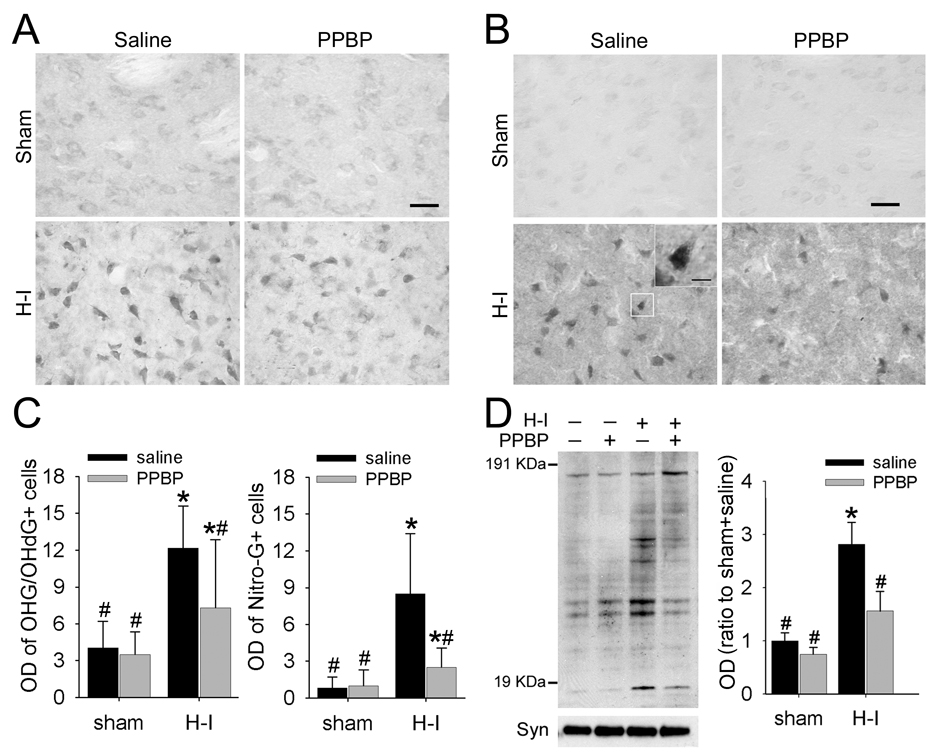
We used immunohistochemistry to study whether PPBP treatment reduces nitrative and oxidative damage to nucleic acids in putamen at 3 h after H–I. In addition, we subjected membrane-enriched extracts from putamen to immunoblot analysis to detect peroxynitrite-mediated oxidative protein damage. OHG/OHdG-positive staining was visible in the putamen of sham-operated and H–I animals (Figure 5A). However, the cellular patterns were quite different in the two groups. Positive cells in both groups had cytoplasmic labeling suggestive of an RNA pattern, although the intensity of cytoplasmic immunoreactivity was much higher in H–I animals. In contrast, intense nuclear labeling could be seen only in H–I–injured cells. Quantification with OD measurements of stained cells in putamen showed that the OD was three-fold greater in the H–I saline group than in the sham-operated saline groups. PPBP treatment did not change the basal OD level in sham animals but significantly decreased the cellular OD of OHG/OhdG immuno-reactivity compared to that of the saline-treated group at 3 h after H–I (Figure 5C).
Figure 5.
PPBP treatment reduces H–I–induced oxidative and nitrative stress in putamen at 3 h of recovery. Representative sections of immunohistochemical staining for 8-hydroxy-2-deoxyguanosine (OHdG)/8-hydroxy-guanosine (OHG) (A) and 8-nitroguanosine (Nitro-G) (B) in putamen of piglets at 3 h of recovery after sham surgery or H–I. Faint staining of cell bodies was present in sham-operated piglets, whereas elevated signals could be observed in both cytosol and nucleus. Inset: representative Nitro-G signal in nucleus. Scale bar = 50 µm; inset scale bar = 10 µm. (C) H–I increased optical density (OD) of OHdG/OHG and Nitro-G positive cells in putamen at 3 h of recovery; both were attenuated by PPBP treatment (n = 85 to 150 cells per group). (D) Western blot analysis showed that PPBP decreased the H–I–induced 3-nitrotyrosine immunoreactivity on multiple protein bands in putamen of sham-operated and H–I piglets at 3 h of recovery. All data are shown as mean ± s.d. *P < 0.05 versus sham+saline group; #P < 0.05 versus H–I+saline group; one-way ANOVA followed by the Student-Newman-Keuls test.
