Abstract
The extent of adult stem cell involvement in embryonic growth is often unclear, as reliable markers or assays for whether a cell is derived from an adult stem cell, such as the Melanocyte Stem Cell (MSC), are typically not available. We have previously shown that two lineages of melanocytes can contribute to the larval zebrafish pigment pattern. The embryo first develops an ontogenetic pattern that is largely composed of ErbB-independent, direct-developing melanocytes. This population can be replaced during regeneration by an ErbB-dependent MSC-derived population following melanocyte ablation. In this study, we developed a melanocyte differentiation assay, used together with drugs that ablate the MSC, to investigate whether MSC-derived melanocytes contribute to the ontogenetic pattern. We found that essentially all melanocytes that develop before 3 dpf arise from the ErbB-independent, direct-developing population. Similarly, late-developing (after 3 dpf) melanocytes of the head are also ErbB independent. In contrast, the melanocytes that develop after three days postfertilization in the lateral and dorsal stripe are sensitive to ErbB inhibitor, indicating that they are derived from the MSC. We show that melanocyte regeneration mutants kitj1e99 and skiv2l2j24e1, that are grossly normal for the overall ontogenetic pattern, also lack the MSC-derived contribution to the lateral stripe. This result suggests that the underlying regeneration defect of these mutations is a defect in MSC regulation. We suggest that the regulative functions of the MSC may serve quality control roles during larval development, in addition to its established roles in larval regeneration and growth and homeostasis in the adult.
Introduction
The life cycle of animals is marked by multiple developmental transitions. Such changes may be morphological, such as the wholesale and coordinated changes in form during insect or amphibian metamorphosis (Truman and Riddiford, 1999; Yaoita and Brown, 1990) or the onset of adult pigment pattern at the larva-to-adult transition in zebrafish (Johnson et al., 1995). Developmental transitions may also be physiological in nature, such has the developmental change in growth response of fins to nutritional insufficiency (Goldsmith et al., 2006). Yet additional developmental transitions may involve the origins of cells that contribute to tissues of the animal. For instance, during embryonic or fetal development of fish, amphibians or mammals, a direct developing (without a renewing stem cell precursor) blood lineage, referred to as primitive hematopoiesis, is replaced by definitive hematopoiesis, through the self-renewing hematopoietic stem cell (Bertrand et al., 2007; Davidson and Zon, 2004). Likewise, the stem cell-derived adult pigment pattern of zebrafish replaces the embryonic and larval pattern of melanocytes that is thought to develop largely without a self-renewing stem cell precursor (Budi et al., 2008; Hultman et al., 2009).
The zebrafish embryonic and larval pigment pattern is an attractive model for studying the relative roles of direct developing and stem cell derived melanocytes (O’Reilly-Pol and Johnson, 2008). During early embryonic development, melanocytes or their precursors begin to migrate from the neural crest beginning at approximately 19 hours (Kelsh et al., 2000), and are largely differentiated and in place by 72 hours (Rawls and Johnson, 2003). When embryonic melanocytes are ablated with lasers (Yang et al., 2004) or chemicals (Yang and Johnson, 2006) they rapidly regenerate from undifferentiated precursors, referred to as melanocyte stem cells (MSCs). Although these MSCs have not yet been directly identified, their properties can be inferred from a variety of experiments. Thus, when embryos are mutant for the EGF-like receptor erbb3b, or treated during early embryonic stages with the ErbB inhibitor AG1478, the ensuing embryonic pigment pattern is grossly normal (Budi et al., 2008; Hultman et al., 2009). However, when these ontogenetic melanocytes are subsequently chemically ablated, no melanocyte regeneration occurs (Hultman et al., 2009), suggesting that the ErbB inhibitor has ablated the stem cell that supports larval melanocyte regeneration. Similarly, new melanocytes fail to develop in these fish during pigment pattern metamorphosis at 14 dpf (Budi et al., 2008), strongly suggesting that larval regeneration melanocytes and metamorphosis melanocytes share a common, AG1478-sensitive, MSC. These results led to a model of two distinct types of lineages that can contribute to the larval melanocyte pattern: (1) direct-developing melanocytes that do not go through an AG1478-sensitive MSC intermediate, that are responsible for the ontogenetic pattern, and (2) MSC-derived melanocytes that contribute melanocytes during regeneration (Hultman et al., 2009). In this study, we explore the notion that MSC-derived melanocytes also contribute to the ontogenetic pattern.
Although the embryonic pattern is relatively stable from 3 dpf to the onset of metamorphosis at 14 dpf, approximately 20 new melanocytes are known to appear after 3 dpf in the lateral stripe (Milos and Dingle, 1978a). This second wave of lateral stripe melanocyte differentiation contributes approximately 50% of the melanocytes in the lateral stripe at 8 dpf. Further analysis revealed that this second wave regulates and fills in the gaps either introduced by manipulation (Milos and Dingle, 1978b) or left by first wave melanocytes (Milos et al., 1983). This regulative behavior is similar to that observed during melanocyte regeneration where the additional melanocytes will reform the normal larval pattern (Yang et al., 2004).
