Abstract
Sleep is a crucial biological process is regulated through complex interactions between multiple brain regions and neuromodulators. As sleep disorders can have deleterious impacts on health and quality of life, a wide variety of pharmacotherapies have been developed to treat conditions of excessive wakefulness and excessive sleepiness. The neurotransmitter norepinephrine (NE), through its involvement in the ascending arousal system, impacts the efficacy of many wake- and sleep-promoting medications. Wake-promoting drugs such as amphetamine and modafinil increase extracellular levels of NE, enhancing transmission along the wake-promoting pathway. GABAergic sleep-promoting medications like benzodiazepines and benzodiazepine-like drugs that act more specifically on benzodiazepine receptors increase the activity of GABA, which inhibits NE and the wake-promoting pathway. Melatonin and related compounds increase sleep by suppressing the activity of the neurons in the brain’s circadian clock, and NE influences the synthesis of melatonin. Antihistamines block the wake-promoting effects of histamine, which shares reciprocal signaling with NE. Many antidepressants that affect the signaling of NE are also used for treatment of insomnia. Finally, adrenergic antagonists that are used to treat cardiovascular disorders have considerable sedative effects. Therefore, NE, long known for its role in maintaining general arousal, is also a crucial player in sleep pharmacology. The purpose of this review is to consider the role of NE in the actions of wake- and sleep-promoting drugs within the framework of the brain arousal systems.
Keywords: norepinephrine, locus coeruleus, sleep, wake, arousal
1. Introduction
1.1 Sleep
Sleep is one of the most universal biological processes in existence. It is highly conserved, and creatures from Drosophila melanogaster and Caenorhabditis elegans to humans experience at least some form of it [1]. Depriving an organism of sleep altogether can be extremely detrimental, and may even lead to death [2]. Sleep is therefore considered necessary for life, but why this is so remains unclear. Sleep is subdivided into rapid eye movement sleep, which is characterized by high- frequency electroencephalogram (EEG) recordings and muscle atonia [3], and non-REM (slow-wave) sleep, characterized by low frequency EEG recordings and body rest [4].
1.2 Sleep disorders
Although sleep is a tightly controlled process orchestrated by multiple regulatory systems, sleep disorders do occur, some as a result of disruptions in sleep circuitry, some secondary to other conditions, and others as a result of modern lifestyles. Sleep complaints are in fact the second-leading cause for seeking medical attention, after pain [5]. Sleep disorders can be categorized broadly into conditions of excessive wakefulness (e.g., insomnia) and excessive sleepiness (narcolepsy, shift-work disorder, jet lag). Insomnia is defined as difficulty falling asleep and maintaining adequate sleep, and is the most commonly reported sleep problem in the United States. In addition to the associated lack of nighttime sleep, there are a host of daytime consequences, as well, such as tiredness, stress, and attentional deficits [5]. Conversely, narcolepsy and hypersomnias involve excessive daytime sleepiness and sleep attacks or unintended napping. This can have a profound impact on an individual’s ability to function and limits even basic tasks like driving a car [5]. In addition to the effects these conditions have on sleep itself, problems such as cardiovascular disease and diabetes are often worsened by insufficient sleep [6]. Furthermore, patients with conditions from depression to Parkinson’s and Alzheimer’s diseases often experience co-morbid sleep disturbances [7–9]. Thus, medications to treat sleep disorders are a necessity in our society, and understanding the pathways and neurotransmitter systems that regulate sleep is crucial.
1.3 Regulation of arousal states
Sleep is a global process, controlled by regions throughout the brain and by multiple neurotransmitters and neuropeptides. The first studies that attempted to localize regions of the brain responsible for sleep maintenance were conducted in the early twentieth century by von Economo, who noticed correlations between sleeping sickness and lesions in certain brain areas [10]. He reported that patients who had encephalitis lethargica and slept for 20 hours a day had lesions at the base of the midbrain, and he hypothesized that this site might be the origin for an ascending arousal pathway. Subsequent studies revealed an arousal system with two main branches (reviewed in [11]). The first branch begins in the cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and projects up, relaying through the thalamus, and into the cortex; the second begins in monoaminergic neurons, the noradrenergic locus coeruleus (LC), dopaminergic neurons in the ventral periaqueductal grey (vPAG), the serotonergic dorsal raphe nucleus (DRN), and also the histaminergic tuberomammillary nucleus (TMN), and progresses to the cerebral cortex. These neurons also receive inputs from orexin neurons in the perifornical area of the lateral hypothalamus [12]. The orexins are especially important for controlling transitions between sleep and wake and between stages of sleep, and as a result, disruptions in this system cause narcolepsy [13]. These two branches also interact with the circadian pacemaker of the brain, the suprachiasmatic nucleus (SCN), mainly via relay through the dorsal medial hypothalamus [14, 15]. This provides the intersection of the homeostatic regulation of sleep, based on accumulated sleep drive or tiredness, and circadian regulation of sleep, based on a 24-hour cycle set by the SCN via integration of light inputs from the retina.
While the cholinergic and monoaminergic systems act to promote wakefulness in conjunction with the orexins, there are other neuronal groups that act to promote sleep. The primary population of sleep-promoting neurons is located in the preoptic area, specifically the ventrolateral preoptic area (VLPO). These neurons express c-Fos protein and have elevated discharge rates specifically during sleep, and lesions to this area prevent sleep [16–18]. In addition, these VLPO neurons project to, and share mutual inhibition with, the neuronal groups involved in the ascending arousal pathways [19, 20]. The majority of these cells are GABAergic, though some contain enkephalin and galanin [20, 21]. The interaction of the VLPO neurons with the ascending arousal systems provides what is described by Saper and colleagues as a “flip-flop switch” that regulates and controls transitions between sleep and wake [11].
1.4 NE and sleep regulation
As described above, norepinephrine (NE) is one of the main neurotransmitters involved in arousal. LC neurons fire in a wake-dependent manner; they are highly active during wake, slow-firing during non-REM sleep, and almost completely quiescent during REM sleep [22]. The A1 and A2 brainstem noradrenergic nuclei also provide input to regions of the hypothalamus known to be involved in sleep regulation [23]. Pharmacological suppression of LC activity leads to sedation and drives forebrain EEG recordings into sleep-like patterns. We know that dopamine β-hydroxylase knockout (Dbh −/−) mice, which lack norepinephrine, have altered sleep and arousal patterns. They show decreased latency to sleep after stress, require stronger stimuli to wake them after sleep deprivation, and have increased overall sleep, albeit with less REM, in a 24-hour period [24–26]. Pharmacological studies have revealed robust wake-promoting effects of α1- and β-adrenergic receptor (AR) agonists when administered to the medial septal area (MSA) and the medial preoptic area (MPOA), both wake-promoting regions, reviewed in detail by Berridge [23, 27]. Conversely, blockade of ARs results in sedation. These effects appear to be mediated mostly by antagonism of α1ARs, though there are synergistic effects when combined with βAR antagonists. While one group has found that microinjection of the α1AR antagonist prazosin into the MPOA induces sleep rather than wake, the magnitude of changes in wake time were relatively small and depended on ambient temperature [28, 29]. Thus, it seems likely that NE primarily acts through α1ARs in the MPOA to increase wake, although stimulation of α1ARs in this brain region may be sedative under some conditions.
