Abstract
Focal adhesion kinase (FAK) is activated in human platelets downstream of integrins, e.g. αIIbβ3, and other adhesion receptors e.g. GPVI. Mice in which platelets lack FAK have been shown to exhibit extended bleeding times and their platelets have been shown to display decreased spreading on fibrinogen-coated surfaces. Recently, a novel FAK inhibitor (PF-573,228) has become available, its selectivity for FAK shown in vitro and in cell lines. We determined the effect of this inhibitor on platelet function and signaling pathways. Like murine platelets lacking FAK, we found that PF-573,228 was effective at blocking human platelet spreading on fibrinogen-coated surfaces but did not affect the initial adhesion. We also found a reduced spreading on CRP-coated surfaces. Further analysis of the morphology of platelets adhered to these surfaces showed the defect in spreading occurred at the transition from filopodia to lamellipodia. Similar to that seen with murine neutrophils lacking FAK, we also observed an unexpected defect in intracellular calcium release in human platelets pre-treated with PF-573,228 which correlated with impaired dense granule secretion and aggregation. The aggregation defect could be partially rescued by addition of ADP, normally secreted from dense granules, suggesting that PF-573,228 has effects on FAK downstream of αIIbβ3 and elsewhere. Our data show that PF-573,228 is a useful tool for analysis of FAK function in cells and reveal that in human platelets FAK may regulate a rise in cell calcium and platelet spreading.
Keywords: FAK; pp125FAK; Platelet; Lamellipodia; PF-573,228; αIIbβ3; Integrin; Focal adhesion kinase
Introduction
Platelets are anucleate cells found in the bloodstream that assist in maintaining the integrity of the cardiovascular system by responding to damaged vessel walls and initiating a complex restorative process, haemostasis. Pathological activation of platelets, thrombosis, can occur at sites of ruptured or eroded atherosclerotic plaques which underlies coronary artery disease, stroke and peripheral artery disease [1]. The integrin αIIbβ3 plays an important role in clot formation by binding soluble ligands such as fibrinogen and vWF facilitating platelet–platelet interactions [2,3]. Diverse platelet agonists converge on this integrin, changing its conformation to allow higher affinity ligand binding [4,5]. Antagonists of this platelet integrin, e.g. abciximab and tirofiban, are sometimes used in treatment of acute coronary syndromes.
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that, in platelets, becomes tyrosine phosphorylated after ligand engagement of αIIbβ3 [6–8]. Recently, a megakaryocyte lineage-specific FAK null mouse has been generated which showed a key role for FAK in megakaryopoiesis, platelet spreading on fibrinogen and haemostasis [9]. A myeloid lineage-specific FAK null mouse has also been generated and revealed that FAK has important roles in adhesion signaling and adhesion-independent signaling in neutrophils [10]. Macrophages from this mouse exhibited altered spreading and migration [11]. Recently, a novel small molecule inhibitor of FAK, PF-573,228 (henceforth referred to as PF-228) has been described [12]. This molecule exhibits selectivity towards FAK, at least in vitro, and inhibits activation of FAK in various cell lines in the range 0.3–3 μM [12].
In this study, we describe the effect of this inhibitor on human platelet function and human platelet signaling pathways. We demonstrate defects in platelet spreading and aggregation, intracellular calcium mobilization and dense granule secretion when platelets are pre-treated with the novel FAK inhibitor PF-228 which is, in part, consistent with data obtained from the haematopoietic cell-type specific FAK knockouts.
