Summary
Respiratory enzyme complex dysfunction is mechanistically involved in mitochondrial failure leading to neurodegenerative disease, but the pathway is unclear. Here, age-related differences in mitochondrial respiration were measured in both whole and permeabilized neurons from 9-month and 24-month adult rat cortex cultured in common conditions. After permeabilization, respiration increased in both ages of neurons with excess substrates. To dissect specific deficiencies in the respiratory chain, inhibitors for each respiratory chain complex were used to isolate their contributions. Relative to neurons from 9-month rats, in neurons isolated from 24-month rats, complexes I, III, and IV were more sensitive to selective inhibition. Flux control point analysis identified complex I in neurons isolated from 24-month rats as the most sensitive to endogenous substrate availability. The greatest age-related deficit in flux capacity occurred at complex IV with a 29% decrease in neurons isolated from 24-month rats relative to those from 9-month rats. The deficits in complexes I and III may contribute to a redox shift in the quinone pool within the electron transport chain, further extending these age-related deficits. Together these changes could lead to an age-related catastrophic decline in energy production and neuronal death.
Keywords: Oxidative phosphorylation, aging, mitochondria, coenzyme Q, NADH, rotenone
Introduction
While neurodegeneration with age is widely documented as a cause of disease [1], there are gaps in understanding of the mechanisms behind it. Many potential pathways of energetic failure have been considered [2]. Among these mechanisms are oxidation of nucleic acids [3, 4, 5, 6], calcium dysregulation [7, 8, 9, 10], redox imbalance [11, 12, 13], reactive oxygen species (ROS) attacks [14, 15, 16], and oxidative phosphorylation deficits [17, 18, 19, 20]. Because the availability of energy from oxidative phosphorylation is so critical to neuron function, here, we investigated further the loss of oxidative phosphorylation by controlling the substrate availability to neurons in situ.
Attempts to elucidate the chain of events leading to neurodegeneration with age have historically been limited by the lack of a viable in situ model of mammalian aging. In homogenized brain tissue, neurons are mixed with the aging environment of the brain, including the aging vascular, hormonal, and immunological systems. Furthermore, brain homogenates do not provide an accurate model of neurons attached to a substrate, forming synapses and transmitting signals [21, 22]. Isolated mitochondria risk considerable degradation during the homogenization and isolation process, and are removed from interaction with nuclear and cytoplasmic signaling [23, 24, 25, 26, 27, 28]. Others have conducted studies in neurons isolated from embryonic [29] or very young (5-7 days) rats [30, 31, 32, 33], precluding age-related comparisons over the life-span. Our method of isolating whole neurons from the brains of adult rats and growing them in common culture conditions has allowed us to apply well-established techniques to an improved model of mammalian aging [34, 35]. Brewer [36] showed that neurons cultured from different ages of rats demonstrate distinct age-related susceptibility to lactate, glutamate, and beta-amyloid. Live neurons isolated from the aging brain environment can be monitored in their endogenous state [17], or permeabilized to allow substrate control and pharmacologic isolation of complexes of the electron transport chain [33].
Redox potential is a greatly under-appreciated source of energy production in neuronal mitochondria [12, 37, 38]. Neurons isolated from old rat brains consume more redox active NADH and glutathione than neurons from middle-age rat brains resulting in redox imbalance with age, but the reason for increased consumption has not been documented [12]. Furthermore, glutathione, part of the most abundant redox pair responsible for redox buffering in the brain, also acts as an antioxidant controlling reactive oxygen species produced during oxidative phosphorylation. Other oxygen-consuming enzymes in the brain such as cyclooxygenase, cytochrome P450, heme oxygenase, lipoxygenase, NADPH oxidase, nitric oxide synthase, phospholipase, and xanthine oxidase are also regulated by redox balance. Reactive oxygen species (ROS) damage enzymes crucial to energy production, and as a result of such damage can propagate further ROS production. Damage to enzyme complexes involved in oxidative phosphorylation is a documented result of excess ROS and cause of age-related neurodegeneration, but previous studies have been limited by their models [39, 40]. ROS damage could affect the inhibitor efficacy for any complex by altering the number of binding sites or their quality of binding.
In a previous study, we found that an age-related deficit in cytochrome C oxidase (complex IV) in whole cells at endogenous levels of cytochrome c was not apparent in substrate-supplemented submitochondrial particles, and that deficits in cardiolipin and upregulation of respiration in response to stress were corrected by estrogen treatment [17]. In this study, we expanded our methods to include substrate supplementation in whole cells, and we studied the three upstream respiratory complexes, NADH-ubiquinone oxidoreductase (complex I), succinate dehydrogenase (complex II), and cytochrome bc1 oxidoreductase (complex III).
Materials and Methods
All reagents were purchased from Sigma Aldrich (St. Louis, Missouri) unless otherwise noted.
Cell Culture
Adult rat neurons were cultured according to the method of Brewer [34, 35]. Male Fisher 344 rats, which have a median life span of 24 months [41], were used for all experiments. The rats were fed rat chow ad libitum and weighed 408 ± 88 g (middle-age) or 403 ± 77 g (old) at the time of sacrifice. All animals were anesthetized with isofluorane prior to decapitation by guillotine. Cortical and hippocampal neurons were extracted from brains of middle-age (9 month) and old (24 month) rats. Once dissected, the cortices were sliced to 0.5 mm with a McIlwain chopper (Campden Instruments, Lafayette, IN), digested in 2 mg/mL papain (Worthington, Lakewood, NJ), and triturated in Hibernate A/B27 (BrainBits, Springfield, IL). Cells were separated from debris on an Optiprep gradient and resuspended in Neurobasal A/B27, 0.5 mM glutamine, 5 ng/mL basic human recombinant FGF2 (Invitrogen, Carlsbad, CA). Cells were then plated on poly-D-lysine -coated 15 mm glass cover slips (Assistent brand, Carolina Biological, Burlington, NC) at a density of 500 cells/mm2 and cultured at 37°C, 5% CO2, 9% O2 for 7 – 12 days. To manually count cells, each culture slip was photographed in 6 consecutive fields measuring 0.15 mm2 using 20x phase optics. Cell counts were used to normalize respiration measurements. Each slip contained 37,700 ± 4,200 (middle-age, n = 44) or 33,390 ± 4,100 (old, n = 37; p=0.47) neurons. Slips were also photographed after respiration measurements to confirm the presence of cells.
Respiration in whole (non-permeabilized) cells
In order to measure respiration from a limited number of neurons in their substrate-attached state, neuronal respiration before and after addition of selective respiratory chain inhibitors was measured using the Oxygraph-2K (Oroboros, Innsbruck, Austria). Two polarographic oxygen sensors connected to temperature-controlled, continuously stirred, sealed chambers measure oxygen concentration within the chamber over time. Early experiments optimized 500 RPM as the best stirring speed for full cell adherence in non-permeablilzed cultures with minimum electrode noise. DatLab4 software (Oroboros) calibrated the instrument using atmospheric oxygen concentration (21%) and 0% oxygen concentration (obtained by addition of a few crystals of sodium dithionite to water) as reference points. We derived oxygen consumption rates from DatLab4-generated graphs of oxygen concentration and flux in real time. Typical measurements in the Oxygraph are taken from cell suspensions, but neuronal bioenergetics rely on attachment to a substrate. We developed 14.5 mm diameter stirbars, topped with silicone (Kwik-cast, World Precision Instruments, Sarasota, FL) with Teflon star bases (Nalgene, Rochester, NY) to accommodate placement of 15 mm diameter glass cover slips on which neuronal cultures had adhered and grown. Complex I was inhibited by rotenone [43, 44], titrated in concentrations from 1 - 10 μM. To inhibit complex II, 3-nitropropionic acid [45, 46] was titrated in concentrations from 1 mM – 10 mM. To inhibit complex III, antimycin A, [47] was titrated in concentrations from 0.1 μM – 1 μM, diluted directly in Neurobasal A/B27 in a 1 mM stock and diluted further in Neurobasal A/B27 to the necessary concentrations. To inhibit complex IV, potassium cyanide [48] was titrated in concentrations from 10 – 100 μM. For example, rotenone was diluted into Neurobasal A/B27 from a 10 mM stock in 100% ethanol and titrated in concentrations from 1 - 10 μM in 2 - 5 minute increments using a 25 μL glass syringe with a 75 mm, 22-gauge needle (Hamilton, Reno, NV). Each addition was 10 μL, 1/100 of the original volume, and additive to the previous concentration. Prior experiments showed no effect on respiration of using only the ethanol vehicle diluted in Neurobasal A/B27 to 0.01% - 0.1% (data not shown).