While NE is important for maintaining normal sleep states, it also plays a role in cataplexy. Cataplexy is a component of narcolepsy in which patients experience abrupt transitions from waking into a state akin to REM sleep, with complete muscle atonia. These cataplexy attacks can be spontaneous or triggered by extreme emotion. α1AR antagonism exacerbates cataplexy, as measured both by the number of attacks and duration of the attacks, whereas activation of these receptors decreases the number of attacks [30]. This indicates that disregulated NE signaling, most likely working in conjunction with acetylcholine, is responsible for cataplexy attacks.
In addition to its role in the regulation of normal and pathological sleep, NE is also critical to the efficacy of sleep pharmacotherapies, both wake- and sleep-promoting, which will be the focus of the rest of this review.
2. Wake-promoting medications
Wake-promoting drugs are among the most commonly used pharmacotherapies in our society. Patients suffering from conditions of excessive daytime sleepiness, such as narcolepsy and shift-work sleep disorder, are often prescribed modafinil or amphetamine, which are used off-label and recreationally, as well. Caffeine, in coffee, soda, and energy drinks, is the most popular psychoactive substance in the world. And while all these work through diverse mechanisms, NE appears to contribute, with the exception of caffeine, either directly or indirectly to their wake-promoting activities.
2.1. Amphetamines
Amphetamines were developed in 1927 and first used to treat narcolepsy in 1935 [31]. The amphetamines include several compounds, each with its own wake-promoting efficacies and abuse liability. Those typically used to treat excessive sleepiness are dexamphetamine (Dexedrine) and methylphenidate (Ritalin). And although amphetamines are highly successful at promoting wakefulness, they have a number of unpleasant side effects that limit their usefulness. Amphetamines induce sympathomimetic effects, such as increased heart rate and blood pressure, and are also known for their addictive properties. Despite this, for many years they remained the frontline of treatment for conditions of excessive sleepiness, such as narcolepsy and phase-shift disorder.
Amphetamine has been shown to increase wakefulness in rats when administered both acutely and chronically [32]. When given acutely, amphetamine increased sleep latency, and decreased the amount of sleep and sleep efficiency compared with saline, as measured by EEG. Furthermore, it eliminated REM sleep for the duration of the three-hour test session. When administered chronically (every day for seven days), amphetamine decreased sleep efficiency on the first day, but efficiency improved gradually over subsequent days. Similarly, whereas amphetamine drastically reduced slow-wave sleep on the first day of testing, this decrease was attenuated over subsequent days, indicating the development of a tolerance to its wake-promoting effects. Sleep latency was increased by amphetamine on each day of the test. In humans, both dexamphetamine and methylphenidate increase sleep latency and improve self-reported sleepiness [31]. Dexamphetamine reduces total sleep, both REM and slow-wave, as measured by EEG, and decreases sleep efficiency. It is effective at sustaining wake in military pilots, in addition to its more traditional use as a narcolepsy treatment. However, administration of amphetamines typically results in rebound hypersomnolence when the individual is allowed to sleep, and because of the associated drop off in sleep efficiency, individuals usually feel residual sleepiness. Furthermore, users can develop a tolerance to both methylphenidate and low-dose dexamphetamine [33, 34].
Amphetamines act on the monoamine system to facilitate release and block reuptake of serotonin (5-HT), dopamine (DA), and NE. As described, all three monoamines are known to play a role in the regulation of arousal state. The DRN, the LC, and DA neurons in the vPAG are primary components of the ascending arousal system. Elevated extracellular levels of these neurotransmitters promote wakefulness through excitatory actions on the ascending arousal pathway, while simultaneously preventing sleep by inhibiting the sleep-promoting neurons of the VLPO.
NE is a particularly important component because, aside from wake-promoting effects of its own, it also acts to promote the firing of DA neurons, thereby increasing their wake-promoting effects [35]. Furthermore, the LC has reciprocal connections with 5-HT neurons in the DRN [36]. The importance of NE is underscored by several findings. For example, the wake-promoting effects of low-dose amphetamine observed in wild-type mice is abolished in Dbh −/− mice that specifically lack NE [25]. Direct infusion of low-dose amphetamine into sleep-related adrenergic projection fields containing both α1 and βARs increases wakefulness and arousal, as measured by EEG (reviewed by Berridge [23]). Microdialysis studies have shown a correlation between NE release in the prefrontal cortex (PFC), which receives direct innervation from the LC, and the time spent awake after administration of low-dose amphetamine. Central infusion of βAR antagonists in anesthetized animals can completely block the effects of low-dose amphetamine on wake, although no effect is seen in unanesthetized rats. This potential discrepancy could be evaluated further in normally sleeping animals, and the addition of α1AR antagonists may yield more insight. Combined, these results indicate that facilitation of NE release is critical for the wake-promoting actions of amphetamine.
2.2 Modafinil
Modafinil (Provigil) was first developed in the early 1990s and was approved for use in the United States in 1998. Since then, it has become the most prescribed medication in the United States for treatment of excessive daytime sleepiness; it is also used off-label for conditions as diverse as attention deficit disorder and cocaine dependence. One reason for the success of modafinil is its lack of negative side effects that are common to other medications used to treat excessive sleepiness. For example, unlike amphetamines, modafinil does not induce sympathomimetic effects, does not cause sleep rebound, and has low abuse liability [37–39].
Initial animal studies revealed that modafinil increased wake in a dose-dependent manner, with no signs of sleep rebound, as measured by EEG recordings [39, 40]. Amphetamine, in comparison, also dose-dependently increased wake, but with a subsequent sleep rebound period and considerable side effects like hyperactivity [40]. When compared with methamphetamine in rats, modafinil increased wake to a similar extent, but with longer bouts of sustained wakefulness and less marked locomotor effects [39]. In humans, modafinil is approved to treat excessive daytime sleepiness associated with narcolepsy, shift-work sleep disorder, and obstructive sleep apnea (reviewed by [38]). In several trials for narcolepsy, modafinil decreased daytime sleepiness without affecting nighttime sleepiness, and when tested for shift-work sleep disorder, it increased sleep latency during nighttime shifts. Modafinil is also well tolerated by patients who experience sleepiness secondary to other conditions or illnesses. When compared with amphetamine, volunteers given modafinil showed less need for recovery sleep after sleep deprivation and fewer sleep disturbances with no REM sleep deficit [41]. Further, modafinil has been shown to boost performance and alertness to a similar level as caffeine [42].
Despite the high prevalence of its use, however, the exact molecular mechanism underlying modafinil’s efficacy has yet to be elucidated. An early screen of possible targets revealed only low-affinity binding to the dopamine transporter (DAT). Subsequent studies confirmed this, and also found potential binding to the norepinephrine transporter (NET) [43–45]. Moreover, DAT knockout, α1AR antagonists, and DA receptor antagonists blunt the wake-promoting effects of modafinil, highlighting the importance of catecholamine signaling. [46].
