Abstract
Mast cells (MCs) are widely distributed in human and animal tissues and have been shown to play an important role in angiogenesis in normal and pathological conditions. Few data are available about the relationship between MCs and blood vessels in the normal human thymus, and there are virtually no data about their distribution and significance in thymoma. The aim of this study was to analyse the spatial distribution of MCs and microvessels in the normal foetal and adult thymus and thymoma. Twenty biopsy specimens of human thymus, including foetal and adult normal thymus and thymoma were analysed. Double staining with CD34 and mast cell tryptase was used to count both mast cells and microvessels in the same fields. Computer-assisted image analysis was performed to characterize the spatial distribution of MCs and blood vessels in selected specimens. Results demonstrated that MCs were localized exclusively to the medulla. Their number was significantly higher in thymoma specimens as compared with adult and foetal normal specimens respectively. In contrast the microvessel area was unchanged. The analysis of the spatial distribution and relationship between MCs and microvessels revealed that only in the thymoma specimens was there a significant spatial association between MCs and microvessels. Overall, these data suggest that MCs do not contribute significantly to the development of the vascular network in foetal and adult thymus, whereas in thymoma they show a close relationship to blood vessels. This could be an expression of their involvement not only in endothelial cells but also in tumour cell proliferation.
Keywords: angiogenesis, mast cell, microvessels, thymoma, thymus
Mast cells (MCs) are characterized by cytoplasmic granules storing a large variety of biological active substances and are normally found in the perivascular space of blood and, to a lesser extent, lymphatic vessels. MCs of different organs are extremely heterogeneous in terms of their size, biochemical properties of specific granules, and function (Galli et al. 2005). Few data are available concerning MC distribution, number and functional significance in the human thymus.
A significant increase in MCs number has been observed in malignant tumours, in both experimental models and human specimens and conflicting results have been published about the prognostic role of mast cells in a large variety of human tumours (Ribatti & Crivellato 2009a). Thymomas are epithelial tumours that originate in the thymus epithelial stroma and the clinical behaviour and therapeutic response to conventional treatment are different even for the same pathological type (Suster & Moran 2008). Several biological aspects of thymomas are not completely clarified, such as the role of angiogenesis in its progression. An increased number of MCs inside the thymic parenchyma was observed in human thymomas and a strong correlation between microvessel density and MCs number has been found (Raica et al. 2007).
The aim of the present study was to evaluate the distribution of MCs in normal foetal and adult human thymus and thymoma and their spatial relationships with blood vessels and to correlate their number with microvessel area.
Material and methods
Tissue samples
A total of 20 biopsy specimens of thymic tissue selected from the archive were examined retrospectively. Foetal thymus specimens were obtained from five cases (6–8 months old) during necropsy. A total of 15 specimens were obtained surgically from five patients (1 month to 5 years old) with cardiac malformations and from 10 patients with thymoma. All specimens were fixed in buffer formalin for 48 h, embedded in paraffin and 5 μm thick sections were stained with haematoxylin-eosin for the routine morphological diagnosis. The classification of cases with thymoma was performed according recommendations of World Health Organization (Rosai 1999). Additional 5 μm thick sections were prepared for the immunohistochemical study. The study protocol was approved by the local research ethic committee, and informed consent was obtained from all subjects according to the World Medical Association (WMA) Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry was performed to visualize blood vessels and MCs on the same specimen. We used sequential application of the routine LSAB and HRP method using two different compatible chromogens. Endothelial cells were shown using a monoclonal mouse anti-human CD34 antibody (QBEnd10, Dako Cytomation, Glostrup, Denmark) followed by visualization with 3,3′ diaminobenzidine as brown staining. MCs were shown using a mouse monoclonal antibody anti-tryptase (clone AA1, Dako Cytomation) followed by visualization of MCs in red with amino-ethyl carbazole. Nuclei were stained with modified Lille’s haematoxylin and the slides were mounted in aqueous mounting media (Glycergel).