The assays that revealed that AG1478 ablates MSCs responsible for larval melanocyte regeneration also led to the identification of melanocyte regeneration mutants. Recessive lethal mutants for skiv2l2j24e1, that encodes an RNA helicase and gfpt1j23e1 that encodes the enzyme for the first step in the hexosamine biosynthetic pathway, develop grossly normal numbers of ontogenetic melanocytes, but fail to regenerate them after chemical ablation (Yang et al., 2007). Similarly, mutants for the temperature-sensitive allele of the receptor tyrosine kinase kit (kitj1e99) also develop grossly normal numbers of melanocytes when reared at the permissive temperature (Rawls and Johnson, 2003), but fail to regenerate them following ablation (Yang and Johnson, 2006; Yang et al., 2004). One model for these mutants is that they are specifically defective for regeneration mechanisms. Alternatively, these mutations may identify defects in regulation of the MSC lineage. These models might be distinguished from each other if we could identify contributions of the MSC lineage to ontogenetic melanocyte pattern.
In this study, we develop a melanocyte differentiation assay to investigate the relative contributions of melanocytes developing at different stages to different regions of the larval pigment pattern. We also examine the effects of ablating the AG1478-sensitive MSC on temporal and regional populations of larval melanocytes. We show that development of early differentiating melanocytes, or melanocytes that develop prior to 3 dpf, is not affected by MSC ablation. Similarly, melanocytes that develop after 3 dpf, that we hereafter refer to as late developing melanocytes (LDMs) in the head are not affected by AG1478-mediated MSC ablation. In contrast, we show that LDMs in the lateral stripe fail to develop following MSC ablation with AG1478, indicating that LDMs of the lateral stripe are derived from the MSC. Examination of larval melanocyte regeneration mutants shows that kitj1e99 and skiv2l2j24e1 are also defective for lateral stripe LDMs, suggesting that their defects are in MSC regulation, rather than specific defects in regeneration.
Materials and Methods
Animal care and stocks
Adult fish were maintained at 25–27°C at 14L:10D light to dark light cycle using standard fish protocols (Westerfield, http://zfin.org/zf_info/zfbook/zfbk.html). Embryos and larval fish were reared at 28°C in 2 ppt marine salt (Coral Life, Carson, CA) in carbon-filtered tap water except for kitj1e99 mutants, which were reared at 25°C.
Spectrophotometric determination of melanin synthesis
Relative melanin content was determined with a modification of the method of (Ozeki et al., 1995). For each treatment, 25 embryos were placed in 0.5 ml Soluene-350 (PerkinElmer) and heated at 95°C for 1 hour. After cooling and centrifugation, melanin content in supernatant was assayed by absorption at 500 nM on a Nanodrop spectrophotometer (Thermo Fisher Scientific Inc.).
PTU melanocyte differentiation assay
In order to determine when and where melanocytes develop, we took advantage of the property of phenylthiourea (PTU), also known as phenylthiocarbamide (Sigma, St. Louis) to inhibit the function of the melanin synthesizing enzyme Tyrosinase (Rawls and Johnson, 2000). Melanin synthesis is inhibited within a few hours of addition of PTU (Figure 1). Thus, melanin-positive melanocytes after the addition of PTU (0.1–0.2 mM) were differentiated prior to drug treatment. To reveal melanocytes that differentiated after PTU treatment, without visible melanin, we took advantage of a transgenic line Tg(Tyrp1:GFP)j900 expressing GFP under the control of the Fugu rubripes Tyrosinase Related Protein 1 promoter. This is a transgenic insertion generated in our lab from a transposon previously described by Zou et al. and is similar to the pt102 insertion allele described therein (Zou et al., 2006). In embryonic and larval stages, this line expresses GFP exclusively in differentiated (melanized melanocytes), or in differentiating melanocytes immediately prior to overt melanization. A small fraction of GFP+ cells are melanin- during melanocyte development (6/175, or 3.3% of GFP+ cells, N=5 embryos at 20 hpf). A smaller fraction of melanin+ cells are observed to be GFP- (1/181 melanin+ cells, or 0.5% of melanin+ cells, N=5 embryos at 20 hpf), indicating that GFP is typically expressed only slightly before the first signs of melanin synthesis in melanocytes. Thus, melanin-positive melanocytes observed after the addition of PTU (0.1–0.2 mM) differentiated prior to PTU treatment. For our differentiation assay, we incubated larvae in PTU beginning at 3, 5 or 7 dpf, and examined them for GFP+, melanin- melanocytes at 5, 7 and 10 dpf, respectively, using a Nikon SMZ1500 stereofluorescence microscope.
Figure 1. PTU exposure blocks melanin synthesis within four hours of treatment.
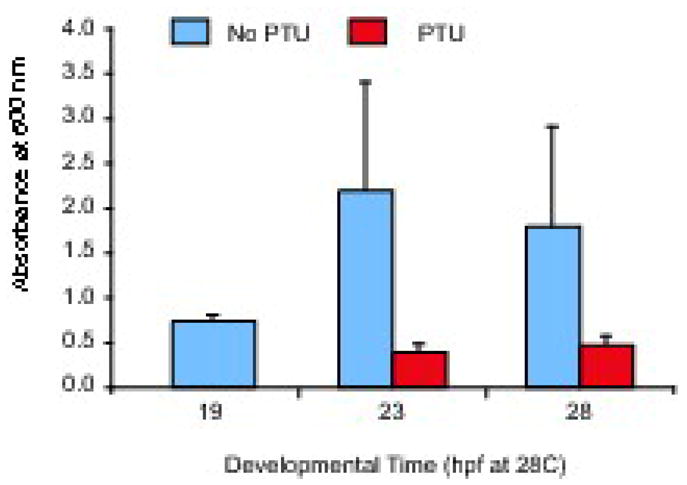
Wild-type embryos were allowed to develop until first signs of melanization (19 hpf). At this time, 3 samples of 25 embryos each were anesthetized and solubilized in Soluene-350 for spectophotometric melanin determination (see Materials and Methods). 0.2 mM PTU was then added to one-half the remaining embryos, and additional samples were prepared from PTU-treated and untreated controls at 23 and 28 hpf. Note that while melanin absorption increased in untreated animals, it stayed the same or decreased in PTU-treated animals, suggesting that PTU blocks melanin synthesis within 4 hours of application to embryos.