As described above, both NE and DA are important components of the ascending arousal pathway, and both are important for modafinil efficacy. Modafinil activates the LC in animal models and in humans [47–49]. NE neurons in both the locus coeruleus and in the A1/A2 groups form reciprocal connections with the sleep-promoting neurons of the VLPO and decrease VLPO neuron firing via presynaptic α2ARs [19, 50]. Wake-active DA neurons in the vPAG also project to the VLPO. [51, 52]. Importantly, these vPAG neurons express and are activated by α1ARs [52, 53]. These data have led us to propose a model in which modafinil blocks both DAT and NET, thereby increasing extracellular DA and NE, which then act downstream. We believe that the NE could promote wake in several different ways in parallel: (1) by providing excitatory input on wake-promoting DA neurons via α1ARs, (2) by activating wake-promoting neurons in the hypothalamus via α1ARs and βARs, and (3) by providing inhibitory input onto sleep-promoting neurons in the hypothalamus (potentially via α2ARs). The higher levels of NE may also be stimulating histamine neurons, as modafinil administration increases histamine release [54]. However, this is most likely a downstream effect, because modafinil does not appear to bind histamine receptors directly [55]. Many aspects of this model remain to be tested.
2.3 Caffeine
Caffeine is the most popular psychoactive substance in the world; eighty percent of the population reports regular use of caffeine. It is present in coffee, tea, chocolate, and sodas, as well as many over-the-counter remedies.
Animal studies have shown that caffeine dose-dependently increases wakefulness at the expense of slow-wave sleep, REM sleep, and total sleep time [56]. Much of caffeine’s effects on sleep and arousal in humans have been self-reported, but there have also been some laboratory-controlled studies (reviewed in [57]). At doses equivalent to a single cup of coffee, caffeine has been found to increase latency to sleep and decrease total sleep time without affecting REM sleep. In a model of chronic caffeine administration, total sleep time was decreased over the course of the study, but some tolerance developed over time. In EEG studies, caffeine decreased slow-wave activity, consistent with “shallower” sleep. When compared with methylphenidate, caffeine had a greater effect on increasing sleep latency and reducing total sleep.
Caffeine is an antagonist for adenosine receptors, blocking the actions of adenosine, a purine that is synthesized throughout the brain. Levels of adenosine accumulate during wakefulness and decrease during sleep, especially in specific brain regions, such as the basal forebrain [58]. Adenosine’s effects on sleep regulation are diffuse, and involve multiple neurotransmitter systems. It acts to inhibit wakefulness by decreasing the frequency of action potentials of orexin neurons, and also inhibits histamine release via excitation of GABA neurons in the TMN [59, 60]. In parallel, it increases sleep by exciting a subset of neurons in the VLPO [61]. The effects of adenosine are mediated by four distinct G protein-coupled receptors (GPCRs) (A1, A2A, A2B, and A3). Those receptors with the most convincing evidence for sleep involvement are the A1 and A2A receptors [58]. Caffeine is a selective antagonist of the A2A receptors, as studies in knockout (KO) mice have revealed that caffeine effectively increases wakefulness in A1 receptor KO mice, but has no effect on A2A receptor KO mice [62]. By preventing the binding of adenosine, caffeine blocks its sleep-promoting effects in the VLPO, and prevents its inhibition of wake-promoting effects on the histamine and orexin systems.
Caffeine is one of the rare stimulant-type drugs that does not appear to act through the noradrenergic system, and there is scant evidence of even an indirect role for NE. Indeed, Dbh −/− mice lacking NE respond normally to caffeine [25]. Rather, caffeine prevents the actions of adenosine on other elements of the sleep regulatory system, such as histamine, orexin, and the GABAergic neurons of the VLPO.
3. Sleep-promoting medications
Just as there is an abundance of medications and treatments for conditions of excessive sleepiness, so it is with insomnia. Insomnia is described as difficulty falling asleep, or maintaining good quality sleep. In the United States, between 10–30% of the general population reports experiencing insomnia, with serious daytime consequences for about half of them [63]. Insomnia can be either primary, or secondary to another disorder, such as stress, depression, or chronic pain. Furthermore, insomnia raises the risk for depression as much as 5-fold, and treating the insomnia itself can ameliorate depression and other psychiatric conditions [64]. Because insomnia can have such a profound impact on daily life, medications that treat it are a real boon for sufferers. These medications fall into several categories: benzodiazepines, benzodiazepine-related compounds, melatonin and melatonin receptor agonists, and medications intended for other uses that also affect sleep.
3.1 Benzodiazepines
Benzodiazepines were first developed as sedatives in the 1960s and quickly gained prominence with drugs such as chlordiazepoxide (Librium) and diazepam (Valium) [63]. These drugs were more effective as sedatives than the previously used class of compounds, the barbiturates, and had fewer side effects. Yet despite the more favorable side-effect profile, benzodiazepines do have the potential for tolerance and dependence, with side effects that include memory impairment and lingering drowsiness.
A rat EEG study compared the benzodiazepines etizolam (Sedekopan) and triazolam (Halcion) with the 5-HT receptor agonist tandospirone (Sediel), both acutely and chronically [65]. Acute administration of all three drugs was found to decrease sleep latency compared to vehicle control. Both etizolam and triazolam increased sleep and decreased wake, whereas tandospirone did not. When given chronically (daily injections for seven days), the benzodiazepines decreased sleep latency on each day. Following one day of drug withdrawal, the etizolam and triazolam groups had a significant increase in sleep latency over the baseline control, while the tandospirone group did not. Decreased sleep time and efficiency, resulting in feelings of withdrawal sleepiness, have also been reported in human benzodiazepine studies [66, 67].
Benzodiazepines act as allosteric modulators of GABAA receptors [68]. GABAA receptors exist as heteropentamers, made up of two α subunits, two β subunits, and one γ subunit. The type and arrangement of subunits determine the function and binding properties of the receptor. Benzodiazepines bind to a site between α and γ subunits on receptors that possess γ2 and either the α1, α2, α3, or α5 subunits. Because GABAA receptors are located throughout the brain and benzodiazepines can act on multiple subtypes of GABAA receptors, the specific location of benzodiazepine-induced sleep is difficult to determine. Benzodiazepines could be acting on receptors directly within the VLPO to promote sleep, or they could be acting more globally to facilitate inhibitory GABA transmission.
Increasing GABA transmission would be expected to have an inhibitory effect on NE transmission through wake-promoting pathways, thereby increasing sedation; indeed, administering midazolam to rats markedly decreases NE release in the prefrontal cortex (PFC) as measured by microdialysis [69]. When used as an anesthetic in humans, midazolam suppresses glucose utilization in the LC [70]. Radiolabeled flunitrazepam binds to benzodiazepine receptors in the LC of human brains, and LC neurons are inhibited by activation of GABAA receptors [71, 72]. The benzodiazepines chlordiazepoxide and diazepam decrease stress-induced releases of NE [73, 74]. However, chronic administration of diazepam increases firing of LC NE neurons, which could contribute to benzodiazepine withdrawal symptoms, similar to the role of noradrenergic neurons in opiate withdrawal [75]. In addition, administration of GABAA antagonists into the LC significantly reduces REM sleep [76].