Evaluation of mast cell number and microvascular density
Microvessel and MC counting was performed according to Weidner’s method (Weidner et al. 1992) on slides stained for CD34 and tryptase. Four to six 200 x fields of each of three sections per sample were examined, and the mean values ± Standard Deviation (SD) was determined for each section, sample and group of samples. Counting was performed by two independent observers. Images were captured as .jpg format and processed to calculate vascular area using Lucia G software (Nikon, Tokyo, Japan) for microscopic image analysis.
Image analysis of MC spatial distribution
Computer-assisted image analysis was performed to characterize the spatial distribution of MCs and blood vessels in selected specimens, using the method described previously [Guidolin et al. 2006, 2009]. The image analysis system included a light microscope (DM-R, Leica Mycrosystems, Wetzlar, Germany) and a high resolution digital camera (CD200, Leica Mycrosystems). Images were transmitted to a PC equipped with software for image acquisition and analysis (Qwin, Leica Mycrosystems, Cambridge, UK). Three fields per sample were acquired at a primary magnification of ×16 and processed to correct the shading and enhance the contrast, and finally stored as .tiff file.
The study area was defined as the minimum rectangle bounding MCs profiles. Colour threshold was applied to discriminate the tryptase-positive structures. The spatial analysis of MCs distribution was performed using two methods. The first one was based on the estimation of the uniformity index that can have any value between 1 (when objects are distributed in a regular array) and 0 (when maximal clustering occurs). The second method involved the calculation of the cumulative frequency distribution [G(d)] of the distances between each cell profile and its nearest neighbour (spatial statistics). To interpret the cell–cell spatial relationship statistically, G(d) has to be compared with the value of [G0(d)] estimated on random point patterns. If G(d) is significantly greater than G0(d) for any range of d, then the cells are clustered and they are closer to each other than could be expected by chance. If G(d) is significantly lower than G0(d), then short cell–cell distances are less frequent than could be expected by chance.
One hundred random point patterns per analysed field were computer generated and each pattern had the same number of points as the number of observed MCs profiles. The analysis were performed to estimate the uniformity index and G0(d) in the case of complete spatial randomness (CSR).
Statistic analysis
Mast cell densities, distances from the nearest blood vessel, uniformity index and blood vessels area were averaged to provide representative value of each parameter for that sample. Differences between the foetal thymus, normal adult thymus and thymoma were tested statistically by one-way anova followed by Bonferroni’s test for multiple comparisons. Two-sample Student’s test was applied to test the statistical difference between the uniformity index values found on tissue samples and the value the parameter assumed in the simulated random point pattern. The GraphPad Prism 3.0 statistical package (GraphPad Software Inc., San Diego, CA, USA) was used for the analysis and P < 0.05 was considered as the limit for statistical significance. The public domain statistical software R 2.6.0 was used to obtain estimators of G(d) and G0(d) (together with the 95% confidence intervals) from the observed and simulated point patterns respectively.