Bioenergetic assessment in permeabilized cells
K2EGTA and CaK2EGTA were made according to Oroboros (www.oroboros.at, Innsbruck, Austria). Permeabilization and respiration media were made according to Safiulina et al. [33] in Table 1. To assess the minimum amount of time needed to permeabilize neurons exposed to 50 μg/mL saponin, cells were incubated at room temperature in permeabilization medium (Table 1) with the membrane-impermeable nuclear stain propidium iodide (4.6 μg/mL). Photographs of propidium iodide fluorescence at 0, 1, 3, and 15 min. intervals were used to assess the time needed for permeabilization. The following additions were made to the respiration medium for each complex measurement: complex I: 5 mM glutamate, 2 mM malate [33]; complex II: 10 mM succinate, 10 μM rotenone [33, 42]; complex III: 5 mM glutamate, 2 mM malate, 10 mM succinate [33, 42]; complex IV: 0.5 mM N,N, N’,N’-tetramethyl-p-phenylenediamine (TMPD), 2 mM ascorbate, 8 μM reduced cytochrome C [33]. ADP was made in a 100x stock concentration of 200 mM in respiration medium; 10 μL were added to each chamber for a final concentration of 2 mM.
Table 1.
Composition of Pre-Permeabibzation Medium and Respiration Medium developed by Safiulina et al. [37]. Concentrations given in mM unless otherwise noted.
| Pre-Permeabilization Medium | Respiration Medium | |
|---|---|---|
| Potassium Methanesulfonate | 81 | |
| BES | 60 | |
| Mg2ATP | 5.69 | |
| KOH | 35 | |
| K-MES | 100 | |
| Imidazole | 20 | |
| MgCl2 | 1.38 | |
| HC1 | 16.25 | |
| BSA | 5 mg/mL | |
| K2EGTA* | 7.23 | 7.23 |
| CaK2EGTA* | 2.77 | 2.77 |
| Taurine | 20 | 20 |
| K2HPO4 | 3 | 3 |
| Dithiothreitol | 0.5 | 0.5 |
| pH | 7.1 | 7.1 |
| Osmolarity | 255 mOsm | 225 mOsm |
Free [Ca2+] in both media was 0.1 μM. K2EGTA and Ca2EGTA were made according to Oroboros (www.oroboros.at, Innsbruck, Austria).
Adherent cells in culture in Neurobasal A/B27 were gently rinsed with permeabilization medium two times before they were transferred to the Oxygraph-2k chambers containing 1 mL permeabilization medium being stirred at 300 rpm. After a baseline respiration measurement was obtained, 10 μL of 5 mg/mL saponin dissolved in permeabilization medium was added to each chamber for a final concentration of 50 μg/mL. After 3 minutes of saponin permeabilization, stirring was stopped. 800 μL of the fluid in the chambers were removed and replaced with 800 μL of respiration solution four times until only a negligible amount (0.16%) of permeabilization solution remained. Stirring resumed at 300 rpm and a basal respiration rate of permeabilized cells was measured before and after the addition of ADP. Inhibitors were added as in non-permeabilized cells. Following each titration, 10 μM diphenylene iodonium was added to determine the contribution of NADPH oxidase to measured oxygen consumption [49, 50].
Data Analysis
Non-linear regression curves were fit to the dose response data using Plot-It software (Scientific Programming Enterprises, Haslett, Michigan). IC50 values were derived algebraically from the non-linear regression equations. Standard errors were generated manually for these values using the coefficient errors for each term generated by Plot-It. Flux threshold plots were generated using the percent inhibition of respiration by each inhibitor in neurons with excess exogenous substrates as a function of the percent of initial respiration remaining after the same dose of inhibitor applied to whole neurons with endogenous substrates [51]. Flux thresholds were determined as the point at which a change in influence of substrates on inhibition of respiration occurred. Plot-It software was used to perform student's t-tests, linear regression, means and S.E.M.
Results
Figure 1 illustrates healthy in situ neurons from A) middle-age and B) old rats, as well as C) the silicone-topped teflon stir bar and glass culture slip on which neurons were cultured for measurement of oxygen consumption. In a previous study in our laboratory, staining for neurofilament, GFAP, and Ox-42 indicated ~80% neurons, 5% astroglia, 10% oligodendroglia, and 5% microglia [52]. Neurons with endogenous substrates were evaluated in culture medium (NeurobasalA/B27), to mimic the extracellular potassium concentration in the brain. Neurons with excess exogenous substrates were permeabilized with saponin and measured in a respiration medium with a potassium concentration similar to intracellular levels.
Figure 1.
Adult neurons were cultured on 15 mm2 glass cover slips and supported in the respiration chamber by a silicone-topped teflon stir bar. A) Neurons prepared from middle-age rats cultured on a glass cover slip. B) Neurons prepared from old rats cultured on a glass cover slip. The fields pictured are 0.04 mm2. C) Silicone-topped teflon stir bar designed to support substrate-adherent neurons on a 15 mm glass culture slip while measuring oxygen consumption.
Intact neurons with endogenous substrates
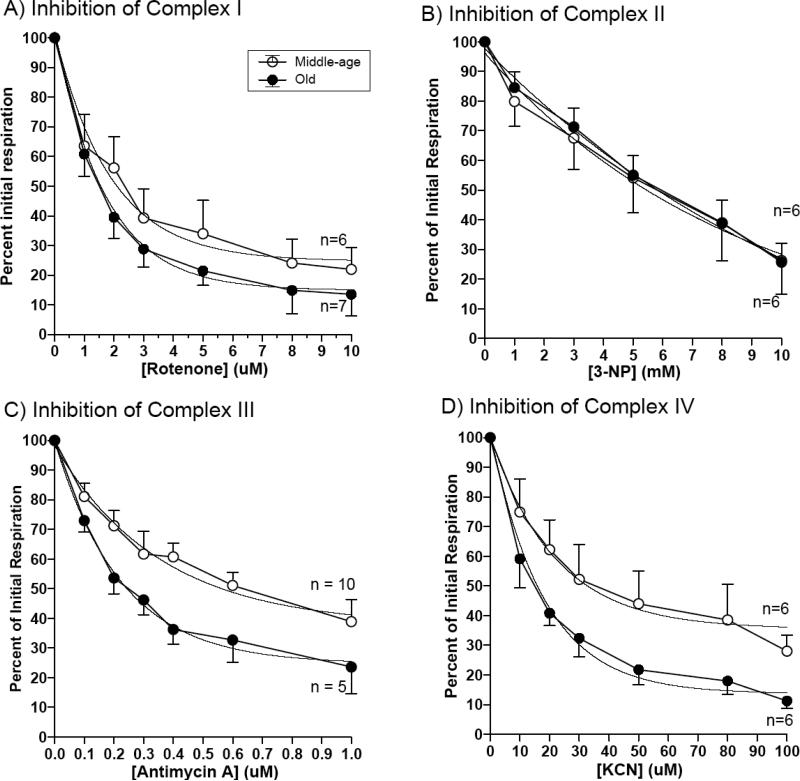
In Figure 2, we measured selective inhibition of respiration in whole neurons at rest in situ. To determine the age contribution of each complex on oxygen consumption at complex IV, electron transport at complexes I – IV was selectively inhibited. The percent of initial respiration was plotted as a function of the inhibitor dose. The IC50 values were calculated to indicate the efficacy of the inhibitor for each complex (Table 2). In Figure 2A, for neurons isolated from middle-age rats, an IC50 of 1.4 μM for rotenone decreased 21% to 1.1 μM in neurons isolated from old rats (p=0.005), indicating a decrease in rotenone sensitivity of complex I in neurons isolated from old brains. The IC50 value for respiratory inhibition by the complex II inhibitor, 3-NP, showed an insignificant 49% increase from 5.7 mM to 8.5 mM with age. For inhibition of complex III by antimycin A, the IC50 decreased 35%, from 0.24 μM to 0.16 μM with age (p=0.006). Finally, the IC50 value for KCN at complex IV decreased 33% from 18 μM to 12 μM with age (p = 0.002). These results reveal increased sensitivity to selective inhibition in complexes I, III, and IV of neurons isolated from old rats relative to those from young rats.
