Abstract
Low-dose chemotherapy drugs can suppress tumours by restraining tumour vessel growth and preventing the repair of damaged vascular endothelial cells. Cisplatin is a broad-spectrum, cell cycle-non-specific drug, but has serious side effects if used at high doses. There have been few reports on the anti-angiogenic effects of low-dose cisplatin and hence the effect of low-dose metronomic (LDM) chemotherapy on the proliferation and neovascularization of H22 hepatocarcinoma cells is discussed in this research. The influence of LDM chemotherapy with cisplatin on human umbilical vascular endothelial cells (HUVECs) and proliferation of the HepG2 human hepatocarcinoma cell line were measured using MTT assays. The LDM group was treated with cisplatin 0.6 mg/kg/day; the control group with saline 0.2 ml; the maximum tolerated dose (MTD) group with cisplatin 9 mg/kg/day. Vascular endothelial growth factor (VEGF) and matrix metallopeptidase 2 (MMP-2) were detected using immunohistochemical staining. A chicken chorio-allantoic membrane (CAM) model was used to check the inhibitory effect of LDM chemotherapy with cisplatin on neovascularization in vivo. Low-dose cisplatin inhibited HUVEC proliferation in a dose- and time-dependent manner, but was ineffective in inhibiting HepG2 cell proliferation. Tumour growth was delayed in mice receiving LDM cisplatin, without apparent body weight loss, compared with mice that received MTD cisplatin. Microvessel density and expression of VEGF and MMP-2 were much lower in mice receiving LDM cisplatin than in the control and MTD groups. Continuous low-dose cisplatin suppressed CAM angiogenesis in vivo. LDM chemotherapy with cisplatin can inhibit the growth of blood vessel endothelial cells in vitro and shows anti-angiogenic ability in vivo.
Keywords: angiogenesis, chicken chorio-allantoic membrane, cisplatin, H22 hepatocarcinoma, HUVECs
Recent studies found that various cytotoxic chemotherapeutic agents could inhibit the angiogenesis of vessels, as the sensitivity of vascular endothelial cells to chemotherapeutic drugs was 10–10,000 times that of tumour cells (Assaraf 2006; Spaner & Masellis 2007). At the same time, studies showed that at usual chemotherapeutic dosages, endothelial cells underwent apoptosis initially, followed by tumour cells, but impaired vascular endothelial cells would be repaired between chemotherapy treatments. Further research also indicated that chemotherapy drugs could inhibit angiogenesis and have persistent therapeutic roles if given at a much lower dose than the effective anti-tumour dose – i.e. at non-cytotoxic concentrations – more frequently and over a longer time.
This new mode of administration redefines chemotherapy drugs’ treatment targets to be vascular endothelial cells (Shaked et al. 2006; Wu et al. 2007). Mathematical models of this method found that inhibiting the growth of new vessels extended the survival time of patients longer than conventional maximum tolerated dose (MTD) chemotherapy (Tamura et al. 2002; Lam et al. 2007); this anti-angiogenic approach may therefore have a significant clinical potential (Lu 2007). Folkman indicated that metastasis and growth of tumour cells depend on neovascularization, indicating a new approach to tumour growth inhibition using anti-neovascularization strategies (Folkman 2003). Some studies in recent years have suggested that many anti-tumour drugs could cause inhibition of tumour neovascularity (Veale & Fearon 2006; Moreira et al. 2007). Low-dose chemotherapy drugs, usually one-tenth to one-third of the MTD, administered continuously and frequently, could selectively suppress vessel growth in tumour tissues and prevent the repair of damaged vascular endothelial cells (VECs). This process, called ‘low-dose metronomic (LDM) chemotherapy,’ might lead to tumour suppression by devascularization.
Cisplatin is a cell cycle-non-specific drug with strong, broad-spectrum effects, but it has serious side effects if used in high doses and over multiple courses of treatment (Schweitzer 1993; Ozdogan et al. 2008). Until now, there have been few reports on the anti-angiogenic effect of low-dose cisplatin; we studied the effect of LDM chemotherapy on the proliferation and neovascularization of H22 hepatocarcinoma cells in this research.
Materials and methods
Materials
Human umbilical vascular endothelial cells (HUVECs) were purchased from Sciencecell Co. (Seattle, WA, USA) and cultured with extracellular matrix. Hepatocarcinoma cell line HepG2, foetal bovine serum and cisplatin for injection were from Gibco Inc. (Billings, MT, USA). Mouse anti-human vascular endothelial growth factor (VEGF), matrix metalloproteinase-2 (MMP-2), tris-ethylene diamine tetra-acetic acid and chicken embryos came from KeyGen Biotechnology Co. (NanJing, JiangSu, China).