Similarly, few 8-nitroguanosine–positive cells were visible in the putamen of sham groups (Figure 5B), and no difference in cellular OD could be discerned between saline- and PPBP-treated sham groups. At 3 h after H–I, intensive 8-nitroguanosine signals were localized in cellular cytoplasm and nucleus (inset of Figure 5B). H–I increased the cellular OD by eight fold in the saline-treated group, and treatment with PPBP largely prevented this increase (Figure 5C).
PPBP administration did not alter the basal level of 3-nitrotyrosine immunoreactivity integrated over multiple protein bands in sham-operated animals (Figure 5D). With H–I, the total amount of 3-nitrotyrosine immunoreactivity increased in the saline group, but this increase was significantly reduced by PPBP.
Discussion
Our results showed that post-treatment of newborn piglets with the sigma receptor ligand PPBP after H–I 1) dose-dependently protected striatal neurons from H–I injury, 2) decreased H–I–induced recruitment of nNOS to the membrane, 3) alleviated the coupling of nNOS to PSD-95, 4) suppressed NOS activity in the membrane, and 5) reduced nitrative and oxidative damage to nucleic acids and proteins.
Previous studies have shown that PPBP provides robust neuroprotection in adult focal ischemia models and in cultured neurons (Goyagi et al., 2001; Harukuni et al., 1998; Takahashi et al., 1996; Yang et al., 2009; Yang et al., 2007a). Although striatum is considered to mature more rapidly than cerebral cortex during development, anatomical structures and some biochemical markers, such as GABAA receptor and dopaminergic markers, have shown apparent differences between newborn and adult striatum (Goetz et al., 2007; Laurie et al., 1992); (Haycock et al., 2003). Newborn pigs are markedly different from adult pigs in striatal cytology (cell density) and neuronal architecture as demonstrated in sections stained with cresyl violet and for calbindin D28 and MAP-2 (Supplementary Figure 1). Therefore, mechanisms of cellular injury drugs action, and efficacy of drug are not necessarily the same in newborn or developing striatum compared to adult fully-differentiated striatum, similar to the idea revealed in mouse cortical neurons (Martin et al., 2009).
Our present work demonstrates for the first time the neuroprotection by a sigma receptor ligand in neonatal H–I brain injury. We focused on putamen, where injury evolves rapidly during early reoxygenation and where excitotoxic mechanisms are prominent (Martin et al., 2000). The degree of protection with the low dose of PPBP was as great as that seen with MK-801. Moreover, protection with the high dose of PPBP was only marginally better possibly because complete blockage of NMDA receptors has counterproductive effects or because sigma receptors may modify the function of multiple receptors and transporters. H–I and reoxygenation lead to the formation of large quantities of oxidants, such as superoxide, hydrogen peroxide, hydroxyl radical, and peroxynitrite, which is also a potent nitrating agent. In our study, PPBP significantly reduced H–I oxidative and nitrative damage to nucleic acids and proteins. These effects of PPBP may underlie its neuroprotective properties in neonatal brain.
Sigma receptor ligands may act by interrupting cascades of NMDA receptor-mediated neuroexcitotoxicity. For example, sigma receptor ligands inhibited postsynaptic glutamate-evoked Ca2+ influx (Klette et al., 1995) and suppressed NO production (Goyagi et al., 2001). However, the molecular details of this modulation are not clear. Sigma receptor ligands may act to downregulate NMDA receptor/channel function by phosphorylation mechanisms, by altering receptor trafficking and localization, or by directly binding to the receptor. PPBP has been reported to inhibit currents of NR1a/2B expressed in Xenopus oocytes with an IC50 of 2 µM (Whittemore et al., 1997). Although we did not measure brain concentrations of PPBP, one might anticipate that the concentration would be <2 µM with an infusion of 1–2 µmol/kg/h, unless PPBP was preferentially distributed across the blood-brain barrier. Thus, we considered the possibility that PPBP may act through other mechanisms, such as alterations in H–I–induced changes of NR1 phosphorylation.
