Abstract
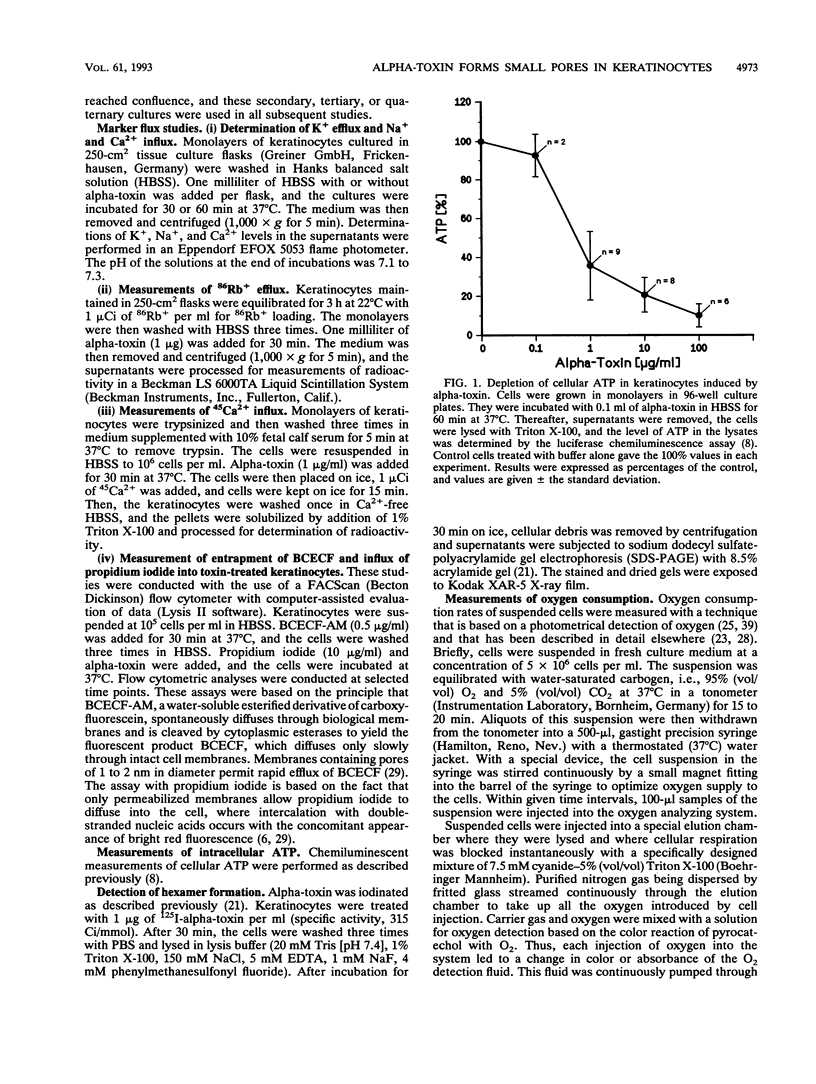
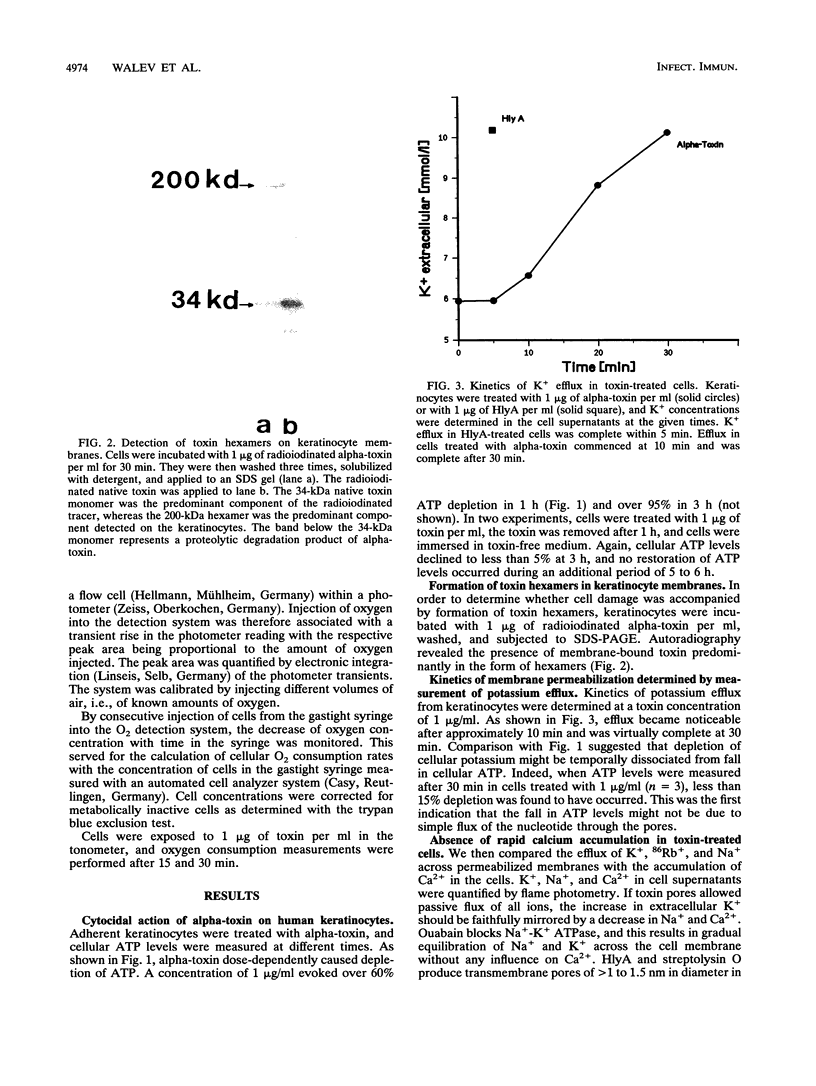
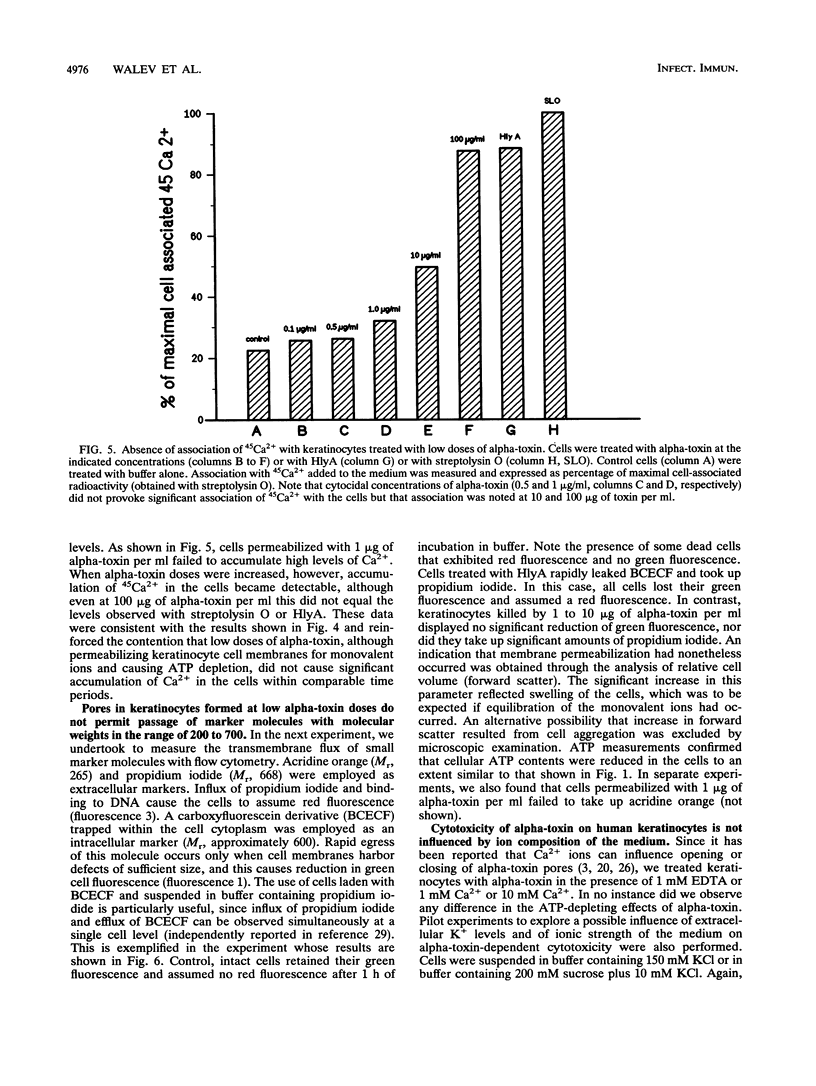
Incubation of human keratinocytes with nanomolar concentrations of Staphylococcus aureus alpha-toxin leads to irreversible depletion of cellular ATP. The toxin forms hexamers in the target cell membranes, and rapid transmembrane flux of K+, Na+, and 86Rb+ is observed. Unexpectedly, pores formed in keratinocytes through application of low but lethal doses of alpha-toxin appeared to be considerably smaller than those formed in erythrocyte membranes. They permitted neither rapid influx of Ca2+ or propidium iodide, nor efflux of carboxyfluorescein. Larger pores allowing flux of all three markers did form when the toxin was applied at high concentrations. Flux of monovalent ions and reduction in cellular ATP levels evoked by low toxin doses correlated temporally with a fall in oxygen consumption, which was interpreted to reflect breakdown of mitochondrial respiration. The lethal event could not be thwarted by manipulating the extracellular K+ or Ca2+ concentrations. Realization that alpha-toxin may form very small pores in nucleated cells is important for future research on cellular toxin effects and membrane repair processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bhakdi S., Gratzl M. Minimal requirements for exocytosis. A study using PC 12 cells permeabilized with staphylococcal alpha-toxin. J Biol Chem. 1985 Oct 15;260(23):12730–12734. [PubMed] [Google Scholar]
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Belmonte G., Cescatti L., Ferrari B., Nicolussi T., Ropele M., Menestrina G. Pore formation by Staphylococcus aureus alpha-toxin in lipid bilayers. Dependence upon temperature and toxin concentration. Eur Biophys J. 1987;14(6):349–358. doi: 10.1007/BF00262320. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991 Sep;59(9):2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Korom S., Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989 Nov;57(11):3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Mannhardt U., Hugo F., Klapettek K., Mueller-Eckhardt C., Roka L. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J Exp Med. 1988 Aug 1;168(2):527–542. doi: 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991 Dec;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Bhakdi S., Weller U., Walev I., Martin E., Jonas D., Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol. 1993 Sep;182(4):167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- Bredel-Geissler A., Karbach U., Walenta S., Vollrath L., Mueller-Klieser W. Proliferation-associated oxygen consumption and morphology of tumor cells in monolayer and spheroid culture. J Cell Physiol. 1992 Oct;153(1):44–52. doi: 10.1002/jcp.1041530108. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Studies on the binding of staphylococcal 125I-labeled alpha-toxin to rabbit erythrocytes. Biochemistry. 1976 Jun 1;15(11):2348–2355. doi: 10.1021/bi00656a016. [DOI] [PubMed] [Google Scholar]
- Forti S., Menestrina G. Staphylococcal alpha-toxin increases the permeability of lipid vesicles by cholesterol- and pH-dependent assembly of oligomeric channels. Eur J Biochem. 1989 May 15;181(3):767–773. doi: 10.1111/j.1432-1033.1989.tb14790.x. [DOI] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S., Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus wood 46. Infect Immun. 1984 Nov;46(2):615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S., Boquet P., Duflot E., Alouf J. E., Montecucco C., Papini E. Staphylococcal alpha-toxin: a study of membrane penetration and pore formation. J Biol Chem. 1989 Sep 5;264(25):14978–14984. [PubMed] [Google Scholar]
- Harshman S., Sugg N. Effect of calcium ions on staphylococcal alpha-toxin-induced hemolysis of rabbit erythrocytes. Infect Immun. 1985 Jan;47(1):37–40. doi: 10.1128/iai.47.1.37-40.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand A., Pohl M., Bhakdi S. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. J Biol Chem. 1991 Sep 15;266(26):17195–17200. [PubMed] [Google Scholar]
