Abstract
The differential diagnosis of soft tissue tumours poses a considerable challenge for pathologists, especially adipocytic tumours, as these may show considerable overlap in clinical presentation and morphological features with many other mesenchymal neoplasms. Hence, a specific and reliable marker that identifies adipocytic differentiation is much sought. We investigated the immunohistochemical expression of PIM-1 kinase in 35 samples of soft tissue tumours using tissue microarray technology and 49 full sections of adipocytic (n = 26) and non-adipocytic tumours (n = 23). Benign and malignant adipocytic tumours showed strong expression of PIM-1 while the non-adipocytic tumours were either negative or showed only weak staining for the protein. In myxoid liposarcomas, PIM-1 showed a distinct, unique vacuolar staining pattern, clearly outlining fine cytoplasmic lipid vacuoles. By contrast, non-adipocytic myxoid tumours (myxoma, chordoma and myxoid chondrosarcoma) did not show this vacuolar pattern of PIM-1 staining, although vacuolated cells were present on H&E. This differential expression was confirmed at a gene expression level in selected cases. Our results indicate that the expression of PIM-1 in adipose tissue may be a useful marker of adipocytic differentiation, in particular if the staining is both of high intensity and present in a unique, vacuolar pattern.
Keywords: adipocytic neoplasms, immunohistochemistry, myxoid liposarcoma, PIM-1, sarcomas, tissue microarray
Introduction
Sarcomas constitute approximately 1% of all malignancies. Difficulties in the differential diagnosis of sarcomas are widely recognized in tissue pathology, owing to the broad spectrum of different histogenetic types and the need to closely append the diagnostic workup with knowledge of the clinical presentation, imaging findings, cytogenetic and molecular analyses (Coindre et al.1996). Unfortunately, sarcomas often have a poor prognosis and often harbour significant metastatic potential. Of the various histological subtypes, liposarcoma is the most common and accounts for 15% of the soft tissue sarcomas (Cormier & Pollock 2004). They are of three major types: atypical lipomatous tumour (well differentiated liposarcoma), pleomorphic liposarcoma (high-grade liposarcoma) and myxoid/round cell liposarcoma. Amongst well-differentiated adipocytic tumours, a retroperitoneal location is related with a poorer outcome as opposed to localization within an extremity. Retroperitoneal tumours often remain asymptomatic for long periods and are large at presentation. Death may occur because of locally recurrent disease without distant metastasis (Goffman et al. 1991).
As a result of considerable morphological overlap between different histogenetic types of soft tissue sarcomas, one of the current challenges faced by surgical pathologists is to be able to accurately subtype the tumour into the most appropriate diagnosis which would best enable the clinician to predict its natural history, and hence manage the patient suitably. Cytogenetic analyses, such as elucidating specific chromosomal translocations in sarcomas and their correlation with outcome have yielded some optimistic results, and these investigations are sometimes used in the diagnosis of cases that cannot be classified clinically and pathologically (Sandberg & Bridge 2002; Borden et al. 2003; Coindre et al. 2003; Folpe et al. 2005; Xia & Barr 2005). However, this quest has yielded conflicting results. One study suggested that fusion types in liposarcoma were not prognostically significant (Antonescu et al. 2001) while another suggested that fusion type influenced clinical outcome in Ewing’s sarcoma (Ginsberg et al. 1999) and alveolar rhabdomyosarcomas (Kelly et al. 1997). Although useful for diagnostic purposes, the significance of the various translocations and their roles in prognostic stratification is still not clearly understood.
In practice, in addition to tissue morphology and immunohistochemistry (IHC), a combination of various techniques is used in the diagnosis of sarcomas; these include karyotyping and cytogenetic analysis, fluorescence in-situ hybridization (FISH) or polymerase chain reaction (PCR) for molecular characterization, but none of these tests can be interpreted in isolation. On the other hand, IHC is the most widely available ‘molecular’ method in the diagnostic armamentarium in the hands of pathologists, with the availability of good quality antibodies and optimization of antigen-retrieval techniques, and is now the mainstay in the diagnostic workup of many soft tissue tumours (Coindre 2003). In this exercise, we aim to study the expression of PIM-1 in sarcomas to investigate its possible diagnostic utility.
PIM-1 is a 34 kDa serine-threonine kinase that is involved in cell proliferation, survival, differentiation, apoptosis and tumourigenesis (Wang et al. 2001). The encoding gene maps to chromosome 6p21 in humans (Cuypers et al. 1986). It is overexpressed in several tissues including B-cell lymphomas (Verbeek et al. 1991), leukaemia’s (Amson et al. 1989; Dreyfus et al. 1990) prostate adenocarcinomas (Xu et al. 2005), various haematopoietic tissues and the testes (Wang et al. 2001). It works in cooperation or is synergistic with other genes such as c-Myc, promoting tumourigenesis in T and B lymphocytes (Berns et al. 1989; van Lohuizen et al.1989). This oncogene is emerging as an important chemotherapeutic target due to its involvement in various cancers (Kumar et al. 2005). PIM-1 is also expressed in low levels in normal prostatic and gastric epithelium and has been found to be upregulated in prostatic and gastric carcinoma (Cibull et al. 2006; Xie et al. 2006).