Figure 2.
Non-permeabilized resting neurons in culture medium showed age-related differences between neurons prepared from old and middle-age rats only at complexes I, III and IV. (A) complex I by rotenone, Middle-age curve fit : Y = 73.6 * e−0.48X + 23.2, Old: Y = 84.8 * e−0.65X + 15.0. (B) complex II by 3-nitropropionic acid, Middle-age: Y = 99.4 * e−0.11X – 3.2, Old: Y = 129 * e−0.079X -31.3. (C) complex III by antimycin A Middle-age: Y = 61.0 * e−2.75X + 37.1, Old: Y = 75.1 * e−4.4X + 24.6. (D) complex IV by KCN, Middle-age: Y = 67.1 * e−0.038X + 31.4, Old: Y = 83.6 * e−0.058X + 15.3. n = 4-6 culture slips. Figure 2D was derived from data published by Jones and Brewer, 2009. IC50 values were derived from this data and summarized in Table 2.
Table 2.
IC50 of complex-specific inhibitors in cortical neurons from old and middle-age rats with and without excess endogenous substrates.
| Complex I (μM) | Complex II (μM) | Complex III (μM) | Complex IV (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | MA | Old | p= | MA | Old | p= | MA | Old | p= | MA | Old | p= |
| Endogenous Substrates | 1.4 | 1.1 | 0.005 | 5.7 | 8.5 | 0.5 | 0.24 | 0.16 | 0.006 | 18 | 12 | 0.002 |
| Exogenous Substrates | 3.0 | 1.0 | 0.02 | 6.1 | 2.1 | 0.9 | 0.13 | 0.052 | 0.0001 | 33 | 26 | 0.5 |
Neuronal permeabilization occurs in < 3 minutes
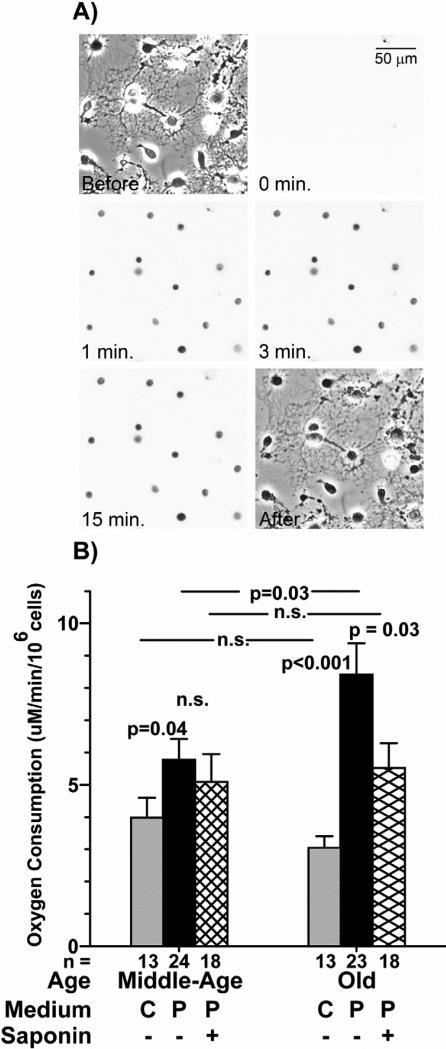
To determine whether the effects of the inhibitors on neurons with endogenous substrate levels was due to a substrate deficit, we permeabilized neurons in situ and provided excess substrates according to the method of Safiulina et al. [33]. They permeabilized neurons with saponin for 15 minutes at 4°C without affecting mitochondrial membranes. In order to avoid cytoskeletal disassembly [55], a pilot experiment showed that after just 3 minutes at 25°C neurons were adequately permeabilized to allow propidium iodide to enter (Figure 3A). The time scale shows that after 15 minutes, no new cells were stained relative to the 3-minute time point, simply the dye had become more dense as it collected within the nuclei.
Figure 3.
Neurons were adequately permeabilized after <3 minutes. A) A healthy cell culture exposed to propidium iodide and incubated with saponin for 0, 1, 3, and 15 minutes showed that all cells were adequately permeabilized for substrate addition after 3 minutes. Phase contrast images before saponin and after 15 minutes of treatment indicate integrity of the permeabilized neurons seen by the intervening nuclear fluorescence images. Scale is 100 μm. B) Neuron respiration in culture medium (C) is not significantly different between old and neurons prepared from middle-age rats. (gray bars; [17]). In pre-permeabilization medium (P), the rate of respiration in neurons prepared from old rats was increased 50% over that in neurons prepared from middle-age rats prior to the addition of saponin (center bars). Following addition of saponin, the rate of respiration in neurons prepared from middle-age rats remained the same, while the rate of respiration in neurons prepared from old rats was reduced to the rate of neurons prepared from middle-age rats (hatched bars). n = 13-24 culture slips, as indicated in graph. Before and after pictures are phase contrast, time scale pictures are red fluorescence.
Old neurons respire at a higher rate than neurons prepared from middle-age rats in pre-permeabilization medium
In preparation for permeabilization, neurons were transferred from culture medium ([K+] = 5.4 mM) to a medium with a depolarizing concentration of K+ (142 mM), similar to the intracellular potassium that is required for mitochondrial function. At depolarizing K+, neurons were expected to respire at higher rates to generate ATP to power the Na+/K+ ATPase that keeps the plasma membrane polarized. Prior to permeabilization by saponin addition and substrate addition, neurons prepared from middle-age rats respired at a rate 50% higher in this depolarizing medium than their respiration rate in culture medium. Respiration of neurons prepared from old rats increased by 180% in the pre-permeabilization medium (Figure 3B). The fact that respiration increased more in neurons prepared from old rats than in neurons prepared from middle-age rats may indicate that ATP production was less efficient in the neurons prepared from old rats in culture medium or that the neurons prepared from old rats had less phosphocreatine reserve to make ATP [56]. Following treatment with saponin for 3 minutes, respiration in the neurons prepared from old rats ceased to be driven maximally as cytoplasmic ADP and other substrates were diluted from the cytoplasm by permeabilization. Respiration in neurons prepared from middle-age rats decreased insignificantly, possibly due to a greater phosphocreatine reserve [56].
Neurons isolated from middle-age and old rats increase respiration in response to substrate supplementation
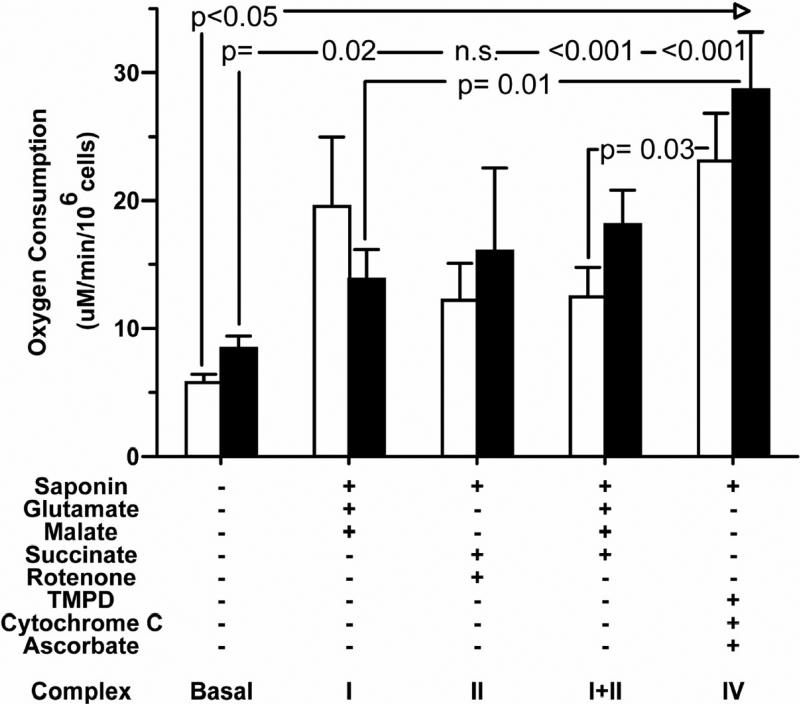
In Figure 4, oxygen consumption was measured in pre-permeabilization medium prior to addition of any substrates to obtain a rate of non-supplemented basal respiration. Following permeabilization and a change to substrate-supplemented respiration medium, middle-age neuron respiration was significantly increased at least two-fold for each complex. Neurons prepared from old rats showed a significant increase in respiration in complexes I, III, and IV, and a trend toward increase for complex II. Complex-specific substrate-supplemented respiration was not, however, significantly different between neurons prepared from old and middle-age rats. This result indicates that, given adequate substrates, neurons prepared from old rats were able to maintain a rate of respiration comparable to neurons prepared from middle-age rats, and that supply of substrate limited native respiration in old neurons.