Results
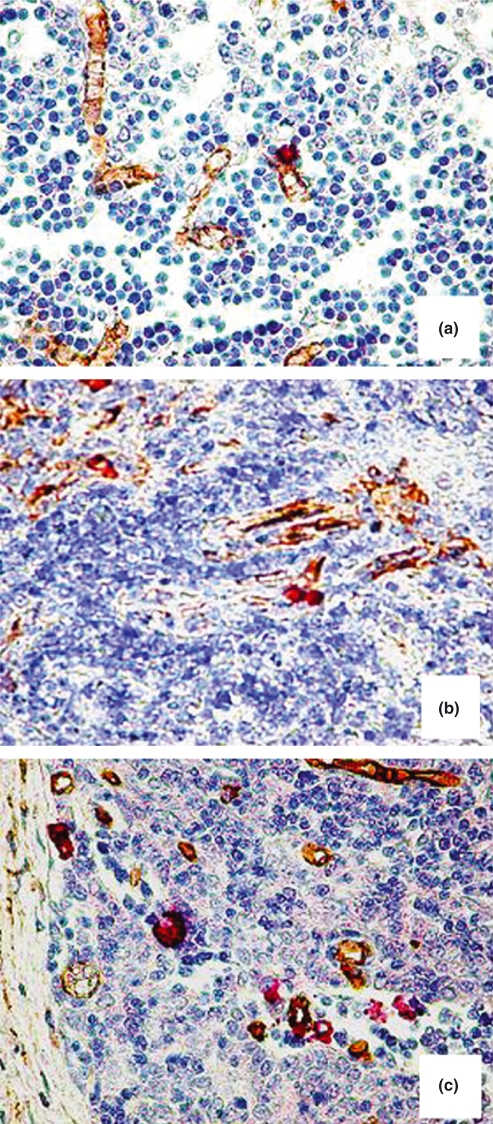
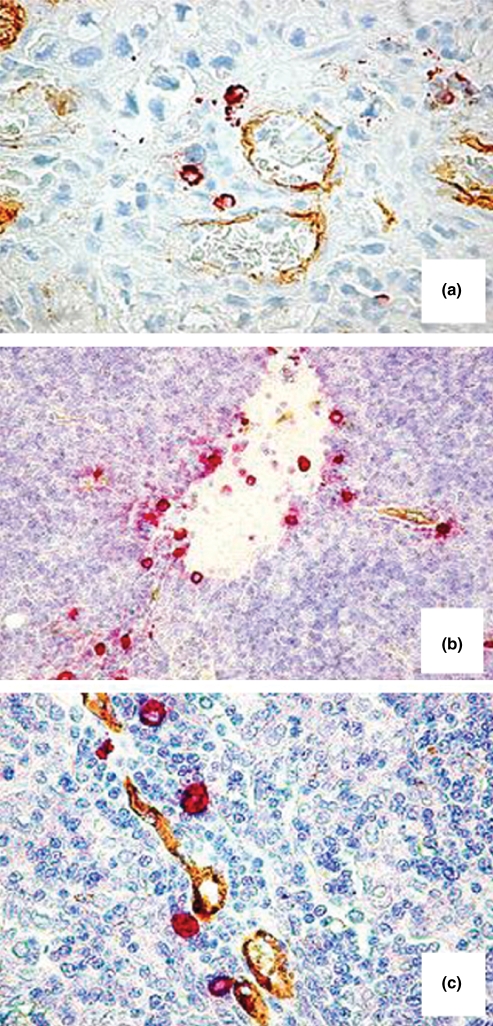
Tryptase-positive MCs and CD34-positive blood vessels were identified by double staining. In the foetal and adult normal thymus, CD34-positive blood vessels were more numerous in the medulla of the thymus parenchyma and at the junction between cortex and medulla and blood vessels were more numerous in adult specimens as compared with foetal ones. MCs were restricted to the thymus medulla and to connective tissue septa and their number increased in adult thymus when compared with foetal thymus (Figures 1a,b and 2a). In thymoma, MCs increased as compared with adult thymus (Figure 1c), they were randomly distributed in the tumour area (Figure 2b) and were preferentially localized in perivascular position (Figure 2c).
Figure 1.
CD34-positive endothelial cells (in brown) and tryptase-positive mast cells (in red) in bioptic specimen of prenatal thymus (a), adult thymus (b) and thymoma (c). Original magnification: (a–c), ×200.
Figure 2.
Double staining CD34-tryptase. Tryptase-positive mast cells in the medulla of a bioptic specimen of adult thymus (a), in the tumour area (b) and in perivascular position (c) in two bioptic specimens of thymoma. Original magnification: (a, c), ×400; (b), ×200.
The morphometric evaluation of the number of MCs and of microvessel area (Table 1) demonstrated that the MC number increased significantly in adult thymus compared with foetal thymus, and in thymoma specimens when compared with adult thymus. In contrast the microvessel area was unchanged.