Animals and subculture
Thirty-six Kunming mice (specific pathogen-free grade; male; aged 5–6 weeks; 20–22 g) were used. H22 hepatocarcinoma cells from KeyGen Biotechnology Co. (NanJing, JiangSu, China) were cultured in ascites fluid; after 7 days, the second generation was used in experiments.
MTT assay
Human umbilical vascular endothelial cells and HepG2 cells were collected in the logarithmic growth phase. They were plated in 96-well plates (0.5 × 104/ml cells/well in 200 μl of medium) and maintained in a humidified incubator with 5% CO2 at 37 °C. After 24 h, the culture medium was replaced by fresh medium containing cisplatin (7.5 ng/ml, 75 ng/ml, 750 ng/ml, or 7.5 μg/ml); the medium was changed without cisplatin in the control group. Each drug concentration was represented by at least three wells and replicated three times. After 24, 48 and 72 h of incubation, 20 μl of MTT (5 g/l) was added to each well and incubated for 4 h. Cells were then collected by centrifugation at 1000 g for 5 min at room temperature. The reaction was stopped by adding 150 μl of dimethyl sulfoxide. Absorbance was measured at 570 nm and the cell proliferation inhibition rate was calculated.
Establishment of animal model
Twenty-four hours after being inoculated, mice were randomly divided into three groups of 12. The LDM group was given cisplatin (0.6 mg/kg/day, five times in one week) and the control group was given 0.9% saline (0.2 ml/day, five times in 1 week), through intraperitoneal injection in both groups. The MTD mice each received one intraperitoneal injection of cisplatin (9 mg/kg). Different regimens of cisplatin dosing were given to different groups, starting on day 3 after tumour vaccination, 1 day at a time, for a two-week period for all groups. Ingestion, status and activity were recorded every day for this period.
Volume, weight and inhibition rate of tumours
Five days after inoculation, maximum diameters (a) and minimum diameters (b) of tumours were measured every 3 days. Tumour volume was measured using the following formula (Ruggeri et al. 2003):
Mice were killed and tumours weighed. Mean tumour weight was calculated. Tumour inhibition rate was calculated using the following formula:
 |
Expression of microvessel density (MVD), VEGF and MMP-2
After formalin fixation and paraffin embedding, stripped tumour tissue was made into sections for immunohistochemical analysis (PowerVision™ two-step). Phosphate-buffered solution was the negative control instead of the first antibody; a known positive section was the positive control. Endothelial cells were identified by marking with CD34 monoclonal antibodies. All brown endothelial cells, or endothelial cell clusters with dyed cytoplasm that were separated from adjacent tumour cells, microvessels or connective tissues, were counted as independent microvessels; Vessels with thick muscle layers or with lumen diameters larger than the width of eight red blood cells were excluded. The ‘hot spot’ method was used for MVD. Tissue sections were scanned with a low-power lens and five vision fields were found in each section in which endothelial cells were clarity under an unambiguous background; the most intense microvessels were chosen at the tumour site and counted for a mean value using an optical microscope (magnification,×400). Positive expression of VEGF and MMP-2 was indicated by brownish-yellow or chocolate-brown granular tumour cells clearly located in the cytoplasm. MMP-2 was also expressed in some interstitial cells. Tissue sections were initially scanned using an optical microscope (magnification, ×100) and three fields of vision in each section with high positive rates were chosen; 500 tumour cells were then counted successively under an optical microscope (magnification, ×400) to calculate the percentage of positive cells.
Chorioallantoic membrane vascular growth experiment in vivo
Fertilized chicken eggs were incubated at 37 °C and relative humidity of 60%. Vascular growth peaked on the seventh day; the chorioallantoic membranes (CAMs) were then exposed. Thin methylcellulose sections were located in the CAM newborn tubule areas. Twenty microlitres of cisplatin solution at different concentrations was dropped into the sections with a micropipetter in the experimental groups; equivalent amounts of saline were dropped into sections in the control group. Sections were incubated after being sealed with sterilized film. The same dose of cisplatin was added every day. The film was uncovered after 72 h and sections were fixed for 15 min at room temperature with a mixture of equal parts (v/v) of methanol and acetone. When the blood solidified, holes with diameters of 3 mm were cut in the CAMs. Capillary growth surrounding the sections was observed under light microscopy. The inhibition rate of a group was the percentage of chicken embryos with inhibited capillaries:
 |
Statistical analysis
The test results were expressed in the form of 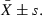 Comparisons between different groups were performed using the Kruskal–Wallis H-test or the Mann–Whitney U-test. P< 0.05 was considered statistically significant.