Figure 4.
Rate of respiration increased when cells were given excess substrates. For complexes I, I+II, and IV with the addition of excess substrates, respiration significantly increased over basal for both ages of neurons. However, no significant age-related differences were observed in basal respiration between middle-age (white bars) and old (black bars) neurons when cells were given excess ADP and substrates for each complex. n = 6 culture slips of each age.
Direct electron supply to complex IV increases respiration more than excess substrates for upstream complexes
Figure 4 shows that, in neurons prepared from middle-age rats given artificial electron donors for complex IV, respiration was increased 1.8-fold higher than in neurons prepared from middle-age rats given substrates for upstream complexes I and II. In neurons prepared from old rats given artificial electron donors for complex IV, respiration was increased 2-fold higher than in neurons prepared from old rats given substrates for complex I. These data suggest that complex IV itself, in both ages, has reserve capacity to respond to excess electron availability revealed by direct electron supply that is limited by indirect supply through the Kreb's cycle. These observations imply that a deficiency in energy production is more likely due to an upstream electron deficiency that becomes more severe with age than to an enzymatic deficiency in complex IV quantity or function.
In permeabilized neurons from old grats with exogenous substrates, complexes I and I+II are more susceptible to selective inhibition relative to neurons from middle-age rats
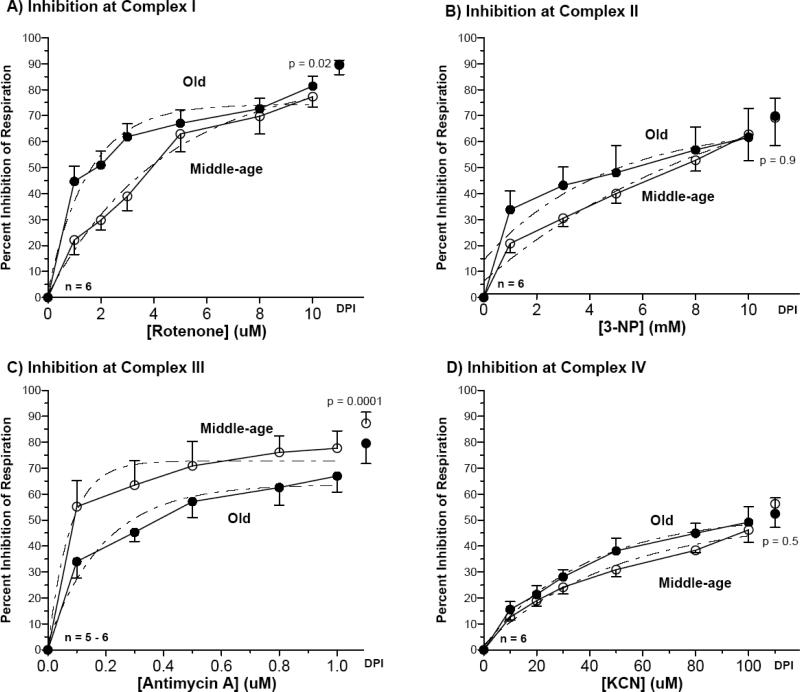
In contrast to the in situ measurements of respiration with endogenous substrates shown in Figure 2, here we investigated with excess substrates in permeabilized neurons in situ how respiration was affected when the individual complexes were inhibited. This allowed us to determine the role of substrate availability on the inhibition of specific complexes in their native state. Figure 5 illustrates the percent inhibition of respiration for increasing doses of selective inhibitors. Table 2 lists the IC50 values for each inhibitor to indicate the efficacy of inhibition for each complex. In Figure 5A, for neurons isolated from middle-age rats, an IC50 of 3.0 μM for rotenone decreased 67% to 1.0 μM in neurons isolated from old rats (p=0.02), indicating an increase in rotenone sensitivity of complex I in neurons isolated from old brains. Since rotenone acts at the quinone oxidation site [53], this increased sensitivity to rotenone may also indicate a deficiency of reduced quinone or the ability of the site to bind reduced quinone. The IC50 value for respiratory inhibition by the complex II inhibitor, 3-NP, showed an insignificant 66% decrease from 6.1 mM to 2.1 mM with age. For inhibition of complex III by antimycin A, the IC50 decreased 60%, from 0.13 μM to 0.052 μM with age (p=0.0001). Finally, the IC50 value for KCN at complex IV decreased 21% from 33 μM to 26 μM with age (p = 0.5). These results reveal increased sensitivity to selective inhibition in complexes I and I+II of permeabilized neurons isolated from old rats relative to those from middle-age rats.
Figure 5.
Permeabilized resting neurons given excess exogenous substrates showed age-related differences in sensitivity to inhibition between neurons prepared from old and middle-age rats at (A) complex I by rotenone, middle-age curve fit Y = -84.7 * e−0.23X + 85.8; old curve fit Y = -72.0 * e−0.65X + 74.4 (B) complex II by 3-nitropropionic acid, middle-age curve fit Y = -87.0 * e−0.10X + 93.2; old curve fit Y = -52.8 * e−0.22X + 67.1 (C) complex III by antimycin A, middle-age curve fit Y =-72.4 * e−13.2X + 72.7; old curve fit Y = -61.0 * e−5.1X + 63.7 and (D) complex IV by KCN, middle-age curve fit Y = -48.6 * e−0.020X + 50.4; old curve fit Y = -50.7 * e−0.026X + 52.0. n = 5-6 culture slips. IC50 values were derived from this data and summarized in Table 2.
Comparisons of endogenous to exogenous excess substrates
Line wise comparisons in Table 2 suggest age-related differences between responses to inhibitors for endogenous and exogenous substrates. For complex I, the IC50 of rotenone increased 114% for neurons isolated from middle-age rats with exogenous substrates, but decreased 9% after addition of excess exogenous glutamate and malate for neurons isolated from old rats. However, for complex II, the IC50 of 3-NP increased by 7% in neurons isolated from middle-age rats and decreased 75% in neurons isolated from old rats when complex I was inhibited and the neurons were supplemented with excess exogenous succinate. IC50 for antimycin A decreased 46% in neurons isolated from middle-age rats supplemented with excess exogenous glutamate, malate, and succinate, but in neurons isolated from old rats the IC50 for antimycin A decreased 68% with the addition of these excess exogenous substrates. Finally, the addition of excess exogenous electron donors for complex IV, TMPD, cytochrome C, and ascorbate, increased the IC50 of KCN by 83% in neurons isolated from middle-age rats and by 117% in neurons isolated from old rats.