3.2 Benzodiazepine-related compounds
As mentioned above, benzodiazepines have several adverse effects, including memory impairment, lingering drowsiness, and potential for abuse. For these reasons, nonselective benzodiazepines have fallen out of favor in recent years, with the advent of more specific medications that have efficacy at targeting a particular type of benzodiazepine receptor.
Studies have revealed that the α1 subunit of the GABA receptor is especially important for benzodiazepine-induced sedation. For example, mice with mutations in the α1 subunit are insensitive to the sedative effects of the traditional benzodiazepine diazepam, but maintain sensitivity to its anxiolytic, myorelaxant, and motor-impairing functions, indicating that the sleep-promoting effects of benzodiazepines are primarily mediated by actions on the α1 subunit [77]. This led to a focus on finding selective drugs for this receptor sub-type, and a new class of medications has been developed with selectivity for receptors containing the α1 subunit [77]. These are often referred to as the “z-drugs,” because they include zolpidem (Ambien), zaleplon (Sonata), zopiclone (Imovane), and eszopiclone (Lunesta). Zaleplon and zolpidem have much higher efficacy at benzodiazepine receptors containing the α1 subunit compared with other types of α subunits, whereas the traditional benzodiazepine triazolam lacks this specificity [78, 79]. The principle differences between the z-drugs is their duration of action, and therefore their clinical indications [79]. Drugs with a longer duration of action, such as zopiclone, are used to induce and maintain sleep, while zaleplon, which has an extremely short duration of action, is used only to induce sleep.α1 subunits are the most widely distributed in the brain of all the α subunits, which makes localizing the brain regions responsible for the hypnotic properties of these drugs difficult [80]. As with the traditional benzodiazepines, z-drug effects on these receptors could involve the direct promotion of sleep via disinhibition of the VLPO, and receptor activation in the VLPO might be promoting sleep directly, or they could be acting more globally to increase inhibitory transmission throughout the brain.
The sleep-promoting effects of the z-drugs have been explored in both rodents and humans. Zaleplon and zopiclone produced a drowsy EEG pattern in rabbits, reminiscent of one produced by a canonical benzodiazepine [81]. Intravenously administered zaleplon produced EEG spectra consistent with physiological sleep. Oral administration of zolpidem to rats reduced sleep fragmentation, causing fewer entries from slow-wave sleep into wakefulness, the same pattern seen with diazepam [82]. In a clinical trial with eszopiclone and zolpidem, patients reported comparable reduced latency to sleep and increased sleep efficiency [83]. Zolpidem increased slow-wave sleep at the expense of wakefulness, but also decreased REM sleep. During a withdrawal day after seven days of zolpidem, rats displayed increased shallow sleep, but reduced slow-wave sleep duration as measured by EEG. REM and active wake were unaffected during this withdrawal period. When zolpidem was readministered after a one-week washout period, its sleep-promoting activity was enhanced compared with the first week with zolpidem. When given to insomnia patients, zolpidem increased self-reported sleep efficacy and decreased time to sleep onset and number of wakings. It also improved sleep quality, and feelings of freshness after sleep [84]. Nonetheless, despite its efficacy at promoting sleep, there have been frequent reports of complex behaviors, such as sleep-eating, sleep-driving, and sleep-conversations, which occur without the patient’s knowledge or remembrance after taking zolpidem [85].
While it is likely that benzodiazepines exert their anxiolytic and sedative effects via inhibition of wake-promoting brain regions, the exact role of their effects on the LC and NE transmission remain to be explored. α1 subunit-containing GABAA receptors are expressed in the LC, and in fact are expressed to a greater degree in the LC in a rodent model of anxiety resilience [86]. Future experiments addressing the functional significance of these receptors in the LC could include examination of LC activity and NE release following administration of z-drugs, or assessment of the sleep-promoting effects of z-drugs in animals with LC lesions or NE depletion.
3.3 Melatonin and melatonin receptor agonists
Melatonin is an endogenous neuromodulator synthesized by the pineal gland, and its secretion is regulated by the SCN, the circadian pacemaker of the brain [87]. Secretion of melatonin is low during the day and high at night, and the onset of melatonin secretion coincides with the onset of nightly sleepiness. Exogenous melatonin crosses the blood-brain barrier, and various over-the-counter melatonin preparations are used to treat insomnia, jet lag, shift work-related sleepiness, and delayed phase syndrome, with various degrees of effectiveness [88]. One reason melatonin is less effective than other regulated sleep treatments is its relatively short duration of action. However, there are now other medications, such as ramelteon (Rozerem) and agomelatine (Valdoxan), which target melatonin receptors with a higher affinity and longer half-life.
A number of animal studies have explored the effects of melatonin and melatonin receptor agonists on sleep. Because melatonin has different effects in nocturnal species compared with diurnal species, these studies are usually not performed in rodents. Melatonin is found to decrease locomotor activity and sleep latency in pigtail macaques, and also induces sleep during the day with an increase in slow-wave EEG activity in pigeons [89, 90]. In a rat study, melatonin increased slow-wave sleep, the number of sleep cycles, and total REM sleep [91]. In humans, studies on melatonin efficacy are somewhat contradictory and depend on the outcomes measured (i.e., sleep quality, number of awakenings, sleep efficiency) and methodology (EEG recordings versus self-reports) (reviewed in [87]). In healthy volunteers, melatonin improved sleep efficiency versus placebo during the light period, as measured by EEG [92]. A range of doses of ramelteon decreased latency to sleep and increased total sleep time, without any effects the subsequent day [93]. In addition to its efficacy as an antidepressant, agomelatine also subjectively improves sleep in depressed patients and decreases sleep latency, waking after sleep onset, and sleep stability, as measured by EEG [94].
Melatonin, ramelteon, and agomelatine are all agonists for melatonin 1 (MT1) and melatonin 2 (MT2) receptors [87]. Ramelteon has an affinity for both receptors that is 3–16 times greater than melatonin, and it has a longer half-life. Agomelatine also has a high affinity for melatonin receptors, in addition to acting as an antagonist at serotonin 5-HT2C receptors to decrease anxiety as well as promote sleep. Both MT1 and MT2 play a role in sleep induction; MT1 activation suppresses firing of SCN neurons, and MT2 receptors are involved in entraining circadian rhythms. Melatonin and the SCN impact sleep and wake in several ways. The SCN receives light signals from the retina, which are transmitted to the dorsal medial hypothalamus (DMH). The DMH acts as a relay center for signals to regions involved in sleep and wake maintenance, including inhibitory inputs to the VLPO and excitatory inputs to the LC [14, 95]. Melatonin acts through MT1 receptors to suppress firing of SCN neurons, thereby disinhibiting the sleep-promoting neurons in the VLPO, suppressing excitatory signals to wake-promoting regions, and increasing sleepiness [87].