Comparisons between different groups were performed using the Kruskal–Wallis H-test or the Mann–Whitney U-test. P< 0.05 was considered statistically significant.
Results
Proliferation inhibition of metronomic chemotherapy with low-dose cisplatin on HUVECs and HepG2 cells
Low-dose cisplatin was cultured with HUVECs for 24, 48 or 72 h. Cisplatin was seen to inhibit the proliferation of HUVECs dose- and time-dependently. Proliferation inhibition for endothelial cells was maximal at cisplatin concentration up to 750 ng/ml. That is, additional cisplatin could make the viable cell count in each well decline slowly. There was no significant difference between the 7.5 μg/ml group and the 750 ng/ml group; absorbance and inhibition are shown in Table 1. There were significant differences (P< 0.01) between the two groups and the control group; under identical conditions, low-dose cisplatin did not inhibit the human hepatocellular carcinoma cell line HepG2; absorbance and inhibition are shown in Table 2. There was no significant difference compared with the control group (P> 0.05).
Table 1.
Proliferation inhibition with different concentrations of cisplatin (DDP) on human umbilical vascular endothelial cells
Absorbance ( ) ) |
Inhibition (%) |
||||||
|---|---|---|---|---|---|---|---|
| Group | [DDP](/ml) | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
| Control | – | 1.53 ± 0.02 | 1.45 ± 0.09 | 1.41 ± 0.03 | – | – | – |
| DDP | 7.5 ng | 1.35 ± 0.02 | 1.25 ± 0.02 | 1.19 ± 0.03 | 11.70 | 13.10 | 15.00 |
| DDP | 75 ng | 1.15 ± 0.02 | 1.08 ± 0.05 | 0.96 ± 0.04 | 25.32 | 26.22 | 31.71 |
| DDP | 750 ng | 0.78 ± 0.04 | 0.72 ± 0.05 | 0.65 ± 0.03 | 49.10 | 51.03 | 53.80 |
| DDP | 7.5 μg | 0.75 ± 0.03 | 0.71 ± 0.02 | 0.63 ± 0.02 | 50.90 | 51.93 | 55.41 |
[DDP] = cisplatin.
Compared with the control group, P< 0.01; compared with DDP 7.5 ng/ml group, P< 0.01; compared with 24 h group P< 0.01, there were significant differences.
Table 2.
Proliferation inhibition with different concentrations of cisplatin (DDP) on HepG2 cells
Absorbance (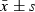 ) ) |
Inhibition (%) |
||||||
|---|---|---|---|---|---|---|---|
| Group | [DDP] (/ml) | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
| Control | – | 1.65 ± 0.03 | 1.67 ± 0.04 | 1.66 ± 0.04 | – | – | – |
| DDP | 7.5 ng | 1.64 ± 0.04 | 1.65 ± 0.07 | 1.64 ± 0.06 | 1.20 | 1.19 | 1.10 |
| DDP | 75 ng | 1.64 ± 0.02 | 1.64 ± 0.03 | 1.63 ± 0.07 | 1.20 | 1.19 | 1.21 |
| DDP | 750 ng | 1.63 ± 0.03 | 1.64 ± 0.02 | 1.63 ± 0.04 | 1.81 | 1.80 | 1.79 |
| DDP | 7.5 μg | 1.63 ± 0.04 | 1.64 ± 0.03 | 1.63 ± 0.07 | 1.85 | 1.81 | 1.82 |
[DDP] = concentration of cisplatin.
Compared with the control group, P> 0.05; compared with DDP 7.5 ng/ml group, P> 0.05; compared with 24 h group, P> 0.05, there were no significant differences.