Flux Control Analysis of Neurons with Endogenous and Excess Exogenous Substrates
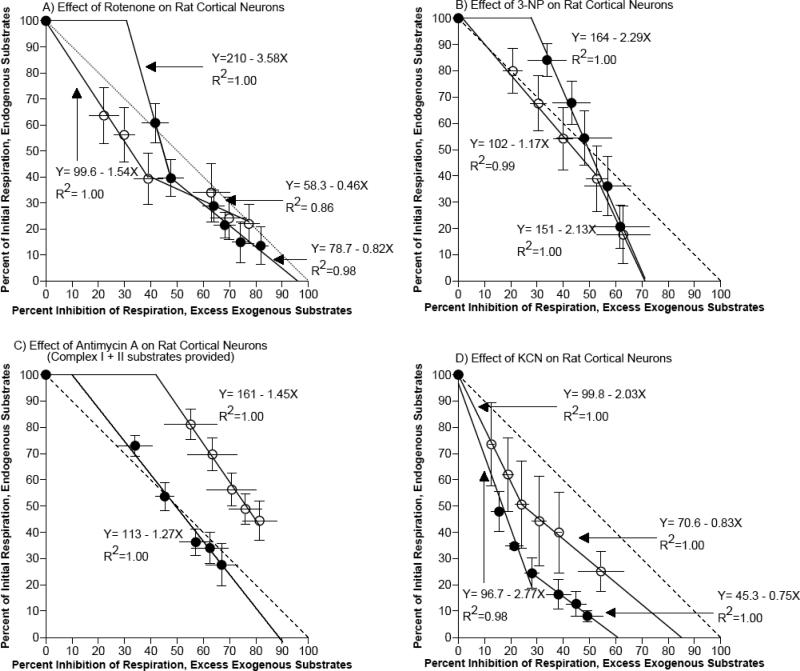
To assess the level of control each complex exerted over electron transport, we compared respiratory flux ratios at the same inhibitor concentrations for neurons with endogenous and excess endogenous substrates [51] (Fig. 6). At each individual complex, we plotted the percent of initial respiration remaining at each dose of inhibitor as a function of the percent inhibition of respiration in neurons with excess exogenous substrates at the same dose. A slope of -1, plotted for reference in Figure 6, was expected if the complex and its substrates exerted no control over global flux. A more negative slope implies substrate limitation at that complex. Less complex capacity is indicated by a lower intersection on the x-axis (complex reaches maximal inhibition with endogenous substrate without reserve to process the excess substrate). Through flux analysis, we determined which complexes were most limiting to electron transport, and whether these limitations were due to substrate or enzyme deficiencies. The slope of the line after the flux threshold point determined whether the activity limitations were due to substrate or enzyme deficiencies. A more negative slope beyond the flux point implied a substrate limitation in the endogenous state.
Figure 6.
Flux control point analysis for neurons with endogenous substrates and excess exogenous substrates compared at A) complex I, B) complex II, C) complex III, and D) complex IV. The slopes of the linear regressions, x-intercepts, and flux threshold points were summarized in Table 3.
For complex I in neurons isolated from middle-age rats, a flux point occurred at 39% of initial respiration with endogenous substrates (3 μM rotenone) with a slope increase from -1.5 to -0.46. At this flux point, respiration in neurons with endogenous substrates was inhibited 61%, while respiration in neurons with excess exogenous substrates was only inhibited 39%, suggesting an endogenous NADH substrate limitation that could be partially corrected by exogenous substrates. Extrapolation of the x-intercept showed that some respiratory capacity would remain in neurons with endogenous substrates given a rotenone dose that would completely inhibit respiration in neurons with excess exogenous substrates. This 20% residual electron flow in neurons with endogenous substrates is likely due to other branches that feed CoQ to complex III, such as complex II, fatty acid dehydrogenase, and glyceraldehyde 3-phosphate dehydrogenase. For neurons isolated from old rats, the data suggest a flux point at 40% of initial respiration with endogenous substrates, but at 8 μM rotenone, where the slope increased from -3.6 to -0.8. Therefore rotenone concentrations in excess of 8 μM were necessary to most effectively inhibit neurons from old rats with excess endogenous substrates. Furthermore, the extrapolated linear regression for the first two rotenone doses shows that rotenone that would inhibit 31% of respiration in neurons with excess exogenous substrates would not affect neurons with endogenous substrates. This result may be due to membrane resistance against rotenone in non-permeabilized neurons from old rats, or excess exogenous substrates may uncover some additional rotenone-sensitive activity. The extrapolated x-intercept was 96%, revealing complete inhibition of respiration by inhibition at complex I enzyme activity in neurons isolated from old rats given excess exogenous substrates.
For complex II, the data suggests that neurons isolated from middle-age rats reached a flux point at 39% of initial rate with endogenous substrates (at) 8 mM 3-NP where the slope decreased from -1.2 to -2.1. This increase in slope beyond the flux point indicated that endogenous substrates became rate-limiting. The extrapolated x-intercept indicated that a concentration of 3-NP that would completely inhibit activity in neurons from middle-age rats with endogenous substrates would only inhibit respiration in neurons with excess exogenous substrates by 72%, revealing a limitation in complex II capacity. Neurons isolated from old rats did not show a defined flux point, but the extrapolated linear regression for all doses of 3-NP shows that a dose of 3-NP that would inhibit 28% of respiration in neurons with excess exogenous substrates would not affect neurons with endogenous substrates. This result may be due to membrane resistance against 3-NP in non-permeabilized neurons from old rats, or excess exogenous substrates may uncover some additional 3-NP-sensitive activity. The extrapolated x-intercept indicated that a concentration of 3-NP that would completely inhibit respiration in neurons from old rats with endogenous substrates would only inhibit respiration in neurons with excess exogenous substrates by 71%, revealing a capacity limitation in complex II in neurons isolated from old rats similar to the limitation revealed in neurons isolated from middle-age rats.
For complex III, neurons isolated from middle-age rats did not reveal a distinct flux point, however the extrapolated linear regression for all doses of antimycin A showed that a dose of antimycin A that would inhibit 42% of respiration in neurons with excess exogenous substrates would not affect neurons with endogenous substrates. This result may be due to membrane resistance against antimycin A in non-permeabilized neurons from middle-age rats, or excess exogenous substrates may uncover some additional antimycin A-sensitive activity. The extrapolated x-intercept exceeding 100% indicated that a dose of antimycin A which would completely inhibit respiration in neurons with excess exogenous substrates would not completely inhibit respiration in neurons with endogenous substrates. Again, this may be due to membrane resistance in non-permeabilized neurons. Neurons isolated from old rats also failed to reveal a distinct flux point, however the extrapolated linear regression for all doses of antimycin A shows that a concentration of antimycin A that would completely inhibit respiration in neurons from old rats with endogenous substrates would only inhibit respiration in neurons with excess exogenous substrates by 89%, revealing a limitation in complex III capacity in neurons isolated from old rats.
For complex IV, neurons isolated from middle-age rats reached a flux point at 51% of initial respiration with endogenous respiration (30 μM KCN) where the slope increased from -2.0 to -0.8. Concentrations of KCN in excess of 30 μM more effectively inhibited neurons from middle-age rats with excess exogenous substrates, revealing a limitation in the capacity of complex IV that is not corrected by substrate supplementation. The extrapolated x-intercept indicated that a concentration of KCN that would completely inhibit activity in neurons from middle-age rats with endogenous substrates would only inhibit respiration in neurons with excess exogenous substrates by 85%, reinforcing that complex IV capacity is limited. Neurons isolated from old rats did not reach a flux point until 25% of initial respiration with endogenous respiration (0.1 μM KCN) where the slope increased from -2.8 to -0.8. Concentrations of KCN up to 30 μM more effectively inhibited neurons from old rats with endogenous substrates, revealing a limitation in the capacity of complex IV in neurons from old rats that is greater than the limitation in neurons from middle-age rats. This difference is marked by an extrapolated x-intercept of 60% inhibition of respiration with excess substrates for neurons isolated from old rats compared to only 85% inhibition for neurons from middle-age rats, at concentrations of KCN that would completely inhibit activity in neurons with endogenous substrates.
Discussion
In a previous study, we discovered that intact, substrate-adherent neurons prepared from old rats in culture medium exhibit no difference in basal respiration relative to neurons prepared from middle-age rats, but do exhibit a decreased capacity to upregulate respiration in response to glutamate stimulation [17]. We also observed a deficit in Complex IV activity in neurons prepared from old rats with endogenous substrate levels that could be corrected in submitochondrial particles with addition of an artificial electron donor. Here, we tested substrate supplementation in complex IV in neurons in situ to confirm the effect of substrate supplementation in whole neurons as a less perturbed condition than submitochondrial particles. We found age-related changes in IC50 for complexes I, III and IV in neurons in situ with endogenous substrates. In situ neurons with excess exogenous substrates exhibited heightened sensitivity to inhibitors at complexes I and III. Thus, the age-related inhibition at complex IV was relieved by excess substrate, suggesting that complex IV was substrate-limited in the native state in neurons isolated from old rats. Furthermore, the common age-related differences at complexes I and III suggest an inherent deficit in these complexes in neurons isolated from old brains that can't be overcome completely with excess substrate.