Materials and methods
Reagents. Generally, laboratory chemicals were purchased from Sigma (Poole, UK) unless otherwise indicated. The focal adhesion kinase (FAK) inhibitor PF-573,228 (3,4-dihydro-6-[[4-[[[3-(methylsulfonyl)phenyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino]-2(1H)-quinolinone) was purchased from Tocris (Bristol, UK). DiOC6 (3,3′-dihexyloxacarbocyanine iodide) was purchased from Alexis Biochemicals (Exeter, UK). Anti-Syk, clone 4D10.1; anti-phosphotyrosine, clone 4G10 were from Millipore (Watford, UK). Anti-FAK (clone 77) and anti-actin antibodies were purchased from BD Biosciences (Oxford, UK). All other primary antibodies were purchased from Cell Signaling Technology (NEB, Hitchin, UK). Anti-mouse and anti-rabbit horse-radish peroxidase (HRP) conjugated antibodies and enhanced chemiluminescent (ECL) reagent was purchased from GE Healthcare (Amersham, UK). Complete mini-protease inhibitor tablets were purchased from Roche (Burgess Hill, UK). Protein-G plus/protein-A agarose beads were purchased from Merck Chemicals (Nottingham, UK). Restore Plus Western blot Stripping Buffer was purchased from Pierce Biotechnology (Cramlington, UK).
Preparation and stimulation of human platelets. Human platelets were obtained from adult volunteers in accordance with the approved guidelines from the local Research Ethics Committee of the University of Bristol, UK; informed consent was obtained in accordance with the World Medical Association Declaration of Helsinki. Venous blood was drawn from volunteers with acid citrate dextrose as anticoagulant, used at a 1:7 (v/v) ratio. Platelet-rich plasma was obtained by centrifugation at 180g for 17 min. Platelets were isolated by centrifugation after treatment with prostaglandin E1 (140 μM) and indomethacin (10 μM) at 550g for 10 min. The platelet pellet was resuspended in modified Tyrode’s-Hepes buffer to a density of 2 × 108/ml.
Cell lysis and immunoprecipitation. For immunoblotting, platelets (400 μl) were lysed into 200 μl of SDS-sample buffer (62.5 mM Tris, pH6.8, 25%(v/v) glycerol, 2%(w/v) SDS and 340 mM DTT). For immunoprecipitation with anti-FAK antibody, platelets were lysed into an equal volume of 2× lysis buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 1%(v/v) NP-40 alternative, 1%(v/v) Triton X-100, 0.2%(w/v) SDS, complete protease inhibitors, 1 mM sodium orthovanadate and 20 mM 2-glycerophosphate). After incubation at 4 °C for 60 min, samples were centrifuged at 12,000g for 15 min and supernatants removed. Immunoprecipitations were performed using antibody precoupled to protein-G plus/protein-A agarose beads. The equivalent of 2 μg of antibody per sample was coupled to beads for 1 h at room temperature before extensive washing in 1× lysis buffer. Samples were then mixed with antibody–protein-G plus/protein-A agarose beads for 60 min at 4 °C. Beads were extensively washed with 1× lysis buffer. After washing, 40 μl of SDS-sample buffer was added followed by incubation at 96 °C for 3 min.
Immunoblotting. Samples for immunoblotting were separated by electrophoresis on linear polyacrylamide gels and then transferred to PVDF membrane by wet transfer. Membranes were blocked with 10%(w/v) BSA in TBSt (10 mM Tris, pH 7.5, 150 mM NaCl and 0.1%(v/v) Tween 20). Primary antibodies were diluted in 10%(w/v) BSA and membranes incubated for 1.5 h at room temperature and washed extensively with TBSt. Membranes were incubated for 1 h at room temperature with secondary antibodies followed by extensive washing with TBSt. ECL detection of bound antibodies was carried out. Where indicated PVDF membranes were stripped by incubating with Restore reagent (Pierce) overnight followed by extensive washing with deionised water and TBSt.
Measurement of platelet adhesion by phase-contrast microscopy. Measurement of static platelet adhesion was performed as previously described. Glass coverslips were coated with CRP (5 μg/ml); blocked with 1%(w/v) essentially fatty acid free BSA and washed with Tyrode’s-Hepes. Washed platelets (2 × 107/ml) were either treated with carrier (DMSO) or PF-228 (1 μM) for 5 min before being allowed to adhere for 20 min followed by fixation with 4%(w/v) paraformaldehyde. Platelets were stained with DiOC6 (2 μM) for 60 min. Adhered platelets were visualized by phase-contrast microscopy with a wide-field microscope DM IRB attached to an ORCA ER camera (40×/1.40 NA oil objective) (Leica Microsystems, Milton Keynes, UK). Numbers of adherent platelets from five randomly selected fields of view per slide/condition were manually counted with the aid of NIH Image J software. Images were processed with Adobe Photoshop Elements.