To date, there has not been significant documentation of PIM-1 expression in soft tissue tumours. We thus aim to elucidate its expression amongst this group of tumours, using both tissue microarrays as well as full tissue sections of specific tumours following the initial microarray analysis.
Materials and methods
Study samples
Formalin fixed paraffin-embedded (FFPE) soft tissue tumours from the year 1994–2005 were obtained from the Department of Pathology, National University Hospital, Singapore upon ethics approval by the National Healthcare Group Review Board (NHG-DSRB B/06/004). A tissue microarray (TMA) of 35 specific translocation-related soft tissue tumours [Ewing sarcoma/peripheral neuroectodermal tumour (n = 7), desmoplastic small round cell tumour (n = 1), synovial sarcoma (n = 4), myxoid chondrosarcoma (n = 3), myxoid liposarcoma (n = 5), neuroblastoma (n = 4), mesenchymal chondrosarcoma (n = 1), dermatofibrosarcoma protuberans (n = 4), clear cell sarcoma (n = 1), rhabdomyosarcoma (n = 5)] was constructed using tissue arrayer (Beecher Instruments Inc, Sun Prairie, WI, USA) and following our previously reported methodology (Salto-Tellez et al. 2004, 2007). Two 4-μm thick tissue sections were cut for each case, one section stained with routine haematoxylin and eosin (H&E) to confirm the diagnosis while the other section was subjected to IHC staining, using the method detailed below.
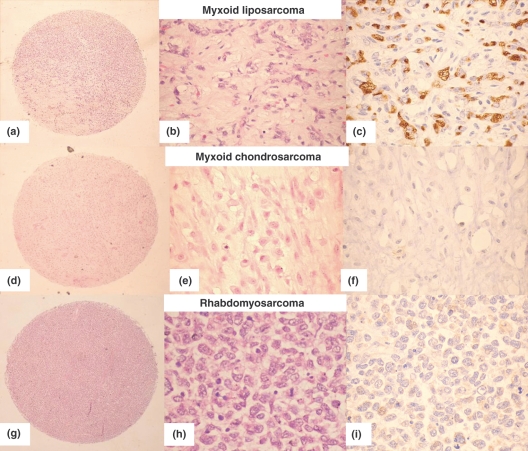
Based on the results of PIM-1 immunostaining on the initial TMA, full sections were cut from a further 49 soft tissue tumours which included a spectrum of 26 adipocytic tumours (benign – 5 typical lipomas, 1 pleomorphic lipoma, 1 hibernoma, 1 lipoblastoma, 4 angiolipomas and 5 spindle cell lipomas; malignant – 4 myxoid liposarcomas and 5 well differentiated liposarcomas, 1 of which showed myxoid features) and 23 morphologically similar, non-adipocytic tumours including eight myxomas (3 cardiac and 5 soft tissue), three myxoid chondrosarcomas, six chordomas and six malignant fibrous histiocytomas. Again, these were investigated for PIM-1 protein expression by IHC. All the cases included in the TMA and in the full section analysis were clear-cut examples from our routine diagnostic setting (their H&E morphology is shown in Figures 2–4), with a full IHC analysis at the time of diagnosis when necessary. Further 3–4 sections (5 μm) from the paraffin blocks of sarcomas were cut to extract RNA for the PIM-1 gene expression analysis.
Figure 2.
PIM-1 staining in benign adipocytic tumours. (a–b) Hibernoma (H&E, and PIM-1 showing strong expression; original magnification ×200), (c–d) Lipoblastoma (H&E and PIM-1 showing strong expression, original magnification ×40), (e–f) Angiolipoma (H&E and PIM-1 showing strong positivity, original magnification ×40 and 200 respectively).
Immunohistochemistry
The TMA and full sections were immunostained using a monoclonal PIM-1 antibody (clone 19F7), recently developed and provided by one of the authors (MBL, Chao Family Comprehensive Cancer Centre, University of California, Irvine, CA, USA). Briefly, the tissue sections were deparaffinized in xylene and rehydrated in alcohol.. They were pretreated in pH6 target retrieval solution (S1699; Dako, Carpinteria, CA, USA) at 119 °C in the pressure cooker for 30 min, cooled in running tap water for 5 min and blocked in 3% hydrogen peroxide solution for 10 min. Sections were then incubated in anti-PIM-1 monoclonal primary antibody (1:5000 dilution) at room temperature for 1 h and with secondary antibody for 30 min (ChemMate™ DAKO Envision™ Detection Kit) followed by a brief incubation of 5 min in diaminobenzidine peroxidase substrate (DAB) and counterstained with the haematoxylin. A colon tissue section with some fat tissue was included in the IHC run as a positive control.