Limitations of the study
In research performed prior to this study, enzyme complexes have been assayed directly for activity to generate flux control coefficients [51]. Due to the nature of our sample, which contained substrate-adherent neurons per cover slip of 37,700 ± 4,200 (middle-age, n = 44) or 33,390 ± 4,100 (old, n = 37; p=0.47), accurate assays of individual enzyme complexes were not possible. Therefore we alternatively chose to compare respiration in neurons with endogenous substrates to respiration in neurons given excess endogenous substrates. In this way, we were able to observe whether deficiencies in respiration in neurons from middle-age and old rat neurons were corrected by substrate supplementation. Furthermore, in hindsight, a lower concentration range for the complex-specific mitochondrial inhibitors may reveal further differences in neuronal response, especially for complexes I and III. This observation requires some reservations in the conclusions about these complexes.
Flux analysis revealed significant age-related changes for each of the complexes I, II, III and IV. The largest change in sensitivity to substrate availability as indicated by the most negative initial slope of the flux curves occurred with complex I for neurons isolated from old rats. These results are consistent with the finding that endogenous NADH is limited in neurons isolated from old rats [12]. For neurons isolated from middle-age rats, the initial slopes maintained a balanced range from -1.2 to -2.0 for complexes I – IV, with complex IV exerting the greatest flux control.
Flux analysis also revealed age-related limits in capacity, as indicated by lower x-intercepts of the flux curves. For complex I, neurons with endogenous substrates from middle-age rats had the ability to compensate for inhibition of electron flow through complex I, possibly via complex II, glyceraldehyde-3-phosphate dehydrogenase, or fatty acid dehydrogenase. Neurons from old rats did not have the same compensatory capability, and with excess substrates in fact were inhibited more by low concentrations of rotenone than neurons from middle-age rats. Because rotenone binds at the quinone oxidation site [57], these results may indicate an age-related alteration in the ability of the neuron to bind coenzyme Q, limiting its energy-producing capacity. The same greater sensitivity of neurons from old rats with endogenous substrates was observed for inhibition at complex II, which also requires reduced coenzyme Q as a substrate.
Age-related alteration of complex I is not a new concept in the field of neurodegeneration. Rotenone toxicity is an accepted model of Parkinson's Disease (PD), implicating complex I deficiency in the neurodegeneration that occurs in PD [59, 60]. Our complex I results support previous proposals that complex I is deficient in its ability to transfer electrons to the quinone pool in aged neurons [61]. Complex I deficiency in the substantia nigra is widely accepted as a possible cause of PD [62], so these results with aged neurons from the mammalian cortex, a selectively vulnerable region in Alzheimer's Disease (AD), suggest that complex I deficiency with age and specifically in the oxidized quinone pool may in part contribute to the aging requirement for neurodegeneration observed in AD [63, 64, 65] and PD [65].
In complex III, neurons from middle-age rats not only were resistant to inhibition by antimycin A, they also had a compensatory protection against complete antimycin A inhibition in the endogenous state. Neurons from old rats, however, were significantly less resistant to inhibition by antimycin A in the endogenous state and had a limited capacity to respond to excess exogenous substrates. Since antimycin A binds to complex III at the quinone reduction site [53], it is possible that the upstream irregularities in the quinone oxidation site previously discussed are responsible for the limited capacity of complex III to respond to excess electron flow from excess substrates provided to complexes I+II. Age-related irregularities in the quinone pool could lead to generation of ROS [71], redox imbalance [20], and catastrophic decline in energy production in the neuron [2]. In fact, Rodriguez-Hernandez et al. [72] recently reported stimulation of mitophagy, and consequently mitochondrial degradation, in patients with coenzyme Q deficiency. Coenzyme Q10 has been shown to be rate-limiting in the electron transport chain in beef heart mitochondria [73], so an age-related decrease in content would have a large effect. Coenzyme Q10 is also a scavenger of superoxide, so the hypothesis of its deficiency lends to the abundant evidence that excess ROS plays a role in neurodegeneration with age [74, 75]. However, the parallel generation of ROS and decline in mitochondrial membrane potential observed in old neurons, and the higher starting ROS and depolarized mitochondrial membrane potential [71] suggest another factor such as redox potential [12] may be upstream and impact all mitochondrial function.
Complex IV was inhibited much more effectively in neurons from old rats with endogenous substrates given low doses of inhibitor than in neurons from middle-age rats with endogenous substrates, indicating an age-related limitation in electron flow to complex IV. This finding was not surprising, given the age-related deficits in electron transport in upstream complexes, and supported our previous finding that complex IV is substrate-limited in neurons from old rats [17]. One possible explanation for the increased inhibition in neurons prepared from old rats with endogenous substrates is the accumulation of nitric oxide (NO), also an inhibitor of complex IV, which attenuates inhibition of complex IV activity by potassium cyanide in brain mitochondria [76, 77]. Furthermore, Clementi et al. [78] have shown that prolonged exposure of mitochondria to NO also leads to inhibition of complex I. It is possible that prolonged generation of NO may also damage complex I in mitochondria over time, resulting in an age-related decline in electron flow to complex IV.
The most significant age-related deficit in capacity to respond to excess substrates was measured at complex IV, responding 29% less in neurons from old rats (60%) than in neurons from middle-age rats (85%). As opposed to the submitochondrial particles we previously studied, complex IV in neurons in situ had an age-related enzymatic deficit when given excess exogenous substrates. The stress of the particle isolation possibly damaged mitochondria from middle-age rats, bringing their activity down to the level of mitochondria from old rats. Furthermore, in intact neurons the age-related cardiolipin damage we observed previously [17] limits electron transport.
In addition to the conclusions derived for each individual complex, we can make conclusions based on this study regarding the overall bioenergetic status of aging rat brain mitochondria. The deficits we observed in complexes I and III are of additional significance because they are considered the main producers of ROS in the mitochondrion [79, 80]. Interestingly, in 30-month rat brain synaptosomes relative to 4-month rat brain synaptosomes, Curti et al. [81] found deficits in complex IV using cytochrome C as substrate. Since we also found age-related deficits in complex IV inhibition by KCN, complex IV appears to accumulate damage at multiple sites with age. Similar to our results in whole neurons with endogenous substrates, Navarro et al. [82] found in isolated cortical mitochondria no age-related deficits in basal respiration supplemented with glutamate/malate or succinate.
The age-related substrate deficits in neurons isolated from the aging brain environment that persist in culture independent of aging hormone levels, vasculature, and immune system could be explained by epigenetic control of metabolism, as has been observed in aging human skeletal muscle [83, 84]. Our cumulative evidence suggests depleted coenzyme Q levels between complexes I and III. Past clinical trials showed that oral coenzyme Q10 supplementation at a dose of 3 mg/kg body weight in human subjects with no known diseases increased plasma concentrations of coenzyme Q10 measured by HPLC, increased platelet levels, and delayed formation of 8-hydroxydeoxy-guanosine [85]. Others have shown healing effects of oral coenzyme Q10 in patients with mitochondrial diseases such as MELAS [86, 87, 88]. Our studies add to the growing body of evidence that dysfunction in the enzyme complexes of the electron transport chain with age lend to age-related neurodegeneration in senescence-related diseases. Our experiments here and previously [12] support an age-related lack of endogenous substrates or a failure of upstream complexes to transport electrons to complex IV, ultimately leading to age-related neurodegeneration [2].
Table 3.