Effect of metronomic chemotherapy with LDM on hepatocarcinoma 22-bearing mice
Comparison of tumour volume and mass among groups during administration
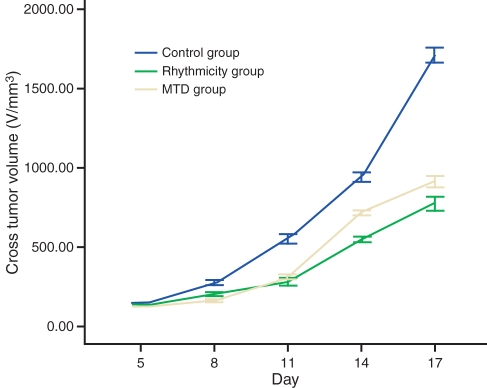
Tumours in the control group continued to grow, whereas those in the LDM group grew slowly. Tumours in the MTD group did not grow for a short period, but picked up rapidly after 10 days. Tumour mass in the LDM group was significantly lower than that in the MTD group and control group with significant differences (P< 0.01); its inhibition was as high as 67.78% (Figure 1).
Figure 1.
The growth curve of tumour volume (V/mm3) in each group.
Comparison of expression levels of MVD, VEGF and MMP-2 in tumour tissues
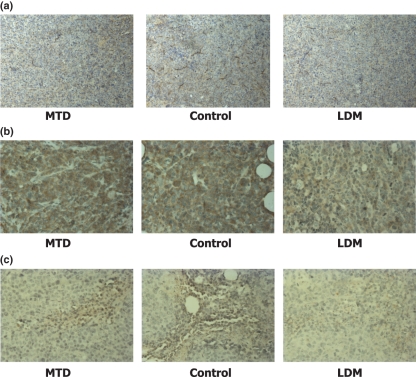
The MVD count and VEGF and MMP-2 levels in the metronomic chemotherapy group were significantly lower than those of the control and MTD groups (Table 3; Figure 2). The difference between the MTD group and the control group was also significant.
Table 3.
Comparison of the results of immunohistochemistry (median, interquartile distance)
| Control | MTD | LDM | H | P | P (groups compared) | |
|---|---|---|---|---|---|---|
| VEGF | 38.98 (10.03) | 29.15 (6.07) | 18.92 (6.16) | 30.39 | 0.00 | MTD to Control, P= 0.000 |
| LMD to Control, P= 0.000 | ||||||
| LDM to MTD, P= 0.000 | ||||||
| MMP-2 | 19.81 (6.66) | 13.72 (3.98) | 9.34 (4.78) | 23.31 | 0.00 | MTD to Control, P= 0.000 |
| LDM to Control, P= 0.000 | ||||||
| LDM to MTD, P= 0.01 | ||||||
| MVD | 29.50 (4.5) | 22.5 (7.75) | 9.5 (3) | 29.10 | 0.00 | MTD to Control, P= 0.000 |
| LDM to Control, P= 0.000 | ||||||
| LDM to MTD, P= 0.000 |
MTD, maximum tolerable dose group; LDM, low-dose metronomic group; H, Statistical analysis: 1. Non-parametric statistics method of Kruskal–Wallis H-test. 2. Non-parametric statistics method of Mann–Whitney U-test for two-group comparison.
Figure 2.
Immunohistochemistry staining in H22 hepatocarcinoma cells. (a) CD34 staining in H22 hepatocarcinoma cells. Groups include cells receiving maximum tolerable doses (MTD) and low-dose metronomic (LDM) amounts of cisplatin and control group (×400). (b) VEGF staining in H22 hepatocarcinoma cells (×400). (c) MMP-2 staining in H22 hepatocarcinoma cells (×400). The microvessel density and VEGF and MMP-2 levels in the LDM group were significantly lower than those in the control and MTD groups.
CAM angiogenesis experiments
Metronomic chemotherapy with low-dose cisplatin was seen to inhibit CAM angiogenesis. New vessels were normal in the saline control group with abundant capillaries that were uniformly dendritic, while the generation of new blood vessels was clearly decreased in the low-dose cisplatin group. Abnormal vessels increased around the methylcellulose area and the density decreased; vascular morphology had changed. Vessels exhibited an uneven diameter and continuity was interrupted. The inhibition of CAM angiogenesis increased with increasing concentration of cisplatin in the experimental groups, reaching a peak at 750 ng/ml. There were significant differences between the control group and experimental groups (P< 0.05) (Table 4).
Table 4.