Table 1.
Mast cells number and microvessel area in foetal and adult thymus and in thymoma
| Mast cells (number/field) | Microvessels (area%) | |
|---|---|---|
| Foetal thymus | 2.2 ± 0.6* | 4.0 ± 0.6 |
| Adult thymus | 4.2 ± 1.1* | 3.9 ± 0.7 |
| Thymoma | 9.7 ± 2.3 | 3.3 ± 0.6 |
P < 0.05 vs. Thymoma group (One-way anova + Bonferroni’s test)
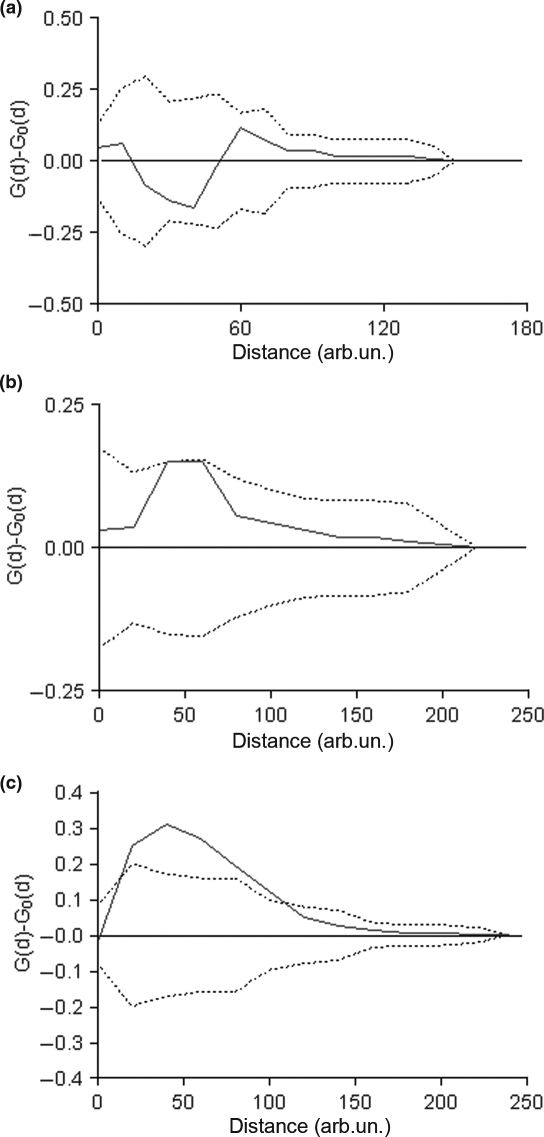
The analysis of the spatial distribution and relationship between MCs and microvessels revealed that only in the thymoma specimens was there a significant spatial association between MCs and microvessels, as indicated by a frequency of short cell-to-vessel distance higher significantly than might be expected by chance (Figure 3).
Figure 3.
Analysis of the spatial relationship between mast cells and microvessels. Solid lines indicate the difference between the observed distribution of cell-to-vessel distances [G(d)] and the estimated distribution [G0(d)] under the hypothesis of complete spatial randomness (CSR). Dotted lines indicate the 95% confidence envelope for CSR. Only in the thymoma group, there is a significant spatial association between mast cells and vessels as indicated by a frequency of short cell-to-vessel distances significantly higher than expected by chance.
Discussion
In the medulla of the thymus parenchyma a variety of cells are found, including T and B lymphocytes, macrophages, eosinophils, dendritic cells, myoid cells and MCs. In normal thymus of humans and other animal species, MCs are localized in the capsule and along the connective tissue septa within the gland (Wight 1970; Frazier 1973; Kendall & Warley 1986). The presence of MCs precursors in thymus was first reported by Ginsburg and Sachs (1963) and further studies supported the possible involvement of MCs in thymus organogenesis (Crivellato et al. 2005) and the influence of removal or stimulation of thymus on the number of tissue MCs distant from the thymic lymphoid tissue (Rao 1972).