Slopes, substrate-supplemented capacity derived from flux analysis, and the flux thresholds have been calculated from Figure 6.
| Complex I | Complex II | Complex III | Complex IV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MA | Old | p= | MA | Old | p= | MA | Old | p= | MA | Old | p= | |
| Slope 1 | −1.54 | −3.58 | 0.001 | −1.17 | −2.29 | <.001 | −1.45 | −1.27 | 0.03 | −2.03 | −2.76 | 0.1 |
| Slope 2 | −0.46 | −0.82 | 0.26 | −2.13 | N/A | N/A | N/A | −0.83 | −0.75 | 0.3 | ||
| Substrate-supplemented capacity | 127% | 96% | 72% | 71% | 111% | 89% | 60% | 85% | ||||
| Flux Threshold (% endogenous, %exogenous) | 39, 39 | 48, 40 | 40, 54 | 28, 100 | 42, 100 | 10, 100 | 24, 51 | 28, 24 | ||||
Acknowledgements
We thank John Torricelli and Elizabeth Kunz for assistance in culturing cortical neurons, and Hannah Helfer for media preparation. We also thank Dr. Steven Verhulst for his advice in statistical analysis. This work was supported by NIA grant R01 AG013435.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keller JN. Age-related neuropathology, cognitive decline, and Alzheimer's disease. Ageing Res. Rev. 2006;5:1. doi: 10.1016/j.arr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Parihar MS, Brewer GJ. Mitoenergetic failure in Alzheimer disease. Am. J. Physiol Cell Physiol. 2007;292:C8. doi: 10.1152/ajpcell.00232.2006. [DOI] [PubMed] [Google Scholar]
- 3.Brink TC, Demetrius L, Lehrach H, Adjaye J. Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontology. 2008 doi: 10.1007/s10522-008-9197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smigrodzki RM, Khan SM. Mitochondrial microheteroplasmy and a theory of aging and age-related disease. Rejuvenation. Res. 2005;8:172. doi: 10.1089/rej.2005.8.172. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz C, Axmacher B, Zunker U, Korr H. Age-related changes of DNA repair and mitochondrial DNA synthesis in the mouse brain. Acta Neuropathol. 1999;97:71. doi: 10.1007/s004010050957. [DOI] [PubMed] [Google Scholar]
- 6.Wallace DC, Shoffner JM, Trounce I, Brown MD, Ballinger SW, Corral-Debrinski M, Horton T, Jun AS, Lott MT. Mitochondrial DNA mutations in human degenerative diseases and aging. Biochim. Biophys. Acta. 1995;1271:141. doi: 10.1016/0925-4439(95)00021-u. [DOI] [PubMed] [Google Scholar]
- 7.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 8.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 9.Brewer GJ, Reichensperger JD, Brinton RD. Prevention of age-related dysregulation of calcium dynamics by estrogen in neurons. Neurobiol. Aging. 2006;27:306. doi: 10.1016/j.neurobiolaging.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Thibault O, Mazzanti ML, Blalock EM, Porter NM, Landfield PW. Single-channel and whole-cell studies of calcium currents in young and aged rat hippocampal slice neurons. J. Neurosci. Methods. 1995;59:77. doi: 10.1016/0165-0270(94)00196-n. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090:35. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J. Neurosci. Res. 2008;86:2339. doi: 10.1002/jnr.21679. [DOI] [PubMed] [Google Scholar]
- 13.Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chem. Biol. Interact. 2006;163:38. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech. Ageing Dev. 2007;128:286. doi: 10.1016/j.mad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech. Ageing Dev. 2001;122:945. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 16.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Jones TT, Brewer GJ. Critical age-related loss of cofactors of neuron cytochrome C oxidase reversed by estrogen. Exp. Neurol. 2009;215:212. doi: 10.1016/j.expneurol.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gellerich FN, Trumbeckaite S, Muller T, Deschauer M, Chen Y, Gizatullina Z, Zierz S. Energetic depression caused by mitochondrial dysfunction. Mol. Cell Biochem. 2004;256-257:391. doi: 10.1023/b:mcbi.0000009885.34498.e6. [DOI] [PubMed] [Google Scholar]
- 19.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000;373:16. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 20.Bowling AC, Mutisya EM, Walker LC, Price DL, Cork LC, Beal MF. Age-dependent impairment of mitochondrial function in primate brain. J. Neurochem. 1993;60:1964. doi: 10.1111/j.1471-4159.1993.tb13430.x. [DOI] [PubMed] [Google Scholar]
- 21.Shivakumar BR, Kolluri SV, Ravindranath V. Glutathione and protein thiol homeostasis in brain during reperfusion after cerebral ischemia. J. Pharmacol. Exp. Ther. 1995;274:1167. [PubMed] [Google Scholar]
- 22.Tialowska B, Kaczor J, Stempak W, Grybos D, Popinigis J. Tightly coupled respiration in rat brain homogenates. Acta Biochim. Pol. 1991;38:165. [PubMed] [Google Scholar]
- 23.Monge C, Beraud N, Kuznetsov AV, Rostovtseva T, Sackett D, Schlattner U, Vendelin M, Saks VA. Regulation of respiration in brain mitochondria and synaptosomes: restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase. Mol. Cell Biochem. 2008;318:147. doi: 10.1007/s11010-008-9865-7. [DOI] [PubMed] [Google Scholar]
- 24.Cakala M, Drabik J, Kazmierczak A, Kopczuk D, Adamczyk A. Inhibition of mitochondrial complex II affects dopamine metabolism and decreases its uptake into striatal synaptosomes. Folia Neuropathol. 2006;44:238. [PubMed] [Google Scholar]
- 25.Brewer GJ, Jones TT, Wallimann T, Schlattner U. Higher respiratory rates and improved creatine stimulation in brain mitochondria isolated with anti-oxidants. Mitochondrion. 2004;4:49. doi: 10.1016/j.mito.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Fonck C, Baudry M. Rapid reduction of ATP synthesis and lack of free radical formation by MPP+ in rat brain synaptosomes and mitochondria. Brain Res. 2003;975:214. doi: 10.1016/s0006-8993(03)02675-1. [DOI] [PubMed] [Google Scholar]
- 27.Sipos I, Tretter L, Adam-Vizi V. The production of reactive oxygen species in intact isolated nerve terminals is independent of the mitochondrial membrane potential. Neurochem. Res. 2003;28:1575. doi: 10.1023/a:1025634728227. [DOI] [PubMed] [Google Scholar]
- 28.Hardy JA, Dodd PR, Oakley AE, Perry RH, Edwardson JA, Kidd AM. Metabolically active synaptosomes can be prepared from frozen rat and human brain. J. Neurochem. 1983;40:608. doi: 10.1111/j.1471-4159.1983.tb08024.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 2006;26:7035. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J. Neurosci. 2007;27:7310. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr. Mol. Med. 2004;4:149. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 32.Jekabsons MB, Nicholls DG. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J. Biol. Chem. 2004;279:32989. doi: 10.1074/jbc.M401540200. [DOI] [PubMed] [Google Scholar]
- 33.Safiulina D, Kaasik A, Seppet E, Peet N, Zharkovsky A, Seppet E. Method for in situ detection of the mitochondrial function in neurons. J. Neurosci. Methods. 2004;137:87. doi: 10.1016/j.jneumeth.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J. Neurosci. Methods. 1997;71:143. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- 35.Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat. Protoc. 2007;2:1490. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- 36.Brewer GJ. Age-related toxicity to lactate, glutamate, and beta-amyloid in cultured adult neurons. Neurobiol. Aging. 1998;19:561. doi: 10.1016/s0197-4580(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 37.Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation. Res. 2006;9:169. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- 38.Maher P. Redox control of neural function: background, mechanisms, and significance. Antioxid. Redox. Signal. 2006;8:1941. doi: 10.1089/ars.2006.8.1941. [DOI] [PubMed] [Google Scholar]
- 39.Fatokun AA, Stone TW, Smith RA. Oxidative stress in neurodegeneration and available means of protection. Front Biosci. 2008;13:3288. doi: 10.2741/2926. [DOI] [PubMed] [Google Scholar]
- 40.Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4820. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solleveld HA, Hollander CF. Animals in aging research: requirements, pitfalls and a challenge for the laboratory animal specialist. Vet. Q. 1984;6:96. doi: 10.1080/01652176.1984.9693919. [DOI] [PubMed] [Google Scholar]
- 42.Almeida A, Medina JM. Isolation and characterization of tightly coupled mitochondria from neurons and astrocytes in primary culture. Brain Res. 1997;764:167. doi: 10.1016/s0006-8993(97)00453-8. [DOI] [PubMed] [Google Scholar]
- 43.Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J. Biol. Chem. 1963;238:418. [PubMed] [Google Scholar]
- 44.Palmer G, Horgan DJ, Tisdale H, Singer TP, Beinert H. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XIV. Location of the sites of inhibition of rotenone, barbiturates, and piericidin by means of electron paramagnetic resonance spectroscopy. J. Biol. Chem. 1968;243:844. [PubMed] [Google Scholar]