Photographic melanocyte differentiation assay
To determine late stage melanocyte development in animals not carrying Tg(Tyrp1:GFP)j900 we photographed the right and left lateral stripe of individually segregated larvae at 3 dpf and at 6 dpf. The number of melanocytes that developed from 3–6 dpf was calculated by subtracting the number of melanocytes at 3 dpf lateral stripe from that at 6 dpf.
ErbB pharmacological inhibitor
The ErbB kinase inhibitor, AG1478 (Calbiochem), was dissolved in dimethyl sulphoxide (DMSO) to make a stock solution (6 mM) and stored at −20°C in a light protected vial. Embryos were incubated in Petri dishes with 3 uM AG1478 with 0.5% DMSO in 2 ppt marine salt water, with 1 embryo per ml. Embryos were kept in the dark during incubation to protect drugs from light degradation. To test whether AG1478 was effectively inhibiting the establishment of the larval melanocyte stem cell, we treated 20 embryos with the melanocytotoxic drug, MoTP (50 uM, (Yang and Johnson, 2006)), from 1–3 dpf and confirmed that no regeneration of the larval pattern occurred by 6 dpf (see Hultman et al., 2009 for MoTP protocol).
Regeneration mutants
The temperature sensitive mutant kitj1e99 has been previously described for embryonic and regenerative melanocyte defects (Rawls and Johnson, 2003; Yang and Johnson, 2006). kitj1e99 homozygous embryos were reared at 25°C, a temperature that is permissive for embryonic kit functions such as migration and differentiation, but is insufficient for larval melanocyte regeneration. Time of experimental manipulations were adjusted to reflect standard development at 28.5°C (Kimmel et al., 1995).
We also tested two recessive lethal mutants that were previously identified in a screen for regeneration-specific melanocyte phenotypes: gfpt1j23e1 and skiv2l2j24e1 (Yang et al., 2007). gfpt1j23e1 heterozygous animals were crossed to obtain ¼ gfpt1j23e1 homozygous embryos, which were identified at 3 dpf by their pale embryonic melanocytes. skiv2l2j24e1 heterozygous animals were crossed to obtain ¼ skiv2l2 homozygous progeny. Since there is no apparent phenotype at 3 dpf, we photographed each animal at 3 and 6 dpf, then identified skiv2l2j24e1 mutants based on their small eye and head phenotype at 6 dpf, and lethality at 8 dpf.
Results
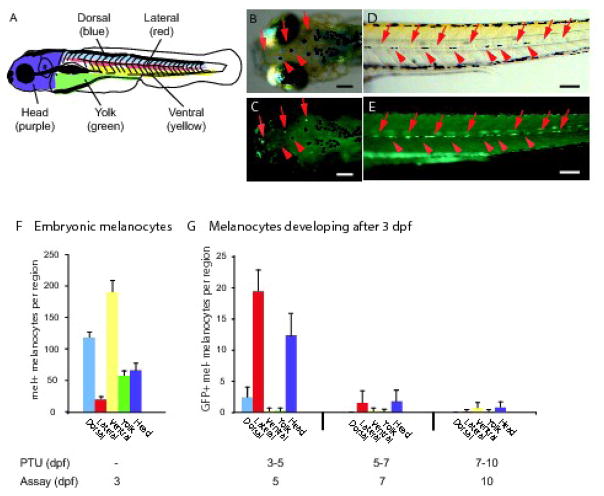
As a first step in exploring the differential role of direct-developing and Melanocyte Stem Cell (MSC)-derived melanocytes in the embryonic and larval pigment pattern we sought to identify the locations in the embryo where melanocytes develop before and after 3 dpf. We chose 3 dpf because although the vast majority of melanocytes in the zebrafish larvae have developed by this stage, Milos and Dingle (1978a) had previously described an early wave of lateral stripe melanocytes that develop before this time, and a second wave of lateral stripe melanocytes that develop after 3 dpf. Whether significant numbers of melanocytes in other regions of the larva develop after 3dpf had not yet been explored. To mark melanocytes for time overt differentiation or melanization, we took advantage of two markers for differentiated melanocytes: a transgenic line expressing GFP driven from the Fugu rubripes tyrosinase-related protein promoter, Tg(Tyrp1:GFP)j900, and melanin. We reasoned that by blocking further melanin synthesis with the tyrosinase inhibitor Phenylthiourea (PTU), we could identify melanocytes that had differentiated prior to PTU treatment by the presence of melanin (See Figure 2B–E, red arrowheads). Conversely, we reasoned that we could identify melanocytes that completed their differentiation after the application of PTU by their GFP expression in the absence melanin (See Figure 2B–E, red arrows).
Figure 2. Location and time of differentiation of larval melanocytes.
(A) Cartoon showing regions of embryonic and larval melanocyte quantitation. (B–E) Photos of Tg(Tyrp1:GFP)j900 larva at 5 dpf after treatment with PTU from 3–5 dpf showing melanocytes that differentiated prior to melanin inhibition by PTU (melanin+, GFP+, red arrowheads) and melanocytes that differentiated after melanin inhibition by PTU (melanin-, GFP+, red arrows). (B, C) Dorsal view of the head. (D, E) Lateral view showing the left-side lateral stripe. Additional, out-of-focus fluorescence is also apparent from melanocytes in the right-side horizontal myoseptum. (F) Quantitation of embryonic melanocytes in various regions at 3 dpf. Average number of melanized cells, error bars represent standard deviation, N=10. (G) Quantitation of melanocytes that develop from 3–5 dpf, 5–7 dpf, and 7–10 dpf in these regions. Average number of GFP-positive, melanin-negative cells at time of assay, error bars represent standard deviation, N=10. Scale bars 100 um.