Dense granule secretion assay. Dense granule secretion was monitored by luminometry using a luciferin/luciferase assay (CHRONO-LUME). Briefly, 245 μl of platelets, at a density of 2 × 108/ml, were incubated under stirring conditions with 5 μl of CHRONO-LUME luciferin/luciferase mix and either carrier and/or PF-228 (1 μM) for 5 min before stimulation with CRP (5 μg/ml) or thrombin (0.1 U/ml). Data were normalised to control (agonist alone) and presented as a percentage of control (mean ± SEM; n = 3).
Measurement of cytosolic free calcium ([Ca2+]i) in platelets. Fura-2AM (3 μM) was added to platelet-rich plasma and was incubated at 30 °C for 45 min in the presence of prostaglandin E1 (140 μM), indomethacin (10 μM) and apyrase (0.02 U/ml). Platelets were pelleted by centrifugation and resuspended in modified Tyrode’s-Hepes buffer. Calcium responses were subsequently measured at 37 °C using a Hitachi F-4500 spectrofluorimeter (Hitachi, London, UK) with fluorescence excitation alternating between 340 and 380 nm and emission at 510 nm. Data are presented as the ratio of fluorescence emission following excitation at 340 and 380 nm (340:380 nm).
Data handling and statistical analyses. Data manipulations were performed using Microsoft Excel and GraphPad Prism software. Statistical analyses were performed with the assistance of GraphPad Prism software. Results were judged to be statistically significant when P < 0.05.
Results
Treatment of platelets with the novel FAK inhibitor PF-228 inhibits platelet aggregation and spreading
The focal adhesion kinase inhibitor PF-228 is a novel inhibitor of FAK family kinases with an IC50 towards FAK in cells of 0.1 μM [12]. In this study it was shown that PF-228 selectively inhibited >85% of FAK activity in vitro and in vivo in the range 0.3–3 μM. We assessed the ability of PF-228 to inhibit platelet aggregation, an integrin (αIIbβ3)-dependent process in platelets that utilizes FAK. Platelets pre-treated with various concentrations of PF-228 were stimulated with either thrombin or collagen-related peptide (CRP); a cross-linked peptide that specifically activates the collagen receptor complex GPVI-FcRγ on human platelets. Platelet aggregation to CRP, and thrombin (data not shown), was inhibited in a concentration-dependent manner, with 1 μM PF-228 being the lowest concentration of inhibitor that inhibited aggregation maximally (Fig. 1A). This is within the concentration range shown in the previous study on this inhibitor to inhibit FAK in cell lines [12]. This concentration was subsequently used throughout this study.
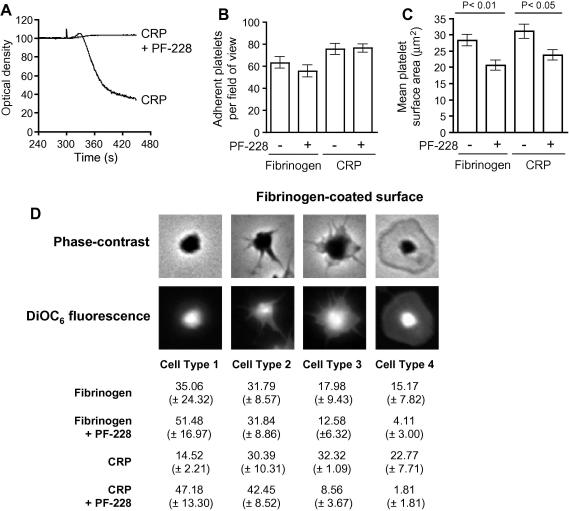
Fig. 1.