- 45.Ludolph AC, Ullrich K, Bick U, Fahrendorf G, Przyrembel H. Functional and morphological deficits in late-treated patients with homocystinuria: a clinical, electrophysiologic and MRI study. Acta Neurol. Scand. 1991;83:161. doi: 10.1111/j.1600-0404.1991.tb04669.x. [DOI] [PubMed] [Google Scholar]
- 46.Ludolph AC, He F, Spencer PS, Hammerstad J, Sabri M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 1991;18:492. doi: 10.1017/s0317167100032212. [DOI] [PubMed] [Google Scholar]
- 47.Potter VR, Reif AE. Inhibition of an electron transport component by antimycin A. J. Biol. Chem. 1952;194:287. [PubMed] [Google Scholar]
- 48.Barber D, Parr SR, Greenwood C. The reactions of Pseudomonas cytochrome c-551 oxidase with potassium cyanide. Biochem. J. 1978;175:239. doi: 10.1042/bj1750239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cross AR, Jones OT. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 1986;237:111. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yea CM, Cross AR, Jones OT. Purification and some properties of the 45 kDa diphenylene iodonium-binding flavoprotein of neutrophil NADPH oxidase. Biochem. J. 1990;265:95. doi: 10.1042/bj2650095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davey GP, Clark JB. Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J. Neurochem. 1996;66:1617. doi: 10.1046/j.1471-4159.1996.66041617.x. [DOI] [PubMed] [Google Scholar]
- 52.Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J. Neurosci. Res. 2003;72:527. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- 53.Huang LS, Cobessi D, Tung EY, Berry EA. Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: a new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. J. Mol. Biol. 2005;351:573. doi: 10.1016/j.jmb.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson MT, Antonini G, Malatesta F, Sarti P, Brunori M. Probing the oxygen binding site of cytochrome c oxidase by cyanide. J. Biol. Chem. 1994;269:24114. [PubMed] [Google Scholar]
- 55.Becker EW. The roles of ATP in the dynamics of the actin filaments of the cytoskeleton. Biol. Chem. 2006;387:401. doi: 10.1515/BC.2006.054. [DOI] [PubMed] [Google Scholar]
- 56.Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 2000;74:1968. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- 57.Estornell E, Tormo JR, Barber T. A deficiency in respiratory complex I in heart mitochondria from vitamin A-deficient rats is counteracted by an increase in coenzyme Q. Biochem. Biophys. Res. Commun. 1997;233:451. doi: 10.1006/bbrc.1997.6480. [DOI] [PubMed] [Google Scholar]
- 58.Huang LS, Sun G, Cobessi D, Wang AC, Shen JT, Tung EY, Anderson VE, Berry EA. 3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006;281:5965. doi: 10.1074/jbc.M511270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J. Neurosci. 2003;23:10756. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 2002;136:317. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 61.Genova ML, Bovina C, Marchetti M, Pallotti F, Tietz C, Biagini G, Pugnaloni A, Viticchi C, Gorini A, Villa RF, Lenaz G. Decrease of rotenone inhibition is a sensitive parameter of complex I damage in brain non-synaptic mitochondria of aged rats. FEBS Lett. 1997;410:467. doi: 10.1016/s0014-5793(97)00638-8. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson's disease pathogenesis. Biochim. Biophys. Acta. 2008 doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braak H, Rub U, Schultz C, Del TK. Vulnerability of cortical neurons to Alzheimer's and Parkinson's diseases. J. Alzheimers. Dis. 2006;9:35. doi: 10.3233/jad-2006-9s305. [DOI] [PubMed] [Google Scholar]
- 64.Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer's disease. Prog. Brain Res. 2002;136:467. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- 65.Ko LW, Sheu KF, Thaler HT, Markesbery WR, Blass JP. Selective loss of KGDHC-enriched neurons in Alzheimer temporal cortex: does mitochondrial variation contribute to selective vulnerability? J. Mol. Neurosci. 2001;17:361. doi: 10.1385/JMN:17:3:361. [DOI] [PubMed] [Google Scholar]
- 66.Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11:573. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- 67.Parihar MS, Brewer GJ. Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J. Neurosci. Res. 2007;85:1018. doi: 10.1002/jnr.21218. [DOI] [PubMed] [Google Scholar]
- 68.Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, Saudou F, Elalouf JM, Hirsch E, Hantraye P, Deglon N, Brouillet E. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol. Biol. Cell. 2006;17:1652. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington's disease brain. Ann. Neurol. 1999;45:25. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 70.Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in Huntington's disease: selective vulnerability of the basal ganglia. Ann. Neurol. 1997;41:646. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 71.Kilbride SM, Telford JE, Davey GP. Age-related changes in H2O2 production and bioenergetics in rat brain synaptosomes. Biochim. Biophys. Acta. 2008;1777:783. doi: 10.1016/j.bbabio.2008.05.445. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Hernandez A, Cordero MD, Salviati L, Artuch R, Pineda M, Briones P, Izquierdo LG, Cotan D, Navas P, Sanchez-Alcazar JA. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009;5 doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- 73.Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti CG, Lenaz G. Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett. 1992;311:107. doi: 10.1016/0014-5793(92)81378-y. [DOI] [PubMed] [Google Scholar]
- 74.Bertram C, Hass R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem. 2008;389:211. doi: 10.1515/BC.2008.031. [DOI] [PubMed] [Google Scholar]
- 75.Harman D. Role of free radicals in aging and disease. Ann. N. Y. Acad. Sci. 1992;673:126. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 76.Pearce LL, Bominaar EL, Hill BC, Peterson J. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J. Biol. Chem. 2003;278:52139. doi: 10.1074/jbc.M310359200. [DOI] [PubMed] [Google Scholar]
- 77.Clementi E, Nisoli E. Nitric oxide and mitochondrial biogenesis: a key to long-term regulation of cellular metabolism. Comp Biochem. Physiol A Mol. Integr. Physiol. 2005;142:102. doi: 10.1016/j.cbpb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 78.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7631. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 80.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 81.Curti D, Giangare MC, Redolfi ME, Fugaccia I, Benzi G. Age-related modifications of cytochrome C oxidase activity in discrete brain regions. Mech. Ageing Dev. 1990;55:171. doi: 10.1016/0047-6374(90)90024-a. [DOI] [PubMed] [Google Scholar]
- 82.Navarro A, Lopez-Cepero JM, Bandez MJ, Sanchez-Pino MJ, Gomez C, Cadenas E, Boveris A. Hippocampal mitochondrial dysfunction in rat aging. Am. J. Physiol Regul. Integr. Comp Physiol. 2008;294:R501–R509. doi: 10.1152/ajpregu.00492.2007. [DOI] [PubMed] [Google Scholar]
- 83.Ling C, Poulsen P, Simonsson S, Ronn T, Holmkvist J, Almgren P, Hagert P, Nilsson E, Mabey AG, Nilsson P, Vaag A, Groop L. Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J. Clin. Invest. 2007;117:3427. doi: 10.1172/JCI30938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronn T, Poulsen P, Hansson O, Holmkvist J, Almgren P, Nilsson P, Tuomi T, Isomaa B, Groop L, Vaag A, Ling C. Age influences DNA methylation and gene expression of COX7A1 in human skeletal muscle. Diabetologia. 2008;51:1159. doi: 10.1007/s00125-008-1018-8. [DOI] [PubMed] [Google Scholar]
- 85.Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. Int. J. Biol. Sci. 2007;3:257. doi: 10.7150/ijbs.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abe K, Fujimura H, Nishikawa Y, Yorifuji S, Mezaki T, Hirono N, Nishitani N, Kameyama M. Marked reduction in CSF lactate and pyruvate levels after CoQ therapy in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) Acta Neurol. Scand. 1991;83:356. doi: 10.1111/j.1600-0404.1991.tb03962.x. [DOI] [PubMed] [Google Scholar]
- 87.Ihara Y, Namba R, Kuroda S, Sato T, Shirabe T. Mitochondrial encephalomyopathy (MELAS): pathological study and successful therapy with coenzyme Q10 and idebenone. J. Neurol. Sci. 1989;90:263. doi: 10.1016/0022-510x(89)90112-3. [DOI] [PubMed] [Google Scholar]
- 88.Nishikawa Y, Takahashi M, Yorifuji S, Nakamura Y, Ueno S, Tarui S, Kozuka T, Nishimura T. Long-term coenzyme Q10 therapy for a mitochondrial encephalomyopathy with cytochrome c oxidase deficiency: a 31P NMR study. Neurology. 1989;39:399. doi: 10.1212/wnl.39.3.399. [DOI] [PubMed] [Google Scholar]