In this study, we have analysed the spatial distribution and their correlation with microvascular density of tryptase-positive MCs in foetal and adult human thymus and in thymoma specimens. MCs were localized exclusively in the medulla, and their number was significantly higher in thymoma specimens compared with adult and foetal normal specimens. Furthermore, MCs were localized in a perivascular position although analysis of the spatial distribution and relationship between MCs and microvessels revealed that only in the thymoma specimens there was spatial association between MCs and microvessels that was significant. However, its microvessel area was unchanged between foetal and adult thymus and thymoma.
These data support the notion of an involvement of MCs in the maintenance of blood vessel homeostasis, but not a specific role in angiogenesis occurring in the thymus.
It is well-known that the crucial role played by inflammatory cells and among these by MCs in regulating tumour progression and angiogenesis. MCs have been associated either with resistance or with a greater susceptibility to tumours. Indeed, MCs accumulate in the stroma surrounding tumours and take part in the inflammatory reaction occurring at the margin of the neoplasia (Ribatti & Crivellato 2009a,b;). MCs can participate in tumour rejection by producing molecules like interleukin-1 (IL-1), IL-4, IL-6 and tumour necrosis factor (TNF)-α, that kill tumour cells. By contrast, MCs can facilitate tumour growth by promoting vascular supply, proteinase-mediated degradation of the tumour extracellular matrix and immunosuppression (Ribatti & Crivellato 2009a,b;).
An increased number of MCs has been reported in angiogenesis associated with vascular neoplasms, like haemangioma and haemangioblastoma (Glowacki & Mulliken 1982), as well as a number of solid and haematopoietic tumours. In general, MC density correlates with angiogenesis and poor tumour outcome. Association between MCs and new vessel formation has been reported in breast cancer (Bowrey et al. 2000), colorectal cancer (Lachter et al. 1995) and uterine cervix cancer (Graham & Graham 1966). Tryptase-positive MCs increase in number and vascularization increases in a linear fashion from dysplasia to invasive cancer of the uterine cervix (Benítez-Bribiesca et al. 2001). An association of vascular endothelial growth factor (VEGF) and MCs with angiogenesis has been demonstrated in laryngeal carcinoma (Sawatsubashi et al. 2000) and in small lung carcinoma, where most intratumoural mast cells express VEGF (Tomita et al. 2000). MC accumulation was correlated with increased neovascularization, mast cell expression of VEGF (Tóth-Jakatics et al. 2000) and fibroblast growth factor-2 (FGF-2) (Ribatti et al. 2003a), tumour aggressiveness and poor prognosis (Ribatti et al. 2003b) in human melanoma. A prognostic significance has been attributed to mast cells and microvascular density also in squamous cell cancer of the oesophagus (Elpek et al. 2001). Angiogenesis has been shown to correlate with tryptase-positive MC count in human endometrial cancer. Both parameters were found to increase in agreement with tumour progression (Ribatti et al. 2005). MC density, new vessel rate and clinical prognosis have also been found to correlate in haematological tumours. In benign lymphadenopathies and B cell non-Hodgkin’s lymphomas, angiogenesis correlates with total and tryptase-positive MC counts, and both increase in step with malignancy grades (Ribatti et al. 1998, 2000). In the bone marrow of patients with inactive and active multiple myeloma as well as those with monoclonal gammopathies of undetermined significance, angiogenesis correlates highly with MC counts (Ribatti et al. 1999). A similar pattern of correlation between bone marrow microvessel count, total and tryptase-positive MC density and tumour progression has been found in patients with myelodysplastic syndrome (Ribatti et al. 2002) and B cell chronic lymphocytic leukaemia (Ribatti et al. 2003c). In the early stages of B cell chronic lymphocytic leukaemia, the density of tryptase-positive MC in the bone marrow has been shown to predict the outcome of the disease (Molica et al. 2003).