Quantitative real-time RT-PCR
Total RNA was extracted from the 5 μm FFPE sections of the sarcomas using High Pure RNA extraction kit (Roche, Basel, Switzerland) and cDNA synthesized. A 77 bp segment of the human Pim-1 gene (from Applied Biosystems, Foster City, CA, USA, Assay ID-Hs00171473_m1; GenBank accession no. NM002648.2) was amplified and quantitated by real-time PCR using GAPDH gene as endogenous control. PCR reaction components included 10 μl of 2 × TaqMan universal PCR master mix without AmpErase UNG, 1 μl of PIM-1 gene expression assay and 9 μl of the cDNA template in a final volume of 20 μl. This was followed by 40 cycles of PCR under standard conditions with an annealing temperature of 56 °C using 7900 HT fast real-time PCR system (Applied Biosystems). For each analysis, a negative control was prepared using all the reagents except the cDNA template. All the reactions were run in duplicates.
Data analysis
IHC
PIM-1 protein expression analysed by IHC in the TMA was scored in terms of intensity of staining (score 0 – no staining; 1 – light intensity; 2 – moderate intensity; 3 – strong intensity) and approximate extent of staining (score 0 – nil; 1 – <25% of tumour cells, 2 – 25–50% of tumour cells, 3 – >50% tumour cells). In the full sections, the staining extent was given as a percentage (from 0% to 100%). For each case, the IHC score was calculated as staining intensity x percentage staining extent. In both the tissue microarray and full section analyses, the results were read and agreed upon by two observers (MEN and SMNN). The results were analysed statistically using Mann–Whitney U-test and P< 0.05 was considered statistically significant.
Quantitative real-time PCR
For the gene expression analysis, cycle threshold values were obtained for PIM-1 and normalized with those of the endogenous control, GAPDH from the same samples. Non-adipocytic tissue was chosen as a calibrator which was equal to 1 and relative expression of the genes was quantitated using the formula: 2 -ΔΔCT, where ΔΔCT = ΔCTadipocytic tissues − ΔCTnon-adipocytic tissues (ΔCT = cycle threshold).
Results
Immunohistochemical analysis
PIM-1 staining pattern and IHC analysis in TMA sections
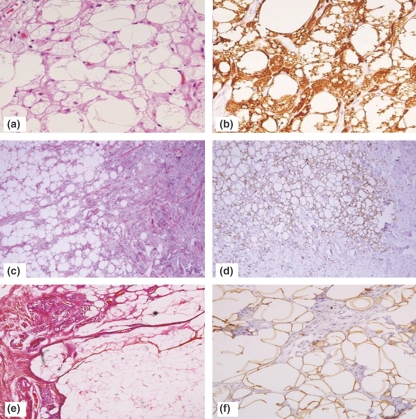
Immunohistochemistry of the sarcoma TMA showed strong positivity for PIM-1 protein in myxoid liposarcomas (MLPS) compared with other tumours, which showed only weak or focal cytoplasmic staining. Figure 1a–i shows the markedly different staining patterns in MLPS vs. myxoid chondrosarcoma and rhabdomyosarcoma. The median staining intensity of the MLPS was 3 while that of the other sarcomas was 0.5 (range 0–2), this difference was statistically significant (P-value < 0.001). The PIM-1 staining extent score in MLPS was also higher than the other sarcomas, although not statistically significant (median 2, range 1–3 vs. 0.5, range 0–3; P = 0.054). The results of the TMA analysis are shown in Table 1. Only the Ewing sarcoma/PNET and desmoplastic small round cell tumour showed significant PIM-1 expression. The former showed weak (mean intensity score 0.71) but more diffuse staining (mean extent score 2) while the single case of desmoplastic small round cell tumour showed weak (intensity score 1) but diffuse staining (extent score 3).
Figure 1.
PIM-1 staining in the TMA. (a–c) Myxoid liposarcoma (H &E, low and high power and PIM-1 showing strong vacuolar cytoplasmic staining). (d–f) Myxoid chondrosarcoma (H&E, low and high power and PIM-1 showing negative staining). (g–i) Rhabdmyosarcoma (H&E, low and high power and PIM-1 showing very pale cytoplasmic non-vacuolar staining).
Table 1.