Using this technique, we examined where LDMs appear in the larval regions shown in Figure 2A, and found that zebrafish embryos develop 452.3 ± 28.8 melanocytes prior to 3 dpf (Figure 2F), and a further 40.6 ± 7.4 melanocytes between 3 dpf and 10 dpf (Figure 2G). We find such late developing melanocytes (LDMs) in most regions that developed melanocytes early, including the dorsal, lateral, ventral and yolk sac stripes as well as in the head (regions shown in Figure 2A). The majority (34.9 ± 6.1 or 86%) of these late melanocytes have differentiated by 5 dpf, and a further 5.7 ± 4.3 late melanocytes differentiate between 5 and 10 dpf.
The majority of LDMs differentiate in the lateral stripe (Figure 2G). Thus, of the approximately 41 melanocytes that differentiate between 3 and 10 dpf, 21.0 ± 3.9, or 52% of LDMs are in the lateral stripe. These LDMs comprise approximately 50% of the lateral stripe melanocytes. The proportion of late lateral stripe melanocytes that we observe is consistent with the numbers of second wave lateral stripe melanocytes described previously (Milos and Dingle, 1978a).
Smaller numbers of LDMs appear in other regions of the body (Figure 2G). Thus, we observe 15.9 ± 4.6 LDMs in the head, 2.4 ± 1.6 LDMs in the dorsal stripe, and 1.5 ± 1.2 LDMs in the ventral and yolk stripes. These LDMs comprise 20, 2.0, and 0.6% of the melanocytes present in each respective region of the 10 dpf larvae. These data indicate that LDMs contribute less than 9% of the total larval melanocyte pattern and only substantially to the lateral stripe and in the head.
Late developing lateral stripe melanocytes, but not late developing head melanocytes, are derived from AG1478-sensitive melanocyte stem cells
We were next interested in determining the relative contribution of direct developing and MSC-derived melanocytes to early (< 3 dpf) and late (> 3 dpf) developing melanocyte populations. Here, we took advantage of the ErbB inhibitor AG1478, that specifically ablates the MSC population when applied during early embryonic development (Budi et al., 2008; Hultman et al., 2009). This treatment has little or no effect on overall development of the embryonic and larval pigment pattern indicating that the majority of these melanocytes develop directly, without a MSC intermediate (Budi et al., 2008; Hultman et al., 2009). However, the effect of AG1478 treatment on development of melanocytes in distinct regions or melanocytes that develop before or after 3 dpf has not been explored. Accordingly, we treated Tg(tyrp:GFP)j900 embryos with AG1478, and used the PTU melanocyte differentiation method described above to determine whether there were specific regional or temporal contributions of MSC-derived melanocytes to the overall larval pigment pattern (Figure 3).
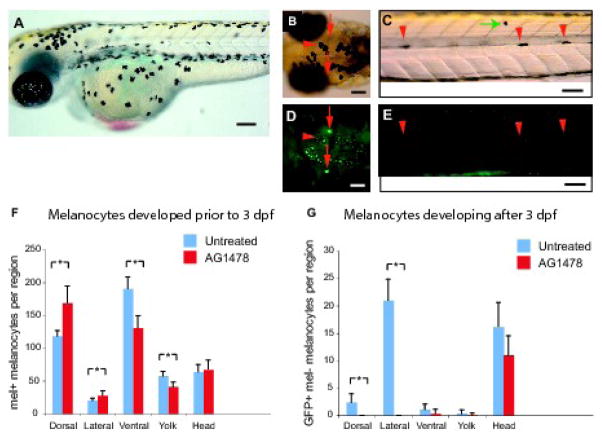
Figure 3. Late-stage melanocytes in the trunk are derived from the ErbB-dependant MSC.
(A) Example of untreated animal at 3 dpf used for quantitative analysis. (B–D) Photos of Tg(Tyrp1:GFP)j900 larva at 5 dpf after treatment with AG1478 from 9–48 hpf and PTU from 3–5 dpf. Red arrows indicate melanin-, GFP+ melanocytes. Red arrowheads indicate melanin+, GFP+ melanocytes. (B, D) Dorsal head view. (C, E) Lateral view. Note absence of GFP+, melanin- cells in horizontal myoseptum of AG1478-treated embryos. GFP fluorescence in mel+ melanocytes (marked by arrowheads) is quenched by melanin in this photograph. (F) Quantitation of embryonic melanocytes at 3 dpf of untreated and AG1478-treated (9–48 hpf) animals. Note that while most melanocytes have reached terminal positions in untreated embryos (A), some melanocytes are still migrating ventrally in AG1478-treated embryo (green arrow in panel C), and are scored as dorsal melanocytes in our analysis. (G) Quantification of larval melanocytes that develop from 3–10 dpf (melanin-, GFP+ following PTU addition at 3 days) in untreated and AG1478-treated animals. In total, 40.6 +/− 7.4 late developing melanocytes are observed in untreated animals, mostly in the lateral stripe and head. In contrast, only 11.6 +/− 4.3 LDM form in AG1478-treated animals, and are notably absent from the lateral stripe. Mean value (N=10) with error bars representing standard deviation. * P-values ≤ 0.05. Scale bars 100 um.