Influence of different concentrations of cisplatin (DDP) on CAM vascular growth
| Group | [DDP] (ng/ml) | Embryos (n) |
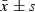 ) ) |
Inhibition (%) |
|---|---|---|---|---|
| Control | 0 | 10 | 76.39 ± 4.13 | – |
| DDP | 7.5 | 10 | 54.50 ± 6.16 | 28.14 |
| DDP | 75 | 10 | 41.85 ± 3.10 | 47.26 |
| DDP | 750 | 10 | 22.48 ± 5.17 | 72.85 |
| DDP | 7500 | 10 | 23.16 ± 3.24 | 70.19 |
[DDP] = concentration of cisplatin.
P< 0.05, compared with control group.
Discussion
Cisplatin is a cell cycle non-specific anti-tumour drug that has been used for treatment of various solid tumours; it has been one of the most effective chemotherapy drugs (Sbovata et al. 2007).
Low-dose cisplatin was shown here to inhibit the proliferation of endothelial cells in vitro, and the angiogenesis of chicken CAM in vivo. These actions proceeded in a dose-dependent manner, reaching a peak at the concentrations of 750 ng/ml.
However, low-dose cisplatin failed to inhibit the proliferation of tumour cells in vitro. Our results show that tumour growth was delayed in the cisplatin LDM chemotherapy group. The ultimate tumour mass in the LDM group was significantly lower than that in the control group and the MTD group, whereas its cumulative dose was less than that in the MTD group. The anti-tumour effect in the MTD group was obvious only during early period, but the MTD mice gradually lost weight and moved slowly; some mice had bloody diarrhoea. At the same time, tumour volume increased significantly during treatment intermissions.
It can be inferred that LDM chemotherapy with cisplatin had a lasting inhibition on tumour growth and had no significant rebounding period and few side effects during long-term application. The growth and metastasis of tumours are vascular-dependent; MVD reflects the extent of tumour angiogenesis and was therefore quantified in our samples, using immunohistochemical techniques. We found that while MVD count declined in both the LDM group and MTD group, it decreased more significantly in LDM group. There was also significant difference between the MTD group and the control group. It could be inferred that although LDM chemotherapy had a little direct effect on tumour cells, it achieved significant anti-tumour effects by inhibiting angiogenesis, destroying tumour vessels and causing ischaemic necrosis of tumour cells. On the other hand, MTD chemotherapy with the same drug achieves its anti-tumour effect by inhibiting tumour cell growth or inducing apoptosis.
Tumour angiogenesis is a complex multistep process comprising various angiogenic factors, matrix proteolytic enzymes and adhesion molecules. In particular, VEGF causes endothelial cells to induce angiogenesis through various proliferative, chemotaxic and metastatic pathways.
Matrix metallopeptidases have a key role in degradation of the basement membrane, cell movement and tube formation. Among MMPs, gelatinase A (MMP-2) has a direct role in promoting angiogenesis by mediating tumour’s angiogenesis ‘switch’. Overexpression of MMP-2 is often present in hepatocellular carcinoma (Guo et al. 2006; Zhang et al. 2006). Some authors found that LDM chemotherapy could indirectly inhibit tumour angiogenesis by inhibiting VEGF secretion in tumour cells. We showed that the expression of VEGF and MMP-2 in the metronomic chemotherapy group was significantly lower than that in the control and MTD groups and that the distribution phases of MVD were similar (Zhang et al. 2006; Albertsson et al. 2006; Buckstein et al. 2006; Li et al. 2006). We speculated that the inhibition of LDM chemotherapy with cisplatin on tumour growth and tumour angiogenesis may be related to its inhibition of the proliferation of vascular endothelial cells and the decreased expression of VEGF and MMP-2.
The anti-angiogenic effect of LDM chemotherapy is a new strategy with few toxic side effects and little drug resistance. The endpoint of therapeutic evaluation is improvement in quality of life and delay in time to progression. The prominent role of metronomic chemotherapy is not the rapid elimination of tumours, but growth inhibition by inhibiting angiogenesis (Franchi et al. 2007; Klement et al. 2007). LDM chemotherapy could become a new palliative treatment for advanced tumours. Incurable tumours could conceivably become controllable, allowing patients with tumours to survive for a long time without lowering their quality of life.