In this study, for the first time we have demonstrated that in the thymoma specimens MCs were closer to each other and to the vessels than in normal thymus. In fact, both the mean distance from vessels and the mean distance from the nearest cell profile were significantly lower than in normal thymus specimens. Shorter intercellular distances and lower cell-to-vessel distances could be a morphological condition important in increasing the rate of signal exchange between the cells and with the vessels and induce higher concentrations of the signalling molecules in the pericellular and periendothelial environment.
Overall, these data suggest that MCs do not significantly contribute to the development of the vascular network in foetal and adult thymus, whereas in thymoma their close relationship to blood vessels, could be expression of their involvement not only in endothelial cell but also in tumour cell proliferation.
Acknowledgments
This work was supported by Grant PNII 41-054/2007 and Grant PNII/41-052/2007 of the Romanian Ministry of Education and Research and the Ministry for Education, the Universities and Research (PRIN 2007), Rome, Italy.
References
- Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J. Histochem. Cytochem. 2001;49:1061–1062. doi: 10.1177/002215540104900816. [DOI] [PubMed] [Google Scholar]
- Bowrey PF, King J, Magarey C, et al. Histamine, mast cells and tumour cell proliferation in breast cancer: does preoperative cimetidine administration have an effect? Br. J. Cancer. 2000;82:167–170. doi: 10.1054/bjoc.1999.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E, Nico B, Battistig M, Beltrami CA, Ribatti D. The thymus is a site of mast cell development in chicken embryos. Anat. Embryol. 2005;209:243–249. doi: 10.1007/s00429-004-0439-5. [DOI] [PubMed] [Google Scholar]
- Elpek GO, Gelen T, Aksoy NH, et al. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J. Clin. Pathol. 2001;54:940–944. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA. Ultrastructure of the chicken thymus. Zeitschrift for Zellforschung. 1973;136:191–205. doi: 10.1007/BF00307440. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Najae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Ginsburg H, Sachs L. Formation of pure suspensions of mast cells in tissue culture by differentiation of lymphoid cells from the mouse thymus. J. Nat. Cancer Inst. 1963;31:1–40. [PubMed] [Google Scholar]
- Glowacki J, Mulliken JB. Mast cells in hemangiomas and vascular malformations. Pediatrics. 1982;70:48–51. [PubMed] [Google Scholar]
- Graham RM, Graham JB. Mast cells and cancer of the cervix. Surg. Gynecol. Obstet. 1966;123:3–9. [PubMed] [Google Scholar]
- Guidolin D, Crivellato E, Nico B, Andreis PG, Nussdorfer GG, Ribatti D. An image analysis of the spatial distribution of perivascular mast cells in human melanoma. Int. J. Mol. Med. 2006;17:981–987. [PubMed] [Google Scholar]
- Guidolin D, Nico B, Crivellato E, Marzullo A, Vacca A, Ribatti D. Tumoral mast cells exhibit a common spatial distribution. Cancer Lett. 2009;273:80–85. doi: 10.1016/j.canlet.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Kendall MD, Warley A. The elemental content of mast cell granules measured by X-ray microanalysis of rat thymic tissue sections. J. Cell Sci. 1986;83:77–87. doi: 10.1242/jcs.83.1.77. [DOI] [PubMed] [Google Scholar]
- Lachter J, Stein M, Lichtig C, Eidelman S, Munichor M. Mast cells in colorectal neoplasias and premalignant disorders. Dis. Colon Rectum. 1995;38:290–293. doi: 10.1007/BF02055605. [DOI] [PubMed] [Google Scholar]
- Molica S, Vacca A, Crivellato E, Cuneo A, Ribatti D. Tryptase-positive mast cells predict clinical outcome of patients with early B-cell chronic lymphocytic leukemia. Eur. J. Haematol. 2003;71:137–139. doi: 10.1034/j.1600-0609.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- Raica M, Cîmpean AM, Encic˘ S, Scridon T, Bârsan M. Increased mast cell density and microvessel density in the thymus of patients with myasthenia gravis. Rom. J. Morphol. Embryol. 2007;48:11–16. [PubMed] [Google Scholar]