Although the number of melanocytes in AG1478-treated embryos at 3 dpf was indistinguishable from untreated animals (442.9 ± 40.7 melanocytes in AG1478-treated larvae vs. 452 ± 28.8 melanocytes in untreated larvae, P = 0.56), regional analysis (see Figure 2A for regions) revealed significant increases in melanocytes in the dorsal region, and an equivalent deficit of melanocytes in ventral and yolk stripes at 3 dpf (Figure 3F). The finding that the deficit in ventral melanocytes is offset by an increase in dorsal melanocytes suggests that the altered distribution is likely due to a delay in melanocyte migration in the AG1478-treated fish. This inference of delayed migration is supported by the observation that melanocytes that would typically have coalesced into the yolk stripe by this time are still scattered on the lateral surface of the yolk, and occasional melanocytes are also observed over the dorsal myotomes, between the dorsal stripe and the lateral stripe in the AG1478-treated embryo (see Figure 3C, green arrow). We conclude that AG1478 treatment and MSC ablation has little or no effect on differentiation of early (< 3dpf) developing melanocytes. This suggests that MSC-derived melanocytes make no substantial contribution to the melanocyte pattern prior to 3 dpf.
In contrast, we find that AG1478 treatment or MSC depletion has substantial deficits in the melanocytes that develop after 3 dpf (Figure 3B–E, G). In total, we find 11.6 ± 4.3 GFP+, melanin- melanocytes at 10 dpf (LDMs) per AG1478-treated larvae in our PTU differentiation studies. This is in contrast to 40.6 ± 7.4 LDMs that typically develop in larvae that have not been treated with AG1478. This result suggests that approximately 29 (40.6 – 11.3) melanocytes, or 73% of those that develop after 3 dpf, arise from AG1478-senstive MSCs. However, these MSC-derived melanocytes contribute less than 6% (29/490 total larval melanocytes) to the overall larval melanocyte pattern at 10 dpf. This small contribution of MSC-derived melanocytes explains earlier studies with AG1478 that concluded that the larval pattern developed by direct development, rather than from MSCs (Budi et al., 2008; Hultman et al., 2009).
We also found region-specific deficits in AG1478-sensitive melanocyte development after 3 dpf (Figure 3B–E, G). Thus, where untreated larvae develop 21 ± 3.9 GFP+, melanin– melanocytes in the lateral stripes after 3 dpf in our PTU differentiation assays, no (0.0 ± 0.0) GFP+, melanin- melanocytes develop in the lateral stripes in AG1478-treated fish (Figure 3C, E, G). Similarly, where 2.4 ± 2.0 GFP+, melanin– melanocytes typically develop in the dorsal stripe after 3dpf, we again find no (0.0 ± 0.0) GFP+, melanin- melanocytes in the dorsal stripe after AG1478 treatment. These results suggest that all LDMs in the lateral and dorsal stripes arise from the AG1478-sensitive MSCs.
In contrast, LDMs in the head are unaffected by AG1478 treatment. Thus, where our differentiation analysis in untreated larvae reveals 15.9 ± 4.6 GFP+, melanin- melanocytes developing after 3 dpf in these regions, AG1478 treatment results in a similar number of late developing head melanocytes (11.1 ± 4.5, P = 0.2, Figure 3B, D, G). Thus, the LDMs in the head are not derived from AG1478-sensitive MSC. Likely, this suggests that these melanocytes are late contributions of the direct developing embryonic melanocyte lineage. We cannot exclude, however, that these late head melanocytes arise from an AG1478-resistant stem cell.
Late-stage lateral stripe melanocyte development is defective in regeneration mutants
We next wished to know whether MSC derived melanocytes developed in melanocyte regeneration mutants. Mutants for kitj1e99, skiv2l2j24e1, and gfpt1j23e1, develop embryonic melanocytes, but fail to regenerate melanocytes following chemical ablation with MoTP. We have tended to interpret these results to indicate defects in the regulation of the MSC lineage, rather than specific defects in regeneration. Our identification of MSC-derived melanocytes in late-developing populations of the lateral stripe now allows us to further challenge this notion.
As a first step to ask whether these mutations affect development of the late-developing, MSC-derived melanocytes we generated 6 dpf mutants and counted their lateral stripe melanocytes (Figure 4). Since MSC-derived lateral stripe melanocytes comprise approximately one-half the normal number at this stage, we expected the regeneration mutants to have one-half the normal complement of larval stripe melanocytes. This was the case for kitj1e99 (Figure 4B) and skiv2l2j24e1 (Figure 4C) mutants, that had 13.1 ± 5.8 and 23.0 ± 11.3 lateral stripe melanocytes, respectively, compared to 39.6 ± 4.4 lateral stripe melanocytes in our control WT fish (Figure 4A). This result is consistent with the model that these mutants lack the MSC-derived contribution in the lateral stripe, although the somewhat lower number of lateral melanocytes observed in the kitj1e99 mutant may also reflect an independent defect in direct-developing melanocyte development (see below). In contrast, mutants for gfpt1j23e1 (Figure 4D) develop 31.0 ± 3.5 lateral stripe melanocytes, or approximately 75% of the normal complement. This number is in excess of the number that typically develops in the lateral stripe from the direct developing lineage, suggesting that the gfpt1j23e1 mutant still has some capacity to develop melanocytes from the MSC lineage.
Figure 4. Late-stage melanocyte development in larval melanocyte regeneration mutants.