PIM-1 Staining in TMA
| Staining intensity | Staining extent | Median intensity | Median extent | ||
|---|---|---|---|---|---|
| MLPS (n = 5) | 3,3,3,3,3 | 1,1,2,2,3 | MLPS | 3 | 2 |
| CCS (n = 1) | 2 | 1 | All non-adipocytic tumours | 0.5 | 0.5 |
| DSRCT (n = 1) | 1 | 3 | |||
| RMS (n = 5) | 0,1,1,1,1 | 0,1,1,1,1 | |||
| EWS/PNET (n = 7) | 0,0,1,1,1,1,1 | 0,0,2,3,3,3,3 | |||
| SS (n = 4) | 0,0,0,0 | 0,0,0,0 | |||
| MCS (n = 3) | 1,1,1 | 1,1,1 | |||
| NB (n = 5) | 0,0,0,1,1 | 0,0,0,1,1 | |||
| DFSP (n = 4) | 0,0,0,0 | 0,0,0,0 | |||
| Mes CS (n = 1) | 0 | 0 | P-value | <0.05 | >0.05 |
MLPS, myxoid liposarcoma; CCS, clear cell sarcoma; DSRCT, desmoplastic round cell tumour; RMS, rhabdomyosarcoma; Sections show: synovial sarcoma; MCS, myxoid chondrosarcoma; EWS/PNET, Ewing sarcoma/Primitive neuroectodermal tumour; NB, neuroblastoma; DFSP, dermatofibrosarcoma protuberans; Mes CS, mesenchymal chondrosarcoma.
PIM-1 staining pattern and IHC analysis in full sections – adipocytic vs. non-adipocytic tumours
All the 26 adipocytic tumours were positive for PIM-1, exhibiting at least moderate staining intensity while only 10 of 23 non-adipocytic tumours expressed PIM-1. This difference was statistically significant in terms of staining intensity and IHC score (P < 0.001). The overall median staining intensity for the adipocytic tumours was 3 (range 2–3) compared with 1 in non-adipocytic tumours (range 0–1), which was statistically significant (P < 0.001). Similarly, the extent of staining was significantly higher in adipocytic tumours with a median of 100% (range 10–100%) compared with the median of 5% (range 0–80%) in non-adipocytic tumours (Table 2). In addition, the overall IHC score was also significantly higher in adipocytic tumours compared with their non-adipocytic counterparts (median of 300 vs. 5, P < 0.001).
Table 2.
Expression of PIM-1 in full sections
| Tumour type | Median intensity | Median % of cell stained with PIM-1 | Median IHC score | ||
|---|---|---|---|---|---|
| Adipocytic tumours (n = 26) | Benign (n = 17) | Lipoma (n = 5) | 3 | 100 | 300 |
| Spindle cell lipoma (n = 5) | 3 | 100 | 200 | ||
| Pleomorphic lipoma (n = 1) | 2 | 100 | 200 | ||
| Angiolipoma (n = 4) | 3 | 100 | 300 | ||
| Hibernoma (n = 1) | 3 | 100 | 300 | ||
| Lipoblastoma (n = 1) | 3 | 100 | 300 | ||
| Malignant (n = 9) | Myxoid liposarcoma (n = 4) | 3 | 45 | 135 | |
| Well-differentiated liposarcoma (n = 5) | 3 | 100 | 300 | ||
| Non-adipocytic tumours (n = 23) | Myxoma (n = 8) | 0 | 0 | 0 | |
| Myxoid chondrosarcoma (n = 3) | 0 | 0 | 0 | ||
| Chordoma (n = 6) | 1 | 24 | 24 | ||
| Malignant fibrous histiocytoma (n = 6) | 1 | 6.5 | 6.5 | ||
We isolated the myxoid tumours as a separate group, owing to the frequent diagnostic difficulties arising from this subset of soft tissue tumours. Our analysis showed that MLPS demonstrated significantly higher staining intensity as well as IHC scores than the other myxoid tumours as a whole group (myxoid chondrosarcoma, myxoma and chordoma) (Table 3). Both benign and malignant adipocytic tumours showed strong expression of PIM-1 (Figures 2 and 3), with a distinct pattern of expression that differed from that seen in non-adipocytic tumours. Adipocytic tumours showed PIM-1 positivity in a cytoplasmic vacuolar pattern, outlining small to large cytoplasmic lipid vacuoles strongly (Figures 1c, 2b,d,f, 3b and 4b). This pattern was not seen in any of the non-lipocytic tumours, which were largely negative for the protein.
Table 3.
PIM-1 staining intensity in full sections of myxoid tumours
| PIM-1 expression | Adipocytic Myxoid liposarcoma (n = 4) | Non-adipocytic MCS, myxoma, chordoma (n = 17) | P-value* |
|---|---|---|---|
| Mean intensity scores | 3 | 0.41 | <0.001 |
| Mean % cell stained | 42.5 | 13.12 | 0.040 |
| Mean IHC score | 127.50 | 13.12 | 0.004 |
IHC, immunohistochemistry.
Mann–Whitney U-test, P-value <0.05 is considered statistically significant.