- Rao KK. Effects of removal and stimulation of thymus on tissue histamine and mast cell contents in rats. Jpn. J. Pharmacol. 1972;22:443–446. doi: 10.1254/jjp.22.443. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E. Immune cells and angiogenesis. J. Cell Mol. Med. 2009a;June 16 doi: 10.1111/j.1582-4934.2009.00810.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E. The controversial role of mast cells in tumor growth. Int. Rev. Cell. Mol. Biol. 2009b;275:89–131. doi: 10.1016/S1937-6448(09)75004-X. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, et al. Do mast cells help to induce angiogenesis in B-cell non-Hodgkin’s lymphomas? Br. J. Cancer. 1998;77:1900–1906. doi: 10.1038/bjc.1998.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Nico B, et al. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br. J. Cancer. 1999;79:451–455. doi: 10.1038/sj.bjc.6690070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Marzullo A, et al. Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in B-cell non-Hodgkin’s lymphomas. Int. J. Cancer. 2000;85:171–175. [PubMed] [Google Scholar]
- Ribatti D, Polimeno G, Vacca A, et al. Correlation of bone marrow angiogenesis and mast cells with tryptase activity in myelodysplastic syndromes. Leukemia. 2002;16:1680–1684. doi: 10.1038/sj.leu.2402586. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Ria R, et al. Neovascularisation, expression of fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur. J. Cancer. 2003a;39:666–674. doi: 10.1016/s0959-8049(02)00150-8. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Ennas MG, Vacca A, et al. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur. J. Clin. Invest. 2003b;33:420–425. doi: 10.1046/j.1365-2362.2003.01152.x. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Molica S, Vacca A, et al. Tryptase-positive mast cells correlate positively with bone marrow angiogenesis in B-cell chronic lymphocytic leukemia. Leukemia. 2003c;17:1428–1430. doi: 10.1038/sj.leu.2402970. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Finato N, Crivellato E, et al. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am. J. Obstet. Gynecol. 2005;193:1961–1965. doi: 10.1016/j.ajog.2005.04.055. [DOI] [PubMed] [Google Scholar]
- Rosai J. Histological Typing of Tumours of the Thymus. Berlin-Heidelberg: World Health Organization, International Histological Classification of Tumours, Springer-Verlag; 1999. [Google Scholar]
- Sawatsubashi M, Yamada T, Fukushima N, Mizokami H, Tokunaga O, Shin T. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch. 2000;436:243–248. doi: 10.1007/s004280050037. [DOI] [PubMed] [Google Scholar]
- Suster S, Moran CA. Histologic classification of thymoma: the World Health Organization and beyond. Hematol. Oncol. Clin. North Am. 2008;22:381–392. doi: 10.1016/j.hoc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Tomita M, Matsuzaki Y, Onitsuka T. Effect of mast cells on tumor angiogenesis in lung cancer. Ann. Thorac. Surg. 2000;69:1686–1690. doi: 10.1016/s0003-4975(00)01160-7. [DOI] [PubMed] [Google Scholar]
- Tóth-Jakatics R, Jimi S, Takebayashi S, Kawamoto N. Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum. Pathol. 2000;31:955–960. doi: 10.1053/hupa.2000.16658. [DOI] [PubMed] [Google Scholar]
- Weidner N, Folkman J, Pozza F, Bevilacqua P, Alfred EN, Moore DH. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J. Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- Wight PAL. The mast cells of Gallus domesticus. I. Distribution and ultrastructure. Acta Anat. 1970;75:100–113. doi: 10.1159/000143444. [DOI] [PubMed] [Google Scholar]