Our results in neonatal putamen show that PPBP potentiated phosphorylation of Ser896 induced by H–I. Although a trend for increased Ser897 phosphorylation was also evident after H–I, this trend was not statistically significant. Consistent with our data, others have shown that intrathecal administration of sigma receptor agonists potentiate NMDA-induced phosphorylation of NR1 at both the PKC-sensitive site, Ser896, and the PKA-sensitive site, Ser897, in the dorsal horn of adult mouse spinal cord (Kim et al., 2008). In striatum, the dopamine- and cAMP-regulated protein-32 plays a prominent role in NR1 phosphorylation at PKA-sensitive sites (Snyder et al., 1998). It is known that PKA-mediated phosphorylation of NR1 Ser897 promotes Ca2+ influx (Dudman et al., 2003; Scott et al., 2003; Skeberdis et al., 2006) and enhances NMDA receptor currents (Maldve et al., 2002). Our previous studies showed that whole-body hypothermia or dopamine D1 receptor antagonist administration attenuated H–I–induced phosphorylation of NR1 at Ser897 in piglet striatum and protected striatal neurons from H–I injury (Mueller-Burke et al., 2008; Yang et al., 2007b). Thus, a potential effect of PPBP on Ser897 phosphorylation during H–I may have been obscured by a strong dopaminergic influence in this brain region. Indeed, the inability of PPBP to provide complete protection in putamen might be related to persistent phosphorylation of Ser896 and Ser897. In addition, Src family tyrosine kinases are involved in NMDA receptor-mediated excitotoxicity and perinatal H–I injury (Jiang et al., 2008). Therefore, further work is required to explore other candidate kinases(Yang et al., 2009) through which sigma receptor ligands might act.
In addition to acting directly on NMDA receptors, sigma receptor ligands may act by attenuating NMDA receptor-induced nNOS activation (Bhardwaj et al., 1998) and reducing ischemia-evoked neuronal NO production (Goyagi et al., 2001). Considering the fact that nNOS is linked to NMDA receptor by PSD-95 (Sattler et al., 1999) and that interrupting this association significantly reduces cell death (Aarts et al., 2002; Cui et al., 2007; Sattler et al., 1999; Sun et al., 2008), we investigated whether the sigma receptor ligand interrupts this association and thereby reduces oxidative and nitrative stress in neurons in newborn brain. Our results revealed an H–I induced association of nNOS with NR2 in piglet putamen. This association was essentially blocked by PPBP treatment. Additional work demonstrated that the increased association of nNOS with NR2 occurred in parallel with an increased coupling of nNOS to PSD-95 and an increase in NOS catalytic activity in the membrane-enriched fraction at 3 h of recovery, all of which were attenuated by PPBP treatment after resuscitation. Moreover, neither H–I nor PPBP treatment affected the association of NR2 with PSD-95. Therefore, these results suggest that the H–I–induced association of NR2 with nNOS and the H–I–induced increase in NOS catalytic activity are driven by changes in nNOS–PSD-95 interactions and not by NR2–PSD-95 interactions. Whether the sigma receptor ligand directly regulates nNOS-PSD-95 interactions or whether the effect of PPBP was mediated indirectly by another mechanism secondary to cell preservation by PPBP was not addressed in here or in other models.
Some evidence implicates phosphorylation of nNOS in modulating the coupling of nNOS to PSD-95. The Ser847 residue of nNOS can be phosphorylated by calcium calmodulin kinase II (CaMKII) and dephosphorylated by phosphatases calcineurin and PP1/PP2A (Rameau et al., 2003; Rameau et al., 2004). An in vivo study showed that brain ischemia enhanced the phosphorylation level of Ser847 in nNOS and the coupling of nNOS to PSD-95; treatment with okadaic acid, an inhibitor of PP1/PP2A, further increased the phosphorylation of Ser847 and enhanced the interaction of nNOS with PSD-95 (Zhou et al., 2008). Moreover, CaMK II binds to NR2B in proximity to nNOS tethered by PSD-95 (Robison et al., 2005). These findings raise the possibility that Ser847-phosphorylated nNOS might be involved in the coupling of nNOS to PSD-95. Our results showed that H–I did increase the level of Ser847-phosphorylated nNOS in proportion to the total increase of nNOS protein in the membrane-enriched fraction. Interestingly, PPBP decreased nNOS Ser847 phosphorylation in proportion to nNOS expression in the membrane fraction after H–I. Therefore, our results indicate that the phosphorylation level of Ser847 has a close relationship with nNOS localization at the membrane. Additional work will be needed to elucidate whether phosphorylation of Ser847 directly modulates nNOS level in the membrane and how PPBP changes the level of Ser847-phosphorylated nNOS.
Two-way ANOVA indicated a highly significant overall effect of PPBP treatment over the 4-day recovery period (P < 0.001). However, neurological function spontaneously improves after cardiac arrest as animals emerge from a clouded state of consciousness. Although PPBP accelerated this improvement, differences from the control group became non-significant by 4 days of recovery possibly because the gross neurological deficit scoring system is not specifically sensitive to striatal damage. We did not continue PPBP throughout the 4-day recovery period because reductions in infarct volume with PPBP treatment after transient focal ischemia in adult rats was found to be lost when treatment extended beyond 1 day (Harukuni et al., 1998).