In benign adipocytic tumours with a significant spindle cell or non-lipocytic component, the staining was largely limited to the adipocytes or to cells that contained small cytoplasmic vacuoles (Figure 2d,f). In PIM-1 positive non-adipocytic tumours, the staining was of low intensity (intensity score 1) and pale, diffuse and cytoplasmic or finely granular (Figures 3e and 4d). The non-adipocytic tumours that showed the most consistent expression were the chordomas and the malignant fibrous histiocytomas. While a significant proportion of chordomas expressed the marker, the staining intensity was low in all the five cases, although the extent was diffuse (Figure 4d).
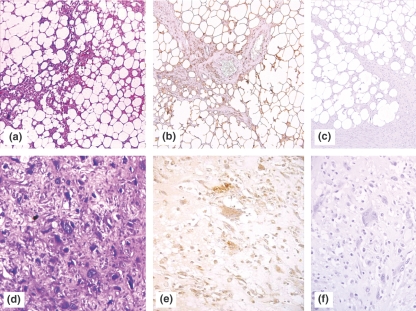
Figure 3.
PIM-1 expression in malignant adipocytic and non-adipocytic tumours. (a–b) Well differentiated liposarcoma (H&E and PIM-1 showing strong vacuolar staining, (c) Negative control without application of antibody (original magnification ×100), (d–e) Malignant fibrous histiocytoma (H&E and PIM-1 showing a cytoplasmic ‘blush’, (f) Negative control without application of PIM-1 antibody, showing no discernible staining (original magnification ×200).
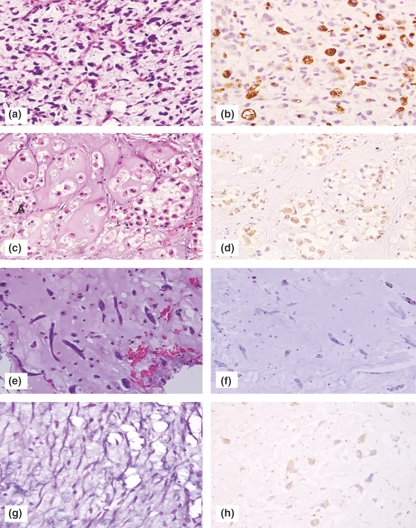
Figure 4.
PIM-1 expression in myxoid adipocytic and non-adipocytic tumours. (a–b) Myxoid liposarcoma (H&E staining and PIM-1 showing strong vacuolar cytoplasmic staining, original magnification ×100), (c–d) Chordoma (H&E and PIM-1 showing only pale diffuse cytoplasmic staining, original magnification ×100), (e–f) Soft tissue myxoma (H&E and PIM-1 showing no appreciable staining, original magnification ×100), (g-h) Cardiac myxoma (H&E and very light PIM-1 staining, original magnification ×100).
In malignant fibrous histiocytomas, expression was preferential in the large, markedly pleomorphic tumour cells which showed a diffuse cytoplasmic ‘blush’ (Figure 3e), even in the vacuolated tumour cells resembling lipoblasts. This was in contrast to the distinct vacuolar pattern of strong cytoplasmic staining present in adipocytic tumours. None of the non-adipocytic tumours exhibited this vacuolar staining pattern. A trend was noticed among the myxomas, where the cardiac tumours showed focal light positivity for PIM-1 (Figure 4h) while the soft tissue counterparts (juxta-articular and intramuscular) exhibited no significant reactivity. However, both the median staining intensity and extent of these tumours, when analysed as a group, was 0.
Quantitative real-time RT-PCR analysis
Gene expression of PIM-1 in adipocytic tumours
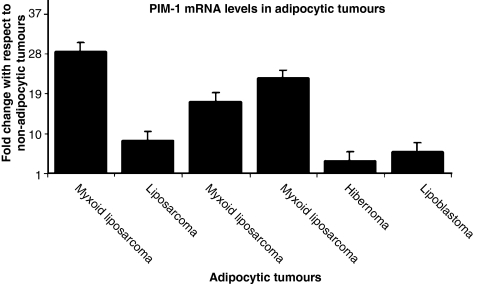
Adipocytic tumours including hibernomas and lipoblastomas and myxoid liposarcomas that showed increased immunohistochemical expression of PIM-1 (strong intensity +3) were analysed quantitatively for PIM-1 gene expression. Both the benign and malignant adipocytic tumours showed PIM-1 upregulation (≥3.0 fold change) compared with the non-adipocytic tumours (Figure 5). PIM-1 expression was also higher (>4 fold change) in myxoid liposarcomas compared with the other benign adipocytic tumours. The results showed a good correlation with the relative levels of PIM-1 expression as determined using immunohistochemistry.
Figure 5.
PIM-1 gene expression in adipocytic tumours.