Attenuation of platelet spreading by PF-228. Human platelets were either pre-treated with DMSO (−) or PF-228 (+) (1 μM) for 5 min in suspension before being allowed to settle on a either a fibrinogen or CRP-coated surface. Unbound platelets were washed off and bound platelets fixed. The numbers of platelets bound were counted with the assistance of Image J software and presented as the mean number bound per field of view (±SEM; 5 replicates, n = 3) (A). The platelet surface area was determined from the same samples using NIH Image J software and was recorded as the mean surface area (±SEM; 5 replicates, n = 3). Statistical significance was determined using a two-way ANOVA with Bonnferoni post-test (B). Bound platelets were subdivided into different classes: class 1, compact shape; class 2, filopodial extensions evident; class 3, filopodial extensions evident and some platelet spreading; class 4, fully spread with no filopodia evident. Platelets in each class were expressed as a mean percentage (±SEM; 5 replicates, n = 3) of the total number of bound platelets examined (C).
A previous study on FAK in murine megakaryocytes and platelets using Pf4-Cre/FAK-floxed mice demonstrated a role for FAK in negative regulation of megakaryopoiesis and a critical requirement for FAK in mediating spreading of platelets on fibrinogen [9]. Therefore, we decided to determine the effect of PF-228 on human platelet adhesion and spreading on both a fibrinogen-coated surface and a CRP-coated surface. We show that pre-treatment of human platelets with 1 μM PF-228 did not significantly alter the number of platelets that adhered to either a fibrinogen- or CRP-coated surface (Fig. 1B). However, we did observe a significant attenuation of platelet spreading, assessed as mean platelet surface area determined by phase-contrast microscopy, on both fibrinogen- and CRP-coated surfaces (Fig. 1C). The mean platelet surface area on fibrinogen-coated surfaces was reduced from 28.31 (±1.71) to 20.63 (±1.60) μm2 which was judged to be significant using one-way ANOVA with Bonferroni’s Multiple Comparison Test (P < 0.01). Similarly, the mean platelet surface area on CRP-coated surfaces was reduced from 31.04 (±2.23) to 23.77 (±1.53) μm2 which was judged to be significant using one-way ANOVA with Bonferroni’s Multiple Comparison Test (P < 0.05). These data are in agreement with data obtained with murine platelets lacking FAK on fibrinogen-coated surfaces [9]. Further analysis of the platelets adhered to fibrinogen- and CRP-coated surfaces, by categorizing them into distinct groups (Fig. 1D), showed that there was a significant defect in the ability of platelets pre-treated with PF-228 to transit from a platelet with filopodial extensions to a fully-spread platelet. These results are consistent with a role for FAK in lamellipodial generation which has been demonstrated for FAK in primary murine macrophages but not previously in human or mouse platelets [11].
Inhibition of FAK with PF-228 leads to inhibition of PAK and AKT but not the GPVI proximal kinase Syk
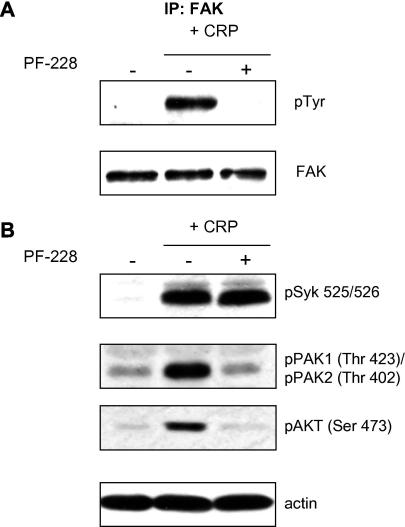
Having confirmed that treatment of platelets with PF-228 was able to reproduce the phenotypes of the limited number of studies using murine cell lineage-specific FAK knockouts [9–11], we analyzed the effect of PF-228 on intracellular signaling pathways in human platelets. We confirmed by immunoprecipitation of FAK and immunoblotting with anti-phosphotyrosine that 1 μM PF-228 was sufficient to completely block tyrosine phosphorylation of FAK induced by CRP stimulation of stirred platelets (Fig. 2A). We also determined if PF-228 perturbed the GPVI-FcRγ signaling axis by assessing activation of the tyrosine kinase Syk using anti-Syk pTyr 525/526 antibody. We showed that PF-228 did not affect Syk activation (Fig. 2B). We examined the phosphorylation/activation status of other platelet signaling proteins that have been shown to play a role in platelet spreading, e.g. p21-activated kinase (PAK) [13], or have been shown to be affected in murine FAK knockout studies, e.g. AKT [10]. Consistent with a defect in lamellipodial formation we also demonstrate a substantial decrease in PAK phosphorylation [13] (Fig. 2B). We also demonstrated a knockdown of AKT (Ser473) consistent with studies in FAK knockouts [10]. Thus we have demonstrated that PF-228 abolishes platelet aggregation, significantly attenuates platelet spreading and appears to selectively inhibit the activity of kinases that have been shown to be involved in FAK-dependent processes.