NE has a reciprocal role in arousal states related to melatonin; it is one of the effector systems impacted by activation of melatonin receptors, and its activity controls the synthesis of melatonin [87]. There is dense innervation of the DMH by the SCN, and this nucleus acts as a positive relay between the SCN and the LC to increase wake [19]. Activation of MT1 receptors in the SCN by melatonin and melatonin receptor agonists decreases firing in these neurons, which suppresses wake drive. Additionally, NE is crucial for the synthesis of melatonin [87]. The SCN projects to the paraventricular nucleus, which then connects to the forebrain bundle and the reticular formation. Neurons from these areas synapse onto preganglionic sympathetic fibers of superior cervical ganglia. These fibers then release NE, which activates β-adrenergic receptors (βARs) in the pineal gland to stimulate the production of cyclic AMP, which in turn promotes the synthesis of melatonin via PKA-mediated transcription. Melatonin secretion can be suppressed by βAR antagonists, thus increasing wake [96].
3.4 Antihistamines
The final classes of pharmacotherapies to be discussed includes those medications developed for other purposes that are also used to treat disorders of sleep and wake because of their actions on arousal systems. Although first-generation antihistamines were developed as anti-allergy medications and for motion sickness, due to their actions on histamine receptors, they have considerable sedative effects. While newer generations of allergy treatments do not cross the blood-brain barrier and lack hypnotic effects, the first-generation antihistamines are still used over-the-counter for insomnia.
Antihistaminergics exert their sedative effects by antagonizing the H1 receptors in the brain. The H1 antagonist cyproheptadine (Periactin) is effective at increasing slow-wave sleep and REM sleep in rats [97], whereas the H1 antagonists diphenhydramine (Benadryl) and chlorpheniramine (Chlor-Trimeton) decreased sleep latency, but had no effect on amount of sleep. In humans, diphenhydramine initially increases subjective sleepiness and reduces latency to sleep compared with placebo, but after four days of administration, this effect is abolished, indicating tolerance to its effects [98]. Furthermore, on the first day after administration, performance on a battery of psychomotor tests was impaired when compared with placebo; this effect, too, was lost by the fourth day of diphenhydramine administration.
Because histamine from the TMN is part of the ascending arousal system, blocking its transmission would decrease wake, and also prevent the inhibitory effects of these neurotransmitters on the sleep-promoting neurons of the VLPO. Since NE and histamine have reciprocal feedback and enhance each other’s release, NE may be important for the sedative effects of antihistamines [99]. Interestingly, blockade of either α1-adrenergic or histaminergic signaling attenuates the wake-promoting and anticonvulsant effects of modafinil, further indicating a potential interaction between these two neuromodulators [100, 101].
3.5 Antidepressants
Although their first-line use is for the treatment of depression, antidepressants are also used off-label to treat insomnia. Depressed patients often suffer from sleep disturbances, and antidepressant therapy can ameliorate these disturbances; however, it is unclear whether this is a direct effect of the antidepressant on sleep, or whether the insomnia fades as mood improves [102]. On the other hand, antidepressants often have unwanted side effects on sleep and wakefulness, ranging from drowsiness to insomnia. Antidepressants are categorized by mechanism, and include tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), and others, which typically act through combinations of transporter and receptor blockade. These variations in mechanism lead to a range of effects on sleep (as reviewed extensively by [103]).
The effects of antidepressants on sleep are diverse, even within a class of medications. Sedation and drowsiness are common side effects of the TCAs (e.g., desipramine (Norpramine), imipramine (Tofranil), amitriptyline (Elavil)). Amitriptyline increases drowsiness and shortens sleep latency compared with placebo, whereas imipramine actually increases sleep latency and decreases total sleep time. MAOIs and SSRIs (e.g., fluoxetine (Prozac), sertraline (Zoloft), citalopram (Celexa)) can cause insomnia and decreased sleep efficiency. Compared with other antidepressants, fluoxetine is associated with significantly more fractured and less efficient sleep. Notably, all these classes of antidepressants suppress REM sleep to some degree.
Trazodone (Desyrel) is an antidepressant that is also commonly prescribed for insomnia [104]. In a study examining its effects on primary insomnia, trazodone improved self-reports of sleep latency, sleep duration, and decreased number of wakings. In another study, trazodone improved self-reports of sleep, but when measured by EEG, had no impact on sleep duration or sleep latency, with only a modest reduction in number of wakings. However, even trazodone has been shown to suppress REM sleep. Besides being observed in the clinic, animal models also show suppression of REM sleep following antidepressant administration [9].
Most antidepressants act through monoamine systems, though their specific mechanisms are variable, which may account for the diverse effects on sleep. Some TCAs are specific for NET blockade (e.g., desipramine), while others block both NET and SERT (e.g., imipramine). TCAs also have interactions with histamine, serotonin, and adrenergic receptors. SSRIs are specific for the SERT [103], whereas MAOIs interfere with monoamine metabolism. Trazodone, the most commonly prescribed antidepressant for insomnia, is a weak inhibitor of serotonin synaptosome reuptake and is also an antagonist at 5-HT1A, 5HT1C, 5-HT2, and α-adrenergic receptors [104]. The contribution of NE to these effects on sleep is not clear. Among the tricyclics, the NET-selective desipramine is the least effective at promoting sleep [103]. However, the dual NET-SERT inhibitor imipramine, which is also less effective at promoting sleep than other TCAs, decreases c-Fos in the LC [105]. In rats, antidepressants that act by inhibiting the NET, either alone or in combination with SERT, showed the highest amount of sleep disruption [9]. These seemingly paradoxical effects could be because treatments that increase extracellular NE, such as NET blockers, increase availability not only at excitatory α1ARs and βARs, which would be expected to increase sleep, but also at α2ARs, which act as inhibitory autoreceptors to decrease LC activity and NE release.
Interestingly, short-term sleep deprivation has antidepressant effects. While the mechanism of this is not yet clear, research indicates that it may be linked to NE from the LC (reviewed in [106]). Sleep deprivation increases the expression of a variety of genes, including brain-derived neurotrophic factor (BDNF) and its receptor TrkB, both of which are hypothesized to play a role in the antidepressant effects of sleep deprivation. However, if the LC is lesioned, the upregulation of these genes following sleep deprivation does not occur. Moreover, sleep deprivation increases LC firing and levels of extracellular NE. Behavioral studies should be conducted in the future to explore the impact of LC lesions on sleep deprivation as an antidepressant therapy.
3.6 Sedative effects of adrenergic drugs
While not typically prescribed for insomnia or other sleep disorders, many medications that suppress the adrenergic system have sedative side effects, lending further support for an essential role of NE in sleep pharmacology. Adrenergic receptor antagonists are most often used to decrease hypertension or treat congestive heart failure, but when these drugs cross the blood-brain barrier, neuropsychiatric effects can be seen [107]. Sedation and fatigue are among the most common side effects in patients taking βAR antagonists, α1AR antagonists, and clonidine, an agonist for α2AR inhibitor autoreceptors that attenuates NE release. Interestingly, prazosin is used to alleviate nightmares in post-traumatic stress disorder patients [108], potentially by acting both as a dual anxiolytic and sedative.
4. Summary
Sleep disorders of both insomnia and excessive sleepiness present major public health problems, making medications that can successfully treat these disorders without side effects most valuable. Sleep is regulated by multiple neurotransmitter systems, and pharmacotherapies act on many aspects of its regulation to treat various disorders.