References
- Albertsson P, Lennernäs B, Norrby K. On metronomic chemotherapy: modulation of angiogenesis mediated by VEGE-A. Acta Oncol. 2006;45:144–155. doi: 10.1080/02841860500417486. [DOI] [PubMed] [Google Scholar]
- Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist. Updat. 2006;9:227–246. doi: 10.1016/j.drup.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Buckstein R, Kerbel RS, Shaked Y, et al. High-dose celecoxib and metronomic “low-dose” cyclophosphamide is an effective and safe therapy in patients with relapsed and refractory aggressive histology non-Hodgkin’s lymphoma. Clin. Cancer Res. 2006;12:5190–5198. doi: 10.1158/1078-0432.CCR-06-0474. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis and apoptosis. Semin. Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- Franchi F, Grassi P, Ferro D, et al. Antiangiogenic metronomic chemotherapy and hyperthermia in the palliation of advanced cancer. Eur. J. Cancer Care. 2007;16:258–262. doi: 10.1111/j.1365-2354.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- Guo RP, Zhong C, Shi M, et al. Expression and clinical significance of certain apoptosis and angiogenesis factors in hepatocellular carcinoma. Zhonghua Wai Ke Za Zhi. 2006;44:1626–1630. [PubMed] [Google Scholar]
- Klement H, St Croix B, Milsom C, et al. Atherosclerosis and vascular aging as modifiers of tumor progression, angiogenesis, and responsiveness to therapy. Am. J. Pathol. 2007;171:1342–1351. doi: 10.2353/ajpath.2007.070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Hetherington JW, Greenman J, Little S, Maraveyas A. Metronomic chemotherapy dosing-schedules with estramustine and temozolomide act synergistically with anti-VEGFR-2 antibody to cause inhibition of human umbilical venous endothelial cell growth. Acta Oncol. 2007;46:1169–1177. doi: 10.1080/02841860701373603. [DOI] [PubMed] [Google Scholar]
- Li T, Li YG, Pu DM. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol. Obstet. Invest. 2006;62:229–235. doi: 10.1159/000094426. [DOI] [PubMed] [Google Scholar]
- Lu QB. Molecular reaction mechanisms of combination treatments of low-dose cisplatin with radiotherapy and photodynamic therapy. J. Med. Chem. 2007;50:2601–2604. doi: 10.1021/jm061416b. [DOI] [PubMed] [Google Scholar]
- Moreira IS, Fernandes PA, Ramos MJ. Vascular endothelial growth factor (VEGF) inhibition – a critical review. Anticancer Agents Med. Chem. 2007;7:223–245. doi: 10.2174/187152007780058687. [DOI] [PubMed] [Google Scholar]
- Ozdogan O, Ertay T, Arslan G, et al. Does cisplatin chemotherapy decrease the MDP uptake of normal bone? An experimental study. Ann. Nucl. Med. 2008;22:357–362. doi: 10.1007/s12149-007-0129-5. [DOI] [PubMed] [Google Scholar]
- Ruggeri B, Singh J, Gingrich D, et al. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–5991. [PubMed] [Google Scholar]
- Sbovata SM, Bettio F, Mozzon M, et al. Cisplatinum and transplatinum complexes with benzyliminoether ligands; synthesis, characterization, structure-activity relationships, and in vitro and in vivo antitumor efficacy. J. Med. Chem. 2007;50:4775–4784. doi: 10.1021/jm070426p. [DOI] [PubMed] [Google Scholar]
- Schweitzer VG. Cisplatin-induced ototoxicity: the effect of pigmentation and inhibitory agents. Laryngoscope. 1993;103:1–52. [PubMed] [Google Scholar]
- Shaked Y, Ciarrocchi A, Franco M, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- Spaner DE, Masellis A. Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia. 2007;21:53–60. doi: 10.1038/sj.leu.2404456. [DOI] [PubMed] [Google Scholar]
- Tamura T, Fujita F, Tanimoto M, et al. Anti-tumor effect of intraperitoneal administration of cisplatin-loaded microspheres to human tumor xenografted nude mice. J. Control. Release. 2002;80:295–307. doi: 10.1016/s0168-3659(02)00003-2. [DOI] [PubMed] [Google Scholar]
- Veale DJ, Fearon U. Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract. Res. Clin. Rheumatol. 2006;20:941–947. doi: 10.1016/j.berh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen H, Hu PC. Circulating endothelial cells and endothelial progenitors as surrogate biomarkers in vascular dysfunction. Clin. Lab. 2007;53:285–295. [PubMed] [Google Scholar]
- Zhang Q, Chen X, Zhou J, et al. CD147, MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence after liver transplantation in hepatocellular carcinoma patients. Cancer Biol. Ther. 2006;5:808–814. doi: 10.4161/cbt.5.7.2754. [DOI] [PubMed] [Google Scholar]