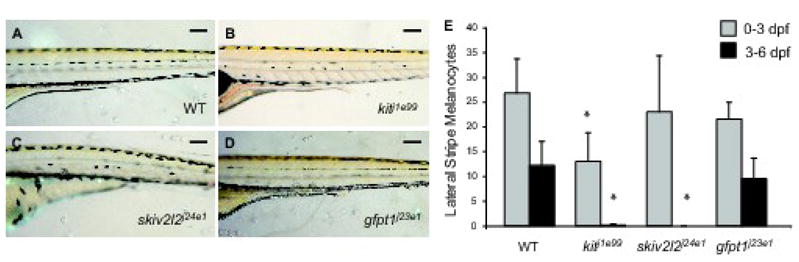
Lateral trunk view at 6 dpf of (A) wild type, (B) kitj1e99, (C) skiv2l2j24e1 and (D) gfpt1j23e1. Scale bars 100 um. (E) Quantitation of embryonic lateral stripe melanocytes at 3 dpf (light gray) and melanocytes that develop from 3–6 dpf (dark gray) in these mutants. Mean value (N=10 for wild type, kitj1e99 and gfpt1j23e1, N=4 for skiv2l2j24e1) with error bars representing standard deviation. * P-values ≤ 0.05.
To further investigate the possible effects of the regeneration mutations on ontogenetic development from MSCs we next examined the relative melanocyte deficits at early (< 3 dpf) and late (> 3 dpf) stages. We reasoned that if the deficits in lateral stripe melanocytes were from the MSC-derived lineages, then we would see few or no deficits in melanocytes that differentiated prior to 3 dpf, and profound deficits in melanocytes that differentiate later. Consistent with this prediction, mutants for skiv2l2j24e1 and gfpt1j23e1 develop normal numbers of lateral stripe melanocytes prior to 3 dpf, indicating that the skiv2l2j24e1 and gfpt1j23e1 mutations have no effect on the direct developing population (Figure 4E). Mutants for kitj1e99 develop approximately 65% of the normal numbers of melanocytes for this period, suggesting that even at this permissive temperature for the kitj1e99 allele, it is not completely sufficient for normal numbers of direct developing melanocytes.
We examined photos of larvae at 3 and 7 dpf to identify how many LDM lateral stripe melanocytes developed in regeneration mutants (Figure 4E). Using this technique for melanocyte differentiation, we observe 19.8 ± 8.2 LDM melanocytes from 3 dpf to 7 dpf in wild-type larvae. This is consistent with the numbers of lateral stripe LDMs we had earlier determined using the PTU differentiation assay from 3 dpf to 7 dpf (20.9 ± 4.6 LDMs, P = 0.72, see Figure 2 for PTU differentiation). We then examined how many LDM lateral stripe melanocytes developed in wild-type and mutant animals from 3 dpf to 6 dpf. In contrast to the wild-type larvae, which developed 12.2 ± 4.9 lateral stripe LDMs, we found mutants for kitj1e99 and skiv2l2j24e1 developed no lateral stripe melanocytes after 3 dpf (0.1 ± 0.3 for kitj1e99 and 0.0 ± 0.0 for skiv2l2j24e1). This result suggests that these mutations cause defects in MSC regulation, rather than defects specific to regeneration. In contrast, gfpt1j23e1 mutants develop approximately 80% the normal complement of late-developing, MSC-derived melanocytes (9.5 ± 4.2 LDMs). Thus, although gfpt1j23e1 mutants have profound defects in melanocyte regeneration, MSC contribution to the lateral stripe is only partially affected. This may indicate that gfpt1j23e1 has specific roles in regeneration that are unrelated to its role in ontogenetic development, or alternatively, reflect the role of gradual loss of maternally provided gene product in melanocyte development in later developing populations, such as LDMs or larval regeneration, between 3 and 6 dpf.
Discussion
Differential contribution of late developing melanocyte stem cell derived melanocytes to the embryonic and larval pigment pattern
We developed a melanocyte differentiation method that allows us to combine time of application of PTU with a gene expression marker to reveal differentiated melanocytes in the absence of melanin, to determine when melanocytes in different regions of the embryo develop. We found that approximately 91% of melanocytes develop before 3 dpf, and a further 9% of melanocytes develop after 3 dpf, but mostly before 5 dpf. We refer to this class as late developing melanocytes (LDMs). Although some LDMs develop in all enumerated regions of the pigment pattern, the largest contribution is in the lateral stripe (52% of LDMs) and head (40% of LDMs). Our data for late development in the lateral stripe is consistent with previous descriptions of an early and late wave of melanogenesis contributing to the lateral stripe (Milos and Dingle, 1978a).
We had previously described two origins for embryonic or larval melanocytes. First, we showed that the melanocytes that regenerate following chemical ablation arise from MSCs, that require ErbB signaling to be established during earlier embryonic stages. The fact that the embryonic melanocyte pattern was grossly normal in mutant or chemical blockade of ErbB signaling suggested that these melanocytes develop directly, without going through an ErbB-dependent stem cell. We asked wither the MSCs derived melanocytes contribute more subtly to temporal or regional subpopulations of the larval melanocyte pattern. Our results showed that ErbB signaling blockade has no significant effect on the number of melanocytes that typically develop prior to 3 dpf. We conclude that essentially all melanocytes that differentiate prior to 3 dpf arise by direct development, without a MSC intermediate.