Wake-promoting medications frequently act by increasing transmission along an ascending arousal pathway, whereas sleep-promoting medications typically inhibit this pathway through neurons in the VLPO, the main sleep-promoting nucleus in the brain. New sleep-promoting treatments are targeting the circadian pathway instead of the homeostatic pathway, and acting as agonists for melatonin receptors.
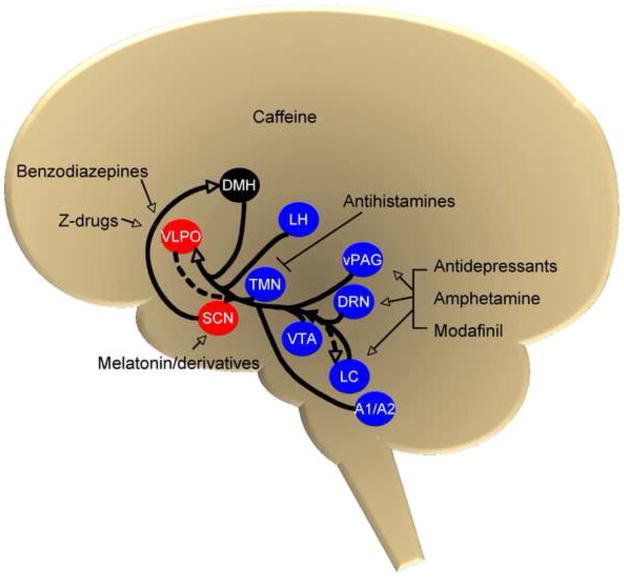
A common thread in the mechanism of action of many sleep pharmacotherapies is NE. A pictorial representation integrating and summarizing the anatomical connections between noradrenergic neurons and the wake- and sleep-promoting systems, as well as potential sites of action of wake- and sleep-promoting drugs, is depicted in Fig. 1. For example, amphetamine directly induces release and blocks reuptake of NE, increasing its extracellular concentration and activating the ascending arousal pathway. And though the mechanism of modafinil has not been determined conclusively yet, its actions are blocked by α1AR antagonists, and there is some evidence that it may act as a NET inhibitor. In contrast, caffeine is one wake-promoting agent that does not appear to have a significant adrenergic component. On the other end of the sleep/wake spectrum, both benzodiazepines and z-drugs act to increase GABA transmission, resulting in inhibition of excitatory signals along arousal pathways, including those from the LC. NE is also involved in the synthesis of melatonin, and thus may help regulate the circadian as well as the homeostatic sleep processes. Finally, most antidepressants, including those used as insomnia treatments, affect the adrenergic system.
Figure 1.
The diversity of the mechanisms of these drugs demonstrates the breadth of transmitter systems involved in sleep regulation. One obstacle to developing effective treatments for sleep disorders is that all these neurotransmitters are crucial for numerous biological processes in addition to sleep, and enhancing or inhibiting their activity often leads to a number of unpleasant or even dangerous side effects. However, as we learn more about how to differentiate the effects on arousal from other systemic effects, we may be able to develop increasingly specific medications that target the arousal system more directly, just as the z-drugs were developed when the specific α1 subunit of the benzodiazepine receptor was revealed as the primary mediator of benzodiazepine-induced sedation. And while the z-drugs have a much-improved safety profile over the benzodiazepines, there still is considerable room for further drug development. This type of specific targeting of receptor subtype or more specific anatomical localization may provide the key to developing safer and more effective medications to treat disorders of the arousal system.
Acknowledgments
We thank C. Strauss for helpful editing of the manuscript. D.W. was supported in part by NIH/NIDA (DA017963). H.M. has been supported by T32 GM008602.
Abbreviations
- 5-HT
serotonin
- AMP
adenosine monophosphate
- AR
adrenergic receptor
- BDNF
brain-derived neurotrophic factor
- DA
dopamine
- DAT
dopamine transporter
- Dbh
dopamine beta hydroxylase
- DMH
dorsal medial hypothalamus
- DRN
dorsal raphe nucleus
- EEG
electroencephalogram
- GABA
gamma-Aminobutyric acid
- GPCR
G protein-coupled receptor
- H
histamine (receptor)
- KO
knockout
- LC
locus coeruleus
- MAOI
monoamine oxidase inhibitor
- MPOA
medial preoptic area
- MSA
medial septal area
- MT
melatonin (receptor)
- NE
norepinephrine
- NET
norepinephrine transporter
- PFC
prefrontal cortex
- PKA
protein kinase A
- REM
rapid eye movement
- SCN
suprachiasmatic nucleus
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
- TMN
tuberomammillary nucleus
- TrkB
tyrosine kinase receptor B
- VLPO
ventrolateral preoptic area
- vPAG
ventral periaqueductal grey
- z-drug
drug that acts specifically on the α1 subunit of the benzodiazepine receptor
Footnotes
Commentary
Ms. Lynn LeCount and Dr. Michael Williams, Biochemical Pharmacology Editorial Office, University of Kansas Medical Center, MRRC Mail Stop 3051, 3901 Rainbow Boulevard, Kansas City, Kansas 66160, BP@kumc.edu
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cirelli C, Tononi G. Is sleep essential? PLoS biology. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep. 1989;12:68–87. [PubMed] [Google Scholar]
- 3.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–22. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 4.Stenberg D. Neuroanatomy and neurochemistry of sleep. Cell Mol Life Sci. 2007;64:1187–204. doi: 10.1007/s00018-007-6530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colten H, Altevogt B. Sleep disorders and sleep deprviation: an unmet public health problem. 2006 [PubMed] [Google Scholar]
- 6.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adolfsson R, Gottfries CG, Roos BE, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry. 1979;135:216–23. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- 8.Comella CL. Sleep disturbances and excessive daytime sleepiness in Parkinson disease: an overview. J Neural Transm Suppl. 2006:349–55. doi: 10.1007/978-3-211-45295-0_53. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez C, Brennum LT, Storustovu S, Kreilgard M, Mork A. Depression and poor sleep: the effect of monoaminergic antidepressants in a pre-clinical model in rats. Pharmacol Biochem Behav. 2007;86:468–76. doi: 10.1016/j.pbb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Reid AH, McCall S, Henry JM, Taubenberger JK. Experimenting on the past: the enigma of von Economo’s encephalitis lethargica. Journal of neuropathology and experimental neurology. 2001;60:663–70. doi: 10.1093/jnen/60.7.663. [DOI] [PubMed] [Google Scholar]
- 11.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 12.Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Current opinion in pharmacology. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 14.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 15.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 18.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 19.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–5. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 21.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 22.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–40. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- 24.Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–6. [PubMed] [Google Scholar]