Fig. 2.
Analysis of platelet kinase signaling pathways. Platelets were either pre-treated with DMSO (−) or PF-228 (+) (1 μM) for 5 min before stimulation with CRP (5 μg/ml) for 3 min under stirred conditions before lysis; basal samples (−) were lysed after 8 min. FAK was immunoprecipitated from platelet lysates before immunoblotting with phosphotyrosine to assess activation (A). Lysates were also prepared by lysis of platelets after treatment in sample buffer. Aliquots (15 μl) from these lysates were resolved by SDS–PAGE and immunoblotted with the activation specific antibodies indicated in (B). Samples were also blotted with anti-actin to confirm equal extraction of protein from platelet samples. All blots are representative of three independent experiments.
Treatment of platelets with PF-228 unexpectedly blocks calcium mobilization and dense granule secretion
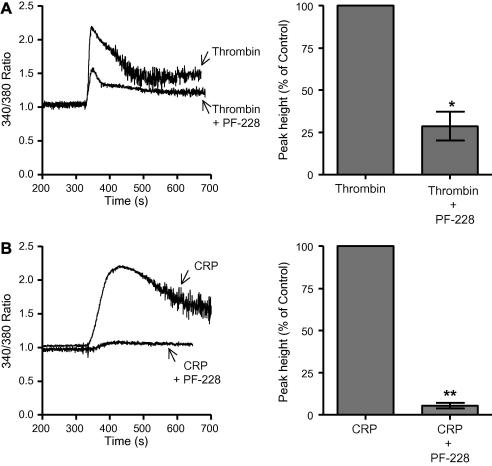
We determined if PF-228 had any effect on the cytosolic calcium response since the report of FAK knockout in murine neutrophils demonstrated an unexpected perturbation of intracellular calcium release in adherent neutrophils [10]. Platelets were loaded with the fluorescent calcium indicator Fura2-AM, pre-treated with PF-228 or carrier followed by stimulation with either thrombin or CRP under continuously stirred conditions. In response to both agonists we observed a significant attenuation of intracellular calcium release in those platelets pre-treated with PF-228 (Fig. 3), although attenuation of the CRP response was more pronounced than for thrombin. We reasoned that this could be, at least in part, due to a failure in secondary mediators, e.g. ADP, feeding back to enhance the calcium signal. We therefore, ascertained whether PF-228 could inhibit dense granule secretion, a source of the important secondary mediator ADP. We showed that 1 μM PF-228 could abolish dense granule secretion to both CRP and thrombin (data not shown).
Fig. 3.
Intracellular calcium mobilization is significantly attenuated after PF-228 treatment. Platelets were loaded with Fura2-AM and were either pre-treated with DMSO (−) or PF-228 (+) (1 μM) for 5 min before addition of either thrombin (0.1 U/ml; A) or CRP (5 μg/ml; B). Representative calcium traces are shown. The mean peak height (±SEM; n = 3) was measured and expressed as a percentage of the control, i.e. agonist alone.