In contrast to its lack of effect on differentiation of early developing melanocytes, ErbB blockade has differential effects on the LDMs of the head and lateral stripes. We found as many LDMs in the head following AG1478 treatment as in untreated animals, suggesting that these approximately 15–20 LDMs arise by direct development. We cannot exclude two alternative possibilities: that a distinct, ErbB-independent stem cell population is responsible for LDMs of the head; or that development of these stem cells are protected from drug treatment. Consistent with the notion of a distinct stem cell in the head is that animals without ErbB activity, after chemical ablation of melanocytes with MoTP to allow regeneration, melanocytes often appeared to regenerate in the head even in fish where we typically observed complete abrogation of trunk and tail melanocyte regeneration. This observation was observed in both AG1478-treated animals as well as erbb3b mutants, indicating that the existence of head regenerating melanocytes is not the result of experimental artifacts due to partial AG1478 treatment. These melanocytes could be regenerated from an erbb3b-independent, AG1478-resistant stem cell, or alternatively, simply be the LDM of the head, that develop too late to be affected by the chemical melanocyte ablation (MoTP treatment from 24–72 hpf). Distinguishing the origin of LDMs in the head will require the development of additional tools, such as gene expression markers specific to either the direct-developing or MSC-derived lineages.
That the LDM of the lateral stripe arise from the MSC is more clear. Here, the development of the approximately 20 melanocytes that arise after 3 dpf are completely sensitive to AG1478 treatment. These late developing, MSC-derived melanocytes contribute approximately one half of the cells in the lateral stripe. Thus, the lateral stripe of the zebrafish larva is a mosaic of equal proportions of direct developing and MSC-derived melanocytes. LDMs make a much smaller contribution to the dorsal, ventral, and yolk stripes. Approximately 3 melanocytes, or ~2% of the cells of the dorsal stripe, arise after 3 dpf. The development of these cells is also completely ablated by AG1478 treatment, indicating a very minor role for MSC-derived melanocytes in finishing the dorsal stripe. The number of LDMs contributing to ventral and yolk stripes was too small for us to determine whether they are significantly affected by AG1478 treatment, and likewise argues that MSC contributes few or no melanocytes to these stripes.
Role of MSC-derived melanocytes in regulation of embryonic pigment pattern
We have previously shown that ablation of either small regions of the ontogenetic pigment pattern with lasers, or wholesale ablation with the melanotoxic agent MoTP, is followed by melanocyte regeneration from the MSC to replace the missing cells (Yang and Johnson, 2006; Yang et al., 2004). Here, we show that the MSC can not only replace or regenerate the pattern, but it also has roles in generating the original pattern. Milos and Dingle (1978b) argued that second round, or late developing lateral stripe melanocytes had regulative properties that allowed them to develop the correct final number of melanocytes and in the correct places. Thus, late developing, MSC-derived melanocytes and MSC-derived regeneration melanocytes share similar properties of regulation of number and pattern of melanocytes. That lateral stripe melanocyte number deficits persist in AG1478-treated fish that lack the MSC-derived component argues that the direct developing lineage lacks this regulative ability. Moreover, that early developing lateral stripe melanocytes in AG1478-treated embryos do not readjust positions to become more evenly spaced in the absence of LDMs (data not shown) tends to argue that the early developing population is also unable to regulate for pattern as well. Thus, it is tempting to speculate that the regulative abilities of the MSC melanocyte lineage, that by yet unidentified mechanisms, recognizes gaps or deficits in the pigment pattern and fills them, has roles both in quality control to perfect the original pattern, as well as restorative roles in regeneration. It remains an interesting question how the MSC knows what the pattern is supposed to be, such that when the melanocytes of the pattern are missing, the MSCs are recruited to fill in those gaps.
We have suggested similar quality control functions distributed across a temporal transition in the regeneration of melanocytes in the regenerating fin. In that case, we have identified two populations of melanocytes that contribute to melanocyte stripe regeneration following fin amputation and regeneration: an early, kit-dependent population that provides the vast majority of melanocytes to the regenerating melanocyte stripes; and a later developing, kit-independent population, that acts to fill in gaps in the pattern generated by the first population (Rawls and Johnson, 2000). In this case, both early and late populations are derived from a MSC, although it is not yet clear whether the same MSC supports both populations, or whether either MSC is dependent on ErbB signaling.
Interpretation of melanocyte regeneration mutants
We were interested in whether the mutations that caused melanocyte regeneration defects identified regeneration specific functions, or more generally, identified defects in MSC regulation. The finding that kitj1e99 and skiv2l2j24e1 mutants are completely defective for late developing, MSC-derived melanocytes of the lateral stripe indicates that they have strong defects in MSC regulation. Thus, it seems likely that it is this defect, rather than a specific defect in regeneration, that is responsible for their failure to regenerate ablated melanocytes. These mutants show few or no cells expressing the late melanoblast marker dct when challenged to regenerate (Yang et al, 2007), suggesting either that they prevent the establishment or maintenance of the MSC, or prevent it from being recruited following melanocyte ablation.
In contrast, the regeneration mutant gfpt1j23e1 develops approximately 80% of the typical number of LDMs. This tends to argue that gfpt1j23e1 may not be a strong block of MSC regulation. It is also unclear whether it is a specific block to regeneration. Because gfpt1j23e1 is a recessive-lethal mutation, homozygous mutants may inherit gene product (mRNA, protein, or downstream products of the hexosamine biosynthetic pathway) from their heterozygous mothers, which may sustain aspects of melanocyte development through the first requirements for the gene. Interestingly, regenerating melanoblasts in gfpt1j23e1 mutants arrest at a late, dct+ stage, as revealed in 4–5 day embryos (Yang et al., 2007). In ontogenetic development, there is no defect before 3 dpf, but after 3 dpf, the melanocytes fail to fully mature and darken (Yang et al, 2007). An increasingly attractive explanation for the gfpt1j23e1 melanocyte defect is not that the gene has specific roles in one lineage or the other, but that these apparently specific defects reveal the role for the hexosamine biosynthetic pathway at the time maternally provided product becomes insufficient, between 3–5 dpf.