- 25.Hunsley MS, Palmiter RD. Altered sleep latency and arousal regulation in mice lacking norepinephrine. Pharmacol Biochem Behav. 2004;78:765–73. doi: 10.1016/j.pbb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–82. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- 27.Berridge CW. Noradrenergic modulation of arousal. Brain research reviews. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar VM, Vetrivelan R, Mallick HN. Noradrenergic afferents and receptors in the medial preoptic area: neuroanatomical and neurochemical links between the regulation of sleep and body temperature. Neurochemistry international. 2007;50:783–90. doi: 10.1016/j.neuint.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Vetrivelan R, Mallick HN, Kumar VM. Unmasking of alpha1 adrenoceptor induced hypnogenic response from medial preoptic area. Physiology & behavior. 2005;84:641–50. doi: 10.1016/j.physbeh.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Mignot E, Guilleminault C, Bowersox S, Rappaport A, Dement WC. Role of central alpha-1 adrenoceptors in canine narcolepsy. The Journal of clinical investigation. 1988;82:885–94. doi: 10.1172/JCI113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee D, Vitiello MV, Grunstein RR. Pharmacotherapy for excessive daytime sleepiness. Sleep Med Rev. 2004;8:339–54. doi: 10.1016/j.smrv.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Andersen ML, Margis R, Frey BN, Giglio LM, Kapczinski F, Tufik S. Electrophysiological correlates of sleep disturbance induced by acute and chronic administration of D-amphetamine. Brain Res. 2009;1249:162–72. doi: 10.1016/j.brainres.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Ross DC, Fischhoff J, Davenport B. Treatment of ADHD when tolerance to methylphenidate develops. Psychiatric services (Washington, DC. 2002;53:102. doi: 10.1176/appi.ps.53.1.102. [DOI] [PubMed] [Google Scholar]
- 34.Strakowski SM, Sax KW, Rosenberg HL, DelBello MP, Adler CM. Human response to repeated low-dose d-amphetamine: evidence for behavioral enhancement and tolerance. Neuropsychopharmacology. 2001;25:548–54. doi: 10.1016/S0893-133X(01)00253-6. [DOI] [PubMed] [Google Scholar]
- 35.Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–51. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 36.Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Minzenberg MJ, Carter CS. Modafinil: A Review of Neurochemical Actions and Effects on Cognition. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 38.Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–66. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 39.Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther. 1997;283:757–69. [PubMed] [Google Scholar]
- 40.Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–26. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- 41.Buguet A, Montmayeur A, Pigeau R, Naitoh P. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. II. Effects on two nights of recovery sleep. J Sleep Res. 1995;4:229–41. doi: 10.1111/j.1365-2869.1995.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 42.Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl) 2002;159:238–47. doi: 10.1007/s002130100916. [DOI] [PubMed] [Google Scholar]
- 43.Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 44.Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–7. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- 45.Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, et al. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–46. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiocchi EM, Lin YG, Aimone L, Gruner JA, Flood DG. Armodafinil promotes wakefulness and activates Fos in rat brain. Pharmacol Biochem Behav. 2009;92:549–57. doi: 10.1016/j.pbb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Hou RH, Freeman C, Langley RW, Szabadi E, Bradshaw CM. Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacology (Berl) 2005;181:537–49. doi: 10.1007/s00213-005-0013-8. [DOI] [PubMed] [Google Scholar]
- 49.Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322:1700–2. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- 50.Matsuo S, Jang IS, Nabekura J, Akaike N. alpha 2-Adrenoceptor-mediated presynaptic modulation of GABAergic transmission in mechanically dissociated rat ventrolateral preoptic neurons. J Neurophysiol. 2003;89:1640–8. doi: 10.1152/jn.00491.2002. [DOI] [PubMed] [Google Scholar]
- 51.Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- 53.Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–68. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003;339:143–6. doi: 10.1016/s0304-3940(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 55.Prast H, Heistracher M, Philippu A. In vivo modulation of the histamine release in the hypothalamus by adrenoreceptor agonists and antagonists. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:183–6. doi: 10.1007/BF00167216. [DOI] [PubMed] [Google Scholar]
- 56.Yanik G, Glaum S, Radulovacki M. The dose-response effects of caffeine on sleep in rats. Brain Res. 1987;403:177–80. doi: 10.1016/0006-8993(87)90141-7. [DOI] [PubMed] [Google Scholar]
- 57.Roehrs T, Roth T. Caffeine: sleep and daytime sleepiness. Sleep Med Rev. 2008;12:153–62. doi: 10.1016/j.smrv.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochemical pharmacology. 2008;75:2070–9. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 59.Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem. 2005;92:1542–9. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97:837–48. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, et al. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134:1377–90. doi: 10.1016/j.neuroscience.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 62.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–9. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 63.Wafford KA, Ebert B. Emerging anti-insomnia drugs: tackling sleeplessness and the quality of wake time. Nature reviews. 2008;7:530–40. doi: 10.1038/nrd2464. [DOI] [PubMed] [Google Scholar]
- 64.Roth T. A physiologic basis for the evolution of pharmacotherapy for insomnia. J Clin Psychiatry. 2007;68 (Suppl 5):13–8. [PubMed] [Google Scholar]
- 65.Hirase M, Ishida T, Kamei C. Rebound insomnia induced by abrupt withdrawal of hypnotics in sleep-disturbed rats. Eur J Pharmacol. 2008;597:46–50. doi: 10.1016/j.ejphar.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 66.Tan X, Uchida S, Matsuura M, Nishihara K, Kojima T. Long-, intermediate- and short-acting benzodiazepine effects on human sleep EEG spectra. Psychiatry Clin Neurosci. 2003;57:97–104. doi: 10.1046/j.1440-1819.2003.01085.x. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki H, Yamadera H, Asayama K, Kudo Y, Ito T, Tamura Y, et al. Study of nocturnal sleep and the carryover effects of triazolam and brotizolam using neurophysiological and subjective methods. Neuropsychobiology. 2003;47:158–64. doi: 10.1159/000070586. [DOI] [PubMed] [Google Scholar]
- 68.Da Settimo F, Taliani S, Trincavelli ML, Montali M, Martini C. GABA A/Bz receptor subtypes as targets for selective drugs. Current medicinal chemistry. 2007;14:2680–701. doi: 10.2174/092986707782023190. [DOI] [PubMed] [Google Scholar]
- 69.Kubota T, Hirota K, Yoshida H, Takahashi S, Anzawa N, Ohkawa H, et al. Effects of sedatives on noradrenaline release from the medial prefrontal cortex in rats. Psychopharmacology (Berl) 1999;146:335–8. doi: 10.1007/s002130051125. [DOI] [PubMed] [Google Scholar]
- 70.Freo U, Dam M, Ori C. The time-dependent effects of midazolam on regional cerebral glucose metabolism in rats. Anesthesia and analgesia. 2008;106:1516–23. doi: 10.1213/ane.0b013e31816a64a8. table of contents. [DOI] [PubMed] [Google Scholar]