Inhibition of aggregation by PF-228 can be partially reversed by addition of ADP
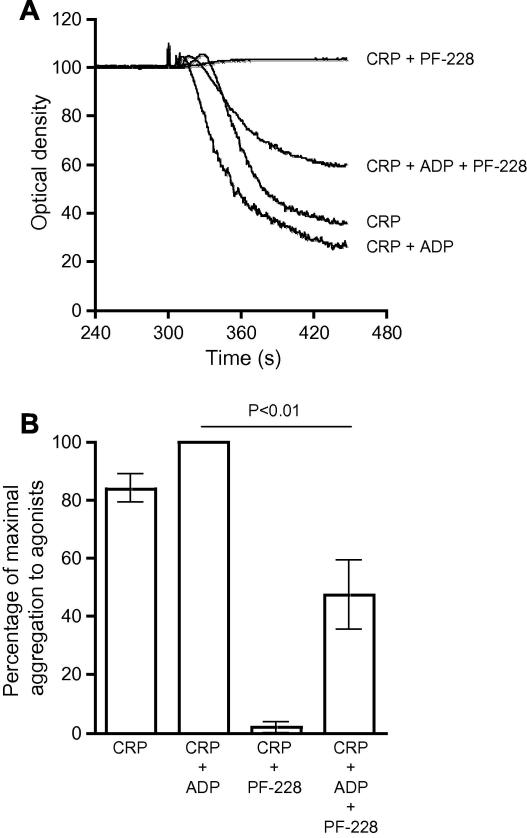
Since dense granule secretion was abolished by pre-treatment of platelets with 1 μM PF-228 we determined the effect of including ADP in the agonist stimulation after pre-treatment of platelets with 1 μM PF-228 (Fig. 4). Addition of ADP and CRP to platelets pre-treated with 1 μM PF-228 resulted in an approximate 50% recovery of the aggregation response compared to stimulation of platelets with CRP alone (Fig. 4B). Thus, the defect in dense granule secretion in platelets treated with PF-228 does not account for all of the affects of PF-228 mediated FAK inhibition.
Fig. 4.
ADP partially rescues the aggregation defect after treatment with PF-228. Platelets were either pre-treated with DMSO (−) or PF-228 (+) (1 μM) for 5 min before the addition of CRP (5 μg/ml), or a combination of CRP (5 μg/ml) and ADP (10 μM), and aggregation monitored (A). Representative aggregation traces are shown. Aggregation after different treatments was determined and normalised to the maximal aggregation induced by CRP (5 μg/ml) and ADP (10 μM). The data was presented as mean percentage of maximal aggregation (±SEM; n = 3).
Discussion
In this study we have characterized the effect of PF-228, a novel small molecule inhibitor, on human platelets. The inhibitor blocked platelet spreading, but not platelet adhesion, on a fibrinogen-coated surface in a manner entirely consistent with the data obtained using the megakaryocytic lineage-specific FAK null mice [9]. Additionally, we demonstrate that PF-228 inhibits spreading on, but not adhesion to, a CRP-coated surface. Further morphological analysis of platelets adhered to fibrinogen- and CRP-coated surfaces showed that PF-228 inhibited the transition from filopodial extensions to a fully spread morphology which is consistent with the role demonstrated for FAK in lamellipodial persistence demonstrated in macrophages from FAK null mice [11]. Analysis of signaling pathways in platelets stimulated with the collagen receptor GPVI-specific agonist CRP showed that, at a concentration of PF-228 that fully blocked platelet aggregation and spreading, FAK tyrosine phosphorylation was absent but Syk tyrosine kinase activation was unaffected indicating that activation of the GPVI pathway was unaffected by this inhibitor. We also determined that p21-activated kinase (PAK) activation was diminished by treatment of platelets with PF-228, which is consistent with the role demonstrated for this kinase in lamellipodial spreading in human platelets [13] and the defect in platelet spreading we observed in this study. We also observed an inhibition of AKT activation when platelets were pre-treated with PF-228 which is similar to the defect in PI(3,4,5)P3/AKT signaling observed in neutrophils lacking FAK [10]. In this study they also showed a defect in intracellular calcium signaling in adherent neutrophils lacking FAK but not in neutrophils in suspension [10]. This is in contrast to our data with PF-228 which shows a significant attenuation in intracellular calcium release in platelets in suspension when stimulated with either thrombin or CRP. However, under our experimental conditions we allowed platelet aggregation to occur in suspension which is dependent upon the integrin αIIbβ3 so the differences in calcium release seen between the two studies may be due to the differences in integrin-dependent signaling between the two cell types. The lack of dense granule secretion seen after treatment with PF-228 