- Ikigai H., Nakae T. Interaction of the alpha-toxin of Staphylococcus aureus with the liposome membrane. J Biol Chem. 1987 Feb 15;262(5):2150–2155. [PubMed] [Google Scholar]
- Kallinowski F., Tyler G., Mueller-Klieser W., Vaupel P. Growth-related changes of oxygen consumption rates of tumor cells grown in vitro and in vivo. J Cell Physiol. 1989 Jan;138(1):183–191. doi: 10.1002/jcp.1041380124. [DOI] [PubMed] [Google Scholar]
- Lang W., Wolf H. U., Zander R. A sensitive continuous and discontinuous photometric determination of oxygen, carbon dioxide, and carbon monoxide in gases and fluids. Anal Biochem. 1979 Jan 15;92(2):255–264. doi: 10.1016/0003-2697(79)90656-0. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Belmonte G., Parisi V., Morante S. Structural features of the pore formed by Staphylococcus aureus alpha-toxin inferred from chemical modification and primary structure analysis. FEMS Microbiol Immunol. 1992 Sep;5(1-3):19–28. doi: 10.1111/j.1574-6968.1992.tb05882.x. [DOI] [PubMed] [Google Scholar]
- Menestrina G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J Membr Biol. 1986;90(2):177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W., Zander R., Vaupel P. A new photometric method for oxygen consumption measurements in cell suspensions. J Appl Physiol (1985) 1986 Aug;61(2):449–455. doi: 10.1152/jappl.1986.61.2.449. [DOI] [PubMed] [Google Scholar]
- Ni J., Watson J. V., Cox H., Karpas A. Multiparameter flow cytometric analysis of a novel cytotoxin (factor 2) induced tumor cell membrane permeability. Cytometry. 1993;14(3):281–286. doi: 10.1002/cyto.990140308. [DOI] [PubMed] [Google Scholar]