Discussion
The differential diagnosis of soft tissue tumours is an ongoing challenge in the practice of modern day pathology, where the prognostication and prediction of tumour response to chemotherapy is of utmost importance in patient management. For the surgical pathologist, the challenge lies in differentiating between similar tumours which can show considerable overlap in clinical presentation, epidemiology and morphological features. Myxoid tumours comprise a heterogeneous group of entities ranging from low grade, locally aggressive tumours (e.g. myxomas, chordomas) to intermediately aggressive malignant tumours (e.g. myxoid liposarcoma, myxoid malignant fibrous histiocytoma) to high grade, aggressive tumours (e.g. myxoid chondrosarcoma, high-grade liposarcoma and pleomorphic malignant fibrous histiocytoma). In the diagnostic workup of tumours with vacuolated cells, a useful test is the Oil Red O stain; however, in practice this is of limited utility as it requires fresh tissue which might not always be readily available and has inherent interpretative problems. To date, there has been a paucity of useful immunostains that are able to select for adipocytic differentiation, compounding the difficulty in the diagnosis of these tumours.
In recent years, a good number of soft tissue tumours have been found to be associated with various cytogenetic aberrations which might be useful in the diagnostic workup; however, access to these methods is limited to several specialized centres and the routine diagnostic pathology laboratory might not have immediate access to specific molecular diagnostic services (Antonescu 2006). Hence, ancillary IHC studies still play a major role in the definitive diagnosis of these tumours. Although other markers have been applied in the diagnosis of lipocytic tumours, these have had limited usefulness, e.g. S100, which is positive in adipocytic tumours but also stains positively in chordoma, chondrosarcoma and liposarcoma (Weiss & Goldblum 2007). Hence, in the field of soft tissue pathology, a suitably specific, reliable marker of adipocytes is still being sought.
PIM-1 is expressed in many cancer types including prostatic carcinoma, pancreatic adenocarcinoma and hamatolymphoid malignancies (Shah et al. 2008). Its hypermutational activity and chromosomal translocation in B-cell non-Hodgkin lymphoma has been shown to be an independent negative prognostic factor suggesting it to be a major contributor in lymphomagenesis (Pasqualucci et al. 2001). PIM-1 is involved in the proliferation of BCR/ABL dependent leukaemogenesis by associating with the anti-apoptotic protein A. It phosphorylates Runx-1 in t (12;21)-positive acute lymphoblastic lymphomas (ALL) ALL. It also inhibits cell death and regulates tumourigenesis by phosphorylating specific target proteins such as BAD (Yan et al. 2003; Aho et al. 2004), HP-1 (Koike et al. 2000), cdc 25A phosphatase (Mochizuki et al. 1999), SOCS-1 (Chen et al. 2002) as well as inactivating cell cycle inhibitors such as p21Waf and C-TAK1 (Wang et al. 2002; Bachmann et al. 2004). In addition, PIM-1 is also a target gene of VEGF and its tyrosine kinase receptor, the foetal liver kinase (Flk-1) that induces vasculogenesis and angiogenesis in differentiating endothelial and mural cells suggesting it to be a new candidate gene for the inhibition of angiogenesis in adults (Zippo et al. 2004).
With respect to immunohistochemical studies, the PIM-1 antibody is a relatively new immunostain which is still undergoing studies to fully understand its place in elucidating the molecular events that give rise to certain neoplasms. Translating these findings into a practical use for this antibody requires detailed, reproducible studies in which its use in the diagnosis of cancer and precancer can be demonstrated.
Prior to this, other studies employing the use of PIM-1 have not demonstrated appreciable reactivity in adipose tissue (Gapter et al. 2006). Gapter et al. described PIM-1 staining in murine mammary tissue; however, in their photomicrographs there was no discernible reactivity in the surrounding adipose stroma. This could be due to several reasons, including a different antibody source (Santa Cruz, CA as documented by Gapter et al.), different methods of tissue fixation (Bouin solution compared wih 4% buffered formalin as used in our laboratory) and differing methods of antigen retrieval (boiling in 0.01 mmol/l sodium citrate in contrast to our method of pressure cooking in commercially prepared pH6 antigen retrieval solution). Using this specific method of immunostaining, our findings of strong, reproducible cytoplasmic vacuolar staining for PIM-1 exclusively in adipocytic tumours indicate that this is a marker that can potentially be used in immunophenotypic panels in the diagnostic workup of soft tissue tumours which have myxoid areas and vacuolated cells.
In the case of myxoid liposarcomas, the diagnostic difficulty lies sometimes in the scarcity of typical lipoblasts, which are universally sought for in the diagnosis of malignant lipocytic tumours. In the cases we examined, PIM-1 staining highlighted isolated cells in these tumours which, on routine H&E staining, were not readily apparent as lipoblasts. On immunostaining, these cells exhibited tiny cytoplasmic vacuoles that showed strong positivity for the protein (Figures 1–4). Thus, PIM-1 appears to be very useful in separating myxoid liposarcomas from other myxoid tumours e.g. myxoma, chordoma, myxoid chrondrosarcoma, which commonly also exhibit vacuolated cells. Even though there was some degree of light cytoplasmic staining in these vacuolated cells (Figures 3e and 4d), there was a complete absence of the specific vacuolar pattern of staining seen in tumours of adipocytic origin. The observation that PIM-1 stained cardiac myxomas favourably over their soft tissue counterparts also suggests a degree of divergent histogenesis/differentiation between these morphologically similar tumour types. Further studies incorporating larger numbers of cases might prove helpful in elucidating these potential differences. Several authors have constructed tissue microarrays for gene expression profiles and immunohistochemical testing in sarcomas; however, to date none have specifically investigated the expression of PIM-1 in this set of tumours (Baird et al. 2005; West & van de Rijn 2006). We thus propose that PIM-1, with its distinct, unique vacuolar staining pattern, could potentially fulfil these criteria.