may be a consequence of the diminished intracellular calcium release. When ADP was added with CRP following PF-228 treatment we were able to recover approximately 50% of the aggregation suggesting that PF-228 has effects other than downstream of αIIbβ3-dependent signaling. Whilst we have shown that treatment of human platelets with PF-228 mimics most of the phenotypes of FAK null platelets [9], neutrophils [10] and macrophages [11] the possibility that PF-228 might be inhibiting other kinases that may play key roles in granule secretion and aggregation cannot be excluded. However, this novel inhibitor has provided some new information on the role of FAK in human platelet functions that remains to be corroborated with further studies on murine platelets lacking FAK.
Acknowledgments
We thank the British Heart Foundation for generous support of our research.
References
- 1.Jennings L.K. Role of platelets in atherothrombosis. Am. J. Cardiol. 2009;103:4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y.Q., Qin J., Plow E.F. Platelet integrin αIIbβ3: activation mechanisms. J. Thromb. Haemost. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 3.Coller B.S., Shattil S.J. The GPIIb/IIIa (integrin αIIbβ3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C., Lau T.L., Ulmer T.S., Ginsberg M.H. Interactions of platelet integrin αIIb and β3 transmembrane domains in mammalian cell membranes and their role in integrin activation. Blood. 2009;113:4747–4753. doi: 10.1182/blood-2008-10-186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau T.L., Kim C., Ginsberg M.H., Ulmer T.S. The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 2009;28:1351–1361. doi: 10.1038/emboj.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipfert L., Haimovich B., Schaller M.D., Cobb B.S., Parsons J.T., Brugge J.S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J. Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shattil S.J., Haimovich B., Cunningham M., Lipfert L., Parsons J.T., Ginsberg M.H., Brugge J.S. Tyrosine phosphorylation of pp125FAK in platelets requires coordinated signaling through integrin and agonist receptors. J. Biol. Chem. 1994;269:14738–14745. [PubMed] [Google Scholar]
- 8.Achison M., Elton C.M., Hargreaves P.G., Knight C.G., Barnes M.J., Farndale R.W. Integrin-independent tyrosine phosphorylation of p125fak in human platelets stimulated by collagen. J. Biol. Chem. 2001;276:3167–3174. doi: 10.1074/jbc.M007186200. [DOI] [PubMed] [Google Scholar]
- 9.Hitchcock I.S., Fox N.E., Prevost N., Sear K., Shattil S.J., Kaushansky K. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood. 2008;111:596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasorn A., Alcaide P., Jia Y., Subramanian K.K., Sarraj B., Li Y., Loison F., Hattori H., Silberstein L.E., Luscinskas W.F., Luo H.R. Focal adhesion kinase regulates pathogen-killing capability and life span of neutrophils via mediating both adhesion-dependent and -independent cellular signals. J. Immunol. 2009;183:1032–1043. doi: 10.4049/jimmunol.0802984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen K.A., Pixley F.J., Thomas K.S., Vicente-Manzanares M., Ray B.J., Horwitz A.F., Parsons J.T., Beggs H.E., Stanley E.R., Bouton A.H. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slack-Davis J.K., Martin K.H., Tilghman R.W., Iwanicki M., Ung E.J., Autry C., Luzzio M.J., Cooper B., Kath J.C., Roberts W.G., Parsons J.T. Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 13.Vidal C., Geny B., Melle J., Jandrot-Perrus M., Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]