In conclusion, our study demonstrates that in a neonatal H–I model, treatment with PPBP, a sigma receptor ligand, after resuscitation reduces neuronal injury in selectively vulnerable putamen. This protection was associated with a marked suppression of NOS activity in the membrane and reduction of nitrosative and oxidative damage to proteins and nucleic acids. These effects may be mediated by a disruption in the association of nNOS with the NMDA receptor. Therapeutic use of sigma receptor ligands in neonatal H–I may provide a means for protecting striatum without provoking the adverse effects of completely blocking NMDA receptors in the developing brain.
Supplementary Material
The neurocytology and chemical organization of the newborn piglet striatum is different from the adult pig striatum. The packing density of putamen neurons the number of calbindin D28 positive cells appears greater in the newborn than those in the adult. Microtubule-associated protein-2 (MAP-2) staining of the putaminal dendrite network was less complex in the newborn than that in the adult. Scale bar = 50 µm.
Acknowledgements
We thank Ellen Gordes for technical assistance and Claire Levine for editorial assistance.
This work was supported by NIH grant NS20020 and NS060703 (R.C.K.) and by an American Heart Association-Phillips Resuscitation Research Fellow Award (Z.-J.Y.)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Agnew DM, Koehler RC, Guerguerian AM, Shaffner DH, Traystman RJ, Martin LJ, Ichord RN. Hypothermia for 24 hours after asphyxic cardiac arrest in piglets provides striatal neuroprotection that is sustained 10 days after rewarming. Pediatr. Res. 2003;54:253–262. doi: 10.1203/01.PDR.0000072783.22373.FF. [DOI] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Vernon DO, Collier L, Pennypacker KR, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3:89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- Akaike T, Okamoto S, Sawa T, Yoshitake J, Tamura F, Ichimori K, Miyazaki K, Sasamoto K, Maeda H. 8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:685–690. doi: 10.1073/pnas.0235623100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A, Sawada M, London ED, Koehler RC, Traystman RJ, Kirsch JR. Potent sigma1-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine modulates basal and N-methyl-D-aspartate-evoked nitric oxide production in vivo. Stroke. 1998;29:2404–2410. [PubMed] [Google Scholar]
- Brambrink AM, Martin LJ, Hanley DF, Becker KJ, Koehler RC, Traystman RJ. Effects of the AMPA receptor antagonist NBQX on outcome of newborn pigs after asphyxic cardiac arrest. J. Cereb. Blood Flow Metab. 1999;19:927–938. doi: 10.1097/00004647-199908000-00012. [DOI] [PubMed] [Google Scholar]
- Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, Garman D, Rabinowitz J, Lu PS, Tymianski M. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J. Neurosci. 2007;27:9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J. Neurochem. 2003;87:922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N. Engl. J. Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Goetz T, Arslan A, Wisden W, Wulff P. GABA(A) receptors: structure and function in the basal ganglia. Prog. Brain Res. 2007;160:21–41. doi: 10.1016/S0079-6123(06)60003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyagi T, Goto S, Bhardwaj A, Dawson VL, Hurn PD, Kirsch JR. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke. 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- Guerguerian AM, Brambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxia-ischemia. Brain Res Mol Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Bhardwaj A, Traystman RJ, Crain B, London ED, Kirsch JR. Neuroprotection from focal ischemia by 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is dependent on treatment duration in rats. Anesth. Analg. 1998;87:1299–1305. doi: 10.1097/00000539-199812000-00016. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert Opin Ther Targets. 2008;12:45–58. doi: 10.1517/14728222.12.1.45. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J. Neurochem. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jiang X, Mu D, Biran V, Faustino J, Chang S, Rincon CM, Sheldon RA, Ferriero DM. Activated Src kinases interact with the N-methyl-D-aspartate receptor after neonatal brain ischemia. Ann. Neurol. 2008;63:632–641. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–240. doi: 10.1111/j.1750-3639.2005.tb00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katnik C, Guerrero WR, Pennypacker KR, Herrera Y, Cuevas J. Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J. Pharmacol. Exp. Ther. 2006;319:1355–1365. doi: 10.1124/jpet.106.107557. [DOI] [PubMed] [Google Scholar]
- Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, Lee JH. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br. J. Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klette KL, DeCoster MA, Moreton JE, Tortella FC. Role of calcium in sigma-mediated neuroprotection in rat primary cortical neurons. Brain Res. 1995;704:31–41. doi: 10.1016/0006-8993(95)01103-x. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc MH, Vig V, Smith B, Parker CC, Evans OB, Smith EE. MK-801 does not protect against hypoxic-ischemic brain injury in piglets. Stroke. 1991;22:1270–1275. doi: 10.1161/01.str.22.10.1270. [DOI] [PubMed] [Google Scholar]
- Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat. Neurosci. 2002;5:641–648. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J. Comp. Neurol. 1997;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Price AC, Kaiser A, Agnew DM, Ichord RN, Traystman RJ. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol. Dis. 2000;7:169–191. doi: 10.1006/nbdi.2000.0282. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cereb Cortex. 2009;19:1273–1293. doi: 10.1093/cercor/bhn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, Junien JL, De Montigny C. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur. J. Pharmacol. 1990;179:441–445. doi: 10.1016/0014-2999(90)90186-a. [DOI] [PubMed] [Google Scholar]
- Mueller-Burke D, Koehler RC, Martin LJ. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurodegeneration after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int. J. Dev. Neurosci. 2008;26:67–76. doi: 10.1016/j.ijdevneu.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau GA, Chiu LY, Ziff EB. NMDA receptor regulation of nNOS phosphorylation and induction of neuron death. Neurobiol. Aging. 2003;24:1123–1133. doi: 10.1016/j.neurobiolaging.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Rameau GA, Chiu LY, Ziff EB. Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J. Biol. Chem. 2004;279:14307–14314. doi: 10.1074/jbc.M311103200. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Bartlett RK, Bass MA, Colbran RJ. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-D-aspartate receptor NR2B subunits and alpha-actinin. J. Biol. Chem. 2005;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Salinas-Zeballos M, Zeballos GA, Gootman PM. A sterotaxic atlas of the developing swine (Sus scrofa) forebrain. New York: Plenum Press; 1986. [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat. Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J. Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HS, Doucette TA, Liu Y, Fang Y, Teves L, Aarts M, Ryan CL, Bernard PB, Lau A, Forder JP, Salter MW, Wang YT, Tasker RA, Tymianski M. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39:2544–2553. doi: 10.1161/STROKEAHA.107.506048. [DOI] [PubMed] [Google Scholar]
- Sunshine P. New York: Epidemiology of perinatal asphyxia Oxford University Press; 1997. [Google Scholar]
- Takahashi H, Kirsch JR, Hashimoto K, London ED, Koehler RC, Traystman RJ. PPBP [4-phenyl-1-(4-phenylbutyl) piperidine], a potent sigma-receptor ligand, decreases brain injury after transient focal ischemia in cats. Stroke. 1995;26:1676–1682. doi: 10.1161/01.str.26.9.1676. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kirsch JR, Hashimoto K, London ED, Koehler RC, Traystman RJ. PPBP [4-phenyl-1-(4-phenylbutyl) piperidine] decreases brain injury after transient focal ischemia in rats. Stroke. 1996;27:2120–2123. doi: 10.1161/01.str.27.11.2120. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Ilyin VI, Woodward RM. Antagonism of N-methyl-D-aspartate receptors by sigma site ligands: potency, subtype-selectivity and mechanisms of inhibition. J. Pharmacol. Exp. Ther. 1997;282:326–338. [PubMed] [Google Scholar]
- Yang S, Alkayed NJ, Hurn PD, Kirsch JR. Cyclic adenosine monophosphate response element-binding protein phosphorylation and neuroprotection by 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) Anesth. Analg. 2009;108:964–970. doi: 10.1213/ane.0b013e318192442c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Bhardwaj A, Cheng J, Alkayed NJ, Hurn PD, Kirsch JR. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth. Analg. 2007a;104:1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. tables of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Torbey M, Li X, Bernardy J, Golden WC, Martin LJ, Koehler RC. Dopamine receptor modulation of hypoxic-ischemic neuronal injury in striatum of newborn piglets. J. Cereb. Blood Flow Metab. 2007b;27:1339–1351. doi: 10.1038/sj.jcbfm.9600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Li C, Yu HM, Zhang F, Han D, Zhang GY. Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J. Neurosci. Res. 2008;86:2973–2983. doi: 10.1002/jnr.21728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The neurocytology and chemical organization of the newborn piglet striatum is different from the adult pig striatum. The packing density of putamen neurons the number of calbindin D28 positive cells appears greater in the newborn than those in the adult. Microtubule-associated protein-2 (MAP-2) staining of the putaminal dendrite network was less complex in the newborn than that in the adult. Scale bar = 50 µm.