According to our findings, non-neoplastic adipose tissue, benign and malignant adipocytic tumours did not show any significant difference in the staining intensity or distribution of PIM-1. Hence we postulate that the expression of this protein in adipose tissue is a marker of adipocytic differentiation rather than tumour progression. Thus the protein is unlikely to serve as a pivotal oncoprotein in the development and progression of adipocytic tumours. However, the increased level of expression in myxoid liposarcomas as compared with benign adipocytic tumours warrants further investigation, as this might point to a PIM-1-related pathway for malignant transformation in at least a subset of adipocytic tumours. Again, further gene expression studies on a more varied group of lipocytic tumours might shed light on the possibility of a contributory mechanism by which PIM-1 overexpression might be related to the evolution of malignant adipocytic tumours. Nevertheless, the potential contribution of this increasingly important marker to the armamentarium of diagnostic antibodies that can be applied to soft tissue tumours is a significant one. Thus, more studies involving larger numbers and a broader range of entities need to be performed to further characterize this apparently novel and unique vacuolar cytoplasmic pattern of PIM-1 staining in adipocytic tumours.
Acknowledgments
Funding support to M. Salto-Tellez was provided by Singapore Cancer Syndicate, Agency for Science, Technology and Research (MN-005 and MN-077) and to M.E. Nga by National Healthcare Group (NHG-SIG/06028), Singapore.
References
- Aho TL, Sandholm J, Peltola KJ, et al. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Amson R, Sigaux F, Przedborski S, et al. The human proto-oncogene product p33 pim is expressed during fetal hematopoiesis and in diverse leukemias. Proc. Natl. Acad. Sci. USA. 1989;86:8857–8861. doi: 10.1073/pnas.86.22.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu CR. The role of genetic testing in soft tissue sarcoma. Histopathology. 2006;48:13–21. doi: 10.1111/j.1365-2559.2005.02285.x. [DOI] [PubMed] [Google Scholar]
- Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of p53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinico-pathologic study of 82 cases. Clin. Cancer Res. 2001;7:3977–3987. [PubMed] [Google Scholar]
- Bachmann M, Hennemann H, Xing PX, Hoffmann I, Moroy T. The oncogenic serine/threonine kinase Pim-1 phosphorylates and inhibits the activity of Cdc25C-associated kinase 1 (CTAK1): a novel role for Pim-1 at the G2/M cell cycle checkpoint. J. Biol. Chem. 2004;279:48319–48328. doi: 10.1074/jbc.M404440200. [DOI] [PubMed] [Google Scholar]
- Baird K, Davis S, Antonescu CR, et al. Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res. 2005;65:9226–9235. doi: 10.1158/0008-5472.CAN-05-1699. [DOI] [PubMed] [Google Scholar]
- Berns A, Breuer M, Verbeek S, et al. Transgenic mice as a means to study synergism between oncogenes. Int. J. Cancer. 1989;4:22–25. doi: 10.1002/ijc.2910440706. [DOI] [PubMed] [Google Scholar]
- Borden EC, Baker LH, Bell RS, et al. Soft tissue sarcomas of the adults: state of the translational science. Clin. Cancer Res. 2003;9:1941–1956. [PubMed] [Google Scholar]
- Chen XP, Losman JA, Cowan S, et al. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc. Natl. Acad. Sci. USA. 2002;99:2175–2180. doi: 10.1073/pnas.042035699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibull TL, Jones TD, Li L, et al. Overexpression of PIM-1 during progression of prostatic adenocarcinoma. J. Clin. Pathol. 2006;59:285–288. doi: 10.1136/jcp.2005.027672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coindre JM. Immunohistochemistry in the diagnosis of soft tissue tumours. Histopathology. 2003;43:1–16. doi: 10.1046/j.1365-2559.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J. Clin. Oncol. 1996;14:869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- Coindre JM, Pelmus M, Hostein I, et al. Should molecular testing be required for diagnosing synovial sarcoma: a prospective study of 204 cases. Cancer. 2003;98:2700–2707. doi: 10.1002/cncr.11840. [DOI] [PubMed] [Google Scholar]