- 71.Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42–8. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- 72.Zhu H, Karolewicz B, Nail E, Stockmeier CA, Szebeni K, Ordway GA. Normal [3H]flunitrazepam binding to GABAA receptors in the locus coeruleus in major depression and suicide. Brain Res. 2006;1125:138–46. doi: 10.1016/j.brainres.2006.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swiergiel AH, Li Y, Wei ZY, Dunn AJ. Effects of chlordiazepoxide on footshock- and corticotropin-releasing factor-induced increases in cortical and hypothalamic norepinephrine secretion in rats. Neurochemistry international. 2008;52:1220–5. doi: 10.1016/j.neuint.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 75.Perez MF, Nasif FJ, Marchesini GR, Maglio LE, Ramirez OA. Hippocampus and locus coeruleus activity on rats chronically treated with diazepam. Pharmacol Biochem Behav. 2001;69:431–8. doi: 10.1016/s0091-3057(01)00517-2. [DOI] [PubMed] [Google Scholar]
- 76.Kaur S, Saxena RN, Mallick BN. GABA in locus coeruleus regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats. Neurosci Lett. 1997;223:105–8. doi: 10.1016/s0304-3940(97)13410-3. [DOI] [PubMed] [Google Scholar]
- 77.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 78.Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–10. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- 79.Sanger DJ. The pharmacology and mechanisms of action of new generation, non–benzodiazepine hypnotic agents. CNS drugs. 2004;18(Suppl 1):9–15. doi: 10.2165/00023210-200418001-00004. discussion 41, 3–5. [DOI] [PubMed] [Google Scholar]
- 80.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 81.Noguchi H, Kitazumi K, Mori M, Shiba T. Binding and neuropharmacological profile of zaleplon, a novel nonbenzodiazepine sedative/hypnotic. Eur J Pharmacol. 2002;434:21–8. doi: 10.1016/s0014-2999(01)01502-3. [DOI] [PubMed] [Google Scholar]
- 82.Renger JJ, Dunn SL, Motzel SL, Johnson C, Koblan KS. Sub-chronic administration of zolpidem affects modifications to rat sleep architecture. Brain Res. 2004;1010:45–54. doi: 10.1016/j.brainres.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 83.Erman MK, Zammit G, Rubens R, Schaefer K, Wessel T, Amato D, et al. A polysomnographic placebo-controlled evaluation of the efficacy and safety of eszopiclone relative to placebo and zolpidem in the treatment of primary insomnia. J Clin Sleep Med. 2008;4:229–34. [PMC free article] [PubMed] [Google Scholar]
- 84.Hajak G, Hedner J, Eglin M, Loft H, Storustovu SI, Lutolf S, et al. A 2-week efficacy and safety study of gaboxadol and zolpidem using electronic diaries in primary insomnia outpatients. Sleep Med. 2009 doi: 10.1016/j.sleep.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 85.Dolder CR, Nelson MH. Hypnosedative-induced complex behaviours : incidence, mechanisms and management. CNS drugs. 2008;22:1021–36. doi: 10.2165/0023210-200822120-00005. [DOI] [PubMed] [Google Scholar]
- 86.Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29:1344–52. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- 87.Pandi-Perumal SR, Srinivasan V, Spence DW, Cardinali DP. Role of the melatonin system in the control of sleep: therapeutic implications. CNS drugs. 2007;21:995–1018. doi: 10.2165/00023210-200721120-00004. [DOI] [PubMed] [Google Scholar]
- 88.Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor-independent actions. Advances in medical sciences. 2007;52:11–28. [PubMed] [Google Scholar]
- 89.Mintz EM, Phillips NH, Berger RJ. Daytime melatonin infusions induce sleep in pigeons without altering subsequent amounts of nocturnal sleep. Neurosci Lett. 1998;258:61–4. doi: 10.1016/s0304-3940(98)00849-0. [DOI] [PubMed] [Google Scholar]
- 90.Zhdanova IV, Cantor ML, Leclair OU, Kartashov AI, Wurtman RJ. Behavioral effects of melatonin treatment in non-human primates. Sleep Res Online. 1998;1:114–8. [PubMed] [Google Scholar]
- 91.Mailliet F, Galloux P, Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology (Berl) 2001;156:417–26. doi: 10.1007/s002130100769. [DOI] [PubMed] [Google Scholar]
- 92.Wyatt JK, Dijk DJ, Ritz-de Cecco A, Ronda JM, Czeisler CA. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–18. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 93.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Zupancic M, Guilleminault C. Agomelatine: a preliminary review of a new antidepressant. CNS drugs. 2006;20:981–92. doi: 10.2165/00023210-200620120-00003. [DOI] [PubMed] [Google Scholar]
- 95.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–7. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Van Den Heuvel CJ, Reid KJ, Dawson D. Effect of atenolol on nocturnal sleep and temperature in young men: reversal by pharmacological doses of melatonin. Physiology & behavior. 1997;61:795–802. doi: 10.1016/s0031-9384(96)00534-3. [DOI] [PubMed] [Google Scholar]
- 97.Tokunaga S, Takeda Y, Shinomiya K, Hirase M, Kamei C. Effects of some H1-antagonists on the sleep-wake cycle in sleep-disturbed rats. Journal of pharmacological sciences. 2007;103:201–6. doi: 10.1254/jphs.fp0061173. [DOI] [PubMed] [Google Scholar]
- 98.Richardson GS, Roehrs TA, Rosenthal L, Koshorek G, Roth T. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol. 2002;22:511–5. doi: 10.1097/00004714-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Bealer SL. Histamine releases norepinephrine in the paraventricular nucleus/anterior hypothalamus of the conscious rat. J Pharmacol Exp Ther. 1993;264:734–8. [PubMed] [Google Scholar]
- 100.Chen CR, Qu WM, Qiu MH, Xu XH, Yao MH, Urade Y, et al. Modafinil exerts a dose-dependent antiepileptic effect mediated by adrenergic alpha1 and histaminergic H1 receptors in mice. Neuropharmacology. 2007;53:534–41. doi: 10.1016/j.neuropharm.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 101.Ishizuka T, Murakami M, Yamatodani A. Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol. 2008;578:209–15. doi: 10.1016/j.ejphar.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Erman MK. Therapeutic options in the treatment of insomnia. J Clin Psychiatry. 2005;66(Suppl 9):18–23. quiz 42–3. [PubMed] [Google Scholar]
- 103.Mayers AG, Baldwin DS. Antidepressants and their effect on sleep. Human psychopharmacology. 2005;20:533–59. doi: 10.1002/hup.726. [DOI] [PubMed] [Google Scholar]
- 104.Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469–76. doi: 10.4088/jcp.v66n0409. [DOI] [PubMed] [Google Scholar]
- 105.de Medeiros MA, Carlos Reis L, Eugenio Mello L. Stress-induced c-Fos expression is differentially modulated by dexamethasone, diazepam and imipramine. Neuropsychopharmacology. 2005;30:1246–56. doi: 10.1038/sj.npp.1300694. [DOI] [PubMed] [Google Scholar]
- 106.Payne JL, Quiroz JA, Zarate CA, Jr, Manji HK. Timing is everything: does the robust upregulation of noradrenergically regulated plasticity genes underlie the rapid antidepressant effects of sleep deprivation? Biol Psychiatry. 2002;52:921–6. doi: 10.1016/s0006-3223(02)01676-1. [DOI] [PubMed] [Google Scholar]
- 107.Huffman JC, Stern TA. Neuropsychiatric consequences of cardiovascular medications. Dialogues in clinical neuroscience. 2007;9:29–45. doi: 10.31887/DCNS.2007.9.1/jchuffman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dierks MR, Jordan JK, Sheehan AH. Prazosin treatment of nightmares related to posttraumatic stress disorder. The Annals of pharmacotherapy. 2007;41:1013–7. doi: 10.1345/aph.1H588. [DOI] [PubMed] [Google Scholar]