- Ostedgaard L. S., Shasby D. M., Welsh M. J. Staphylococcus aureus alpha-toxin permeabilizes the basolateral membrane of a Cl(-)-secreting epithelium. Am J Physiol. 1992 Jul;263(1 Pt 1):L104–L112. doi: 10.1152/ajplung.1992.263.1.L104. [DOI] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Hessz T., Seeger W., Wilke A., Koob R., Lutz F., Drenckhahn D. Bacterial exotoxins and endothelial permeability for water and albumin in vitro. Am J Physiol. 1988 Sep;255(3 Pt 1):C368–C376. doi: 10.1152/ajpcell.1988.255.3.C368. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Dewein E., Bhakdi S., Roka L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: role of calcium. Am J Physiol. 1985 Jan;248(1 Pt 1):C127–C134. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Determination of toxin-induced leakage of different-size nucleotides through the plasma membrane of human diploid fibroblasts. Infect Immun. 1975 Apr;11(4):640–648. doi: 10.1128/iai.11.4.640-648.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobkes N., Wallace B. A., Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985 Apr 9;24(8):1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Bayley H. Functional complementation of staphylococcal alpha-hemolysin fragments. Overlaps, nicks, and gaps in the glycine-rich loop. J Biol Chem. 1993 Mar 5;268(7):5285–5292. [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Zorn L., Bayley H. Assembly of the oligomeric membrane pore formed by Staphylococcal alpha-hemolysin examined by truncation mutagenesis. J Biol Chem. 1992 Oct 25;267(30):21782–21786. [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Zorn L., Kasianowicz J., Bayley H. Functional expression of the alpha-hemolysin of Staphylococcus aureus in intact Escherichia coli and in cell lysates. Deletion of five C-terminal amino acids selectively impairs hemolytic activity. J Biol Chem. 1992 May 25;267(15):10902–10909. [PubMed] [Google Scholar]
- Wolf H. U., Zander R., Lang W. An automated continuous determination of oxygen with high sensitivity. Anal Biochem. 1976 Aug;74(2):585–591. doi: 10.1016/0003-2697(76)90241-4. [DOI] [PubMed] [Google Scholar]