Conclusion
Our results suggest a discrete temporal transition in the origins of cells that contribute to the embryonic and larval pigment pattern. Essentially, all melanocytes that develop prior to 3 dpf are direct developing, without a self-renewing stem cell intermediate. We have yet to identify regulative mechanisms of melanocyte number or pattern for this lineage, other than that their presence is monitored by the MSC to regulate regeneration. In contrast, the late developing cells of the lateral and dorsal stripes are MSC-derived. In addition to a shared origin with the larval regeneration melanocytes from AG1478-sensitive stem cells, the MSC-derived LDMs of the lateral stripe are also able to regulate for pattern and number (Milos and Dingle, 1978a; Milos and Dingle, 1978b). We suggest that the primary role for the transition from direct-developing melanocytes to MSC-derived melanocytes in development of the embryonic and larval pigment pattern is the quality control functions of the MSC lineage, which allows the larva to fill gaps in the pigment pattern left by the direct-developing embryonic population.
Acknowledgments
We would like to thank Tom O’Reilly-Pol for his help with skiv2l2j24e1 and gfpt1j23e1 mutants. This work was supported by NIH grant RO1-GM56988 to SLJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–56. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development. 2008;135:2603–14. doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–46. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- Goldsmith MI, Iovine MK, O’Reilly-Pol T, Johnson SL. A developmental transition in growth control during zebrafish caudal fin development. Dev Biol. 2006;296:450–7. doi: 10.1016/j.ydbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hultman KA, Budi EH, Teasley DC, Gottlieb AY, Parichy DM, Johnson SL. Defects in ErbB-dependent establishment of adult melanocyte stem cells reveal independent origins for embryonic and regeneration melanocytes. PLoS Genet. 2009;5:e1000544. doi: 10.1371/journal.pgen.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Africa D, Walker C, Weston JA. Genetic control of adult pigment stripe development in zebrafish. Dev Biol. 1995;167:27–33. doi: 10.1006/dbio.1995.1004. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Schmid B, Eisen JS. Genetic analysis of melanophore development in zebrafish embryos. Dev Biol. 2000;225:277–93. doi: 10.1006/dbio.2000.9840. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Milos N, Dingle A. Dynamics of pigment pattern formation in the zebrafish, Brachydanio rerio. I. Establishment and regulation of the lateral line melanophore stripe during the first eight days of development. J Exp Zool. 1978a;205:204–216. [Google Scholar]
- Milos N, Dingle A. Dynamics of pigment pattern formation in the zebrafish, Brachydanio rerio. II. Lability of lateral line stripe formation and regulation of pattern defects. J Exp Zool. 1978b;205:217–224. doi: 10.1002/jez.1402270112. [DOI] [PubMed] [Google Scholar]
- Milos N, Dingle AD, Milos JP. Dynamics of pigment pattern formation in the zebrafish, Brachydanio rerio. III. Effect of anteroposterior location of three-day lateral line melanophores on colonization by the second wave of melanophores. J Exp Zool. 1983;227:81–92. doi: 10.1002/jez.1402270112. [DOI] [PubMed] [Google Scholar]
- O’Reilly-Pol T, Johnson SL. Melanocyte regeneration reveals mechanisms of adult stem cell regulation. Semin Cell Dev Biol. 2008 doi: 10.1016/j.semcdb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki H, Ito S, Wakamatsu K, Hirobe T. Chemical characterization of hair melanins in various coat-color mutants of mice. J Invest Dermatol. 1995;105:361–6. doi: 10.1111/1523-1747.ep12320792. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Johnson SL. Zebrafish kit mutation reveals primary and secondary regulation of melanocyte development during fin stripe regeneration. Development. 2000;127:3715–24. doi: 10.1242/dev.127.17.3715. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Johnson SL. Temporal and molecular separation of the kit receptor tyrosine kinase’s roles in zebrafish melanocyte migration and survival. Dev Biol. 2003;262:152–61. doi: 10.1016/s0012-1606(03)00386-5. [DOI] [PubMed] [Google Scholar]
- Truman JW, Riddiford LM. The origins of insect metamorphosis. Nature. 1999;401:447–52. doi: 10.1038/46737. [DOI] [PubMed] [Google Scholar]
- Yang CT, Hindes AE, Hultman KA, Johnson SL. Mutations in gfpt1 and skiv2l2 cause distinct stage-specific defects in larval melanocyte regeneration in zebrafish. PLoS Genet. 2007;3:e88. doi: 10.1371/journal.pgen.0030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CT, Johnson SL. Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development. 2006;133:3563–73. doi: 10.1242/dev.02533. [DOI] [PubMed] [Google Scholar]
- Yang CT, Sengelmann RD, Johnson SL. Larval melanocyte regeneration following laser ablation in zebrafish. J Invest Dermatol. 2004;123:924–9. doi: 10.1111/j.0022-202X.2004.23475.x. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990;4:1917–24. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Zou J, Beermann F, Wang J, Kawakami K, Wei X. The Fugu tyrp1 promoter directs specific GFP expression in zebrafish: tools to study the RPE and the neural crest-derived melanophores. Pigment Cell Res. 2006;19:615–27. doi: 10.1111/j.1600-0749.2006.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]