- Cormier JN, Pollock RE. Soft tissue tumours. CA Cancer J. Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- Cuypers HT, Selten G, Quint W, et al. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1986;37:141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- Dreyfus F, Sola B, Fichelson S, et al. Rearrangements of the Pim-1, c-myc and p53 genes in Friend helper virus-induced mouse erythroleukemias. Leukemia. 1990;4:590–594. [PubMed] [Google Scholar]
- Folpe AL, Goldblum JR, Rubin BP, et al. Morphologic and immunophenotypic diversity in Ewing family tumours: a study of 66 genetically confirmed cases. Am. J. Surg. Pathol. 2005;29:1025–1033. [PubMed] [Google Scholar]
- Gapter LA, Magnuson NS, Ng KY, et al. Pim-1 kinase expression during murine mammary development. Biochem. Biophys. Res. Commun. 2006;345:989–997. doi: 10.1016/j.bbrc.2006.04.110. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, de Alava E, Ladanyi M, et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcoma. J. Clin. Oncol. 1999;17:1809–1814. doi: 10.1200/JCO.1999.17.6.1809. [DOI] [PubMed] [Google Scholar]
- Goffman T, Tochner Z, Glatstein E. Primary treatment of large and massive adult sarcomas with iododeoxyuridine and aggressive hyperfractionated irradiation. Cancer. 1991;67:572–576. doi: 10.1002/1097-0142(19910201)67:3<572::aid-cncr2820670308>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Womer RB, Sorensen PH, et al. Common and variant gene fusions predict distinct clinical phenotypes in rhabdomyosarcoma. J. Clin. Oncol. 1997;15:1831–1836. doi: 10.1200/JCO.1997.15.5.1831. [DOI] [PubMed] [Google Scholar]
- Koike N, Maita H, Taira T, Ariga H, Iguchi-Ariga SM. Identification of heterochromatin protein 1 (HP1) as a phosphorylation target by Pim-1 kinase and the effect of phosphorylation on the transcriptional repression function of HP1 (1) FEBS Lett. 2000;467:17–21. doi: 10.1016/s0014-5793(00)01105-4. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mandiyan V, Suzuki Y, et al. Crystal structures of proto-oncogene kinase Pim1: a target of aberrant somatic hypermutations in diffuse large cell lymphoma. J. Mol. Biol. 2005;348:183–193. doi: 10.1016/j.jmb.2005.02.039. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M, Verbeek S, Krimpenfort P, et al. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc murine leukemia virus-induced tumours. Cell. 1989;56:673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1- mediated activation of the c-Myc signaling pathway. J. Biol. Chem. 1999;274:18659–18666. doi: 10.1074/jbc.274.26.18659. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Neumeister P, Goossens T, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- Salto-Tellez M, Lee SC, Chiu LL, et al. Microsatellite instability in colorectal cancer – considerations for molecular diagnosis and high-throughput screening of archival tissues. Clin. Chem. 2004;50:1082–1086. doi: 10.1373/clinchem.2003.030700. [DOI] [PubMed] [Google Scholar]
- Salto-Tellez M, Nga ME, Han HC, et al. Molecular information arrays characterizes the clinical significance of a VEGF-A protein expression signature in gastrointestinal stromal tumours. Br. J. Cancer. 2007;98:776–782. doi: 10.1038/sj.bjc.6603551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumours: synovial sarcoma. Cancer Genet. Cytogenet. 2002;133:1–23. doi: 10.1016/s0165-4608(01)00626-4. [DOI] [PubMed] [Google Scholar]
- Shah N, Pang B, Yeoh KG, et al. Potential roles for the Pim-1 kinase in human cancer – a molecular and therapeutic appraisal. Eur. J. Cancer. 2008;44:2144–2151. doi: 10.1016/j.ejca.2008.06.044. [DOI] [PubMed] [Google Scholar]
- Verbeek S, van Lohuizen M, van der Valk M, et al. Mice bearing the Eμ-myc and Eμ-pim-1 transgenes develop pre-B-cell leukemia prenatally. Mol. Cell. Biol. 1991;11:1176–1179. doi: 10.1128/mcb.11.2.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bhattacharya N, Weaver M, et al. A serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumourigenesis. J. Vet. Sci. 2001;2:167–179. [PubMed] [Google Scholar]
- Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim. Biophys. Acta. 2002;1593:45–55. doi: 10.1016/s0167-4889(02)00347-6. [DOI] [PubMed] [Google Scholar]
- Weiss SW, Goldblum JR. Immunohistochemistry for analysis of soft tissue tumours. In: Folpe AL, Gown AM, editors. Weiss Enzinger and Weiss’s Soft Tissue Tumours. 5th edn. Philadelphia, PA: Mosby Elsevier; 2007. pp. 129–174. [Google Scholar]
- West RB, van de Rijn M. The role of microarray technologies in the study of soft tissue tumours. Histopathology. 2006;48:22–31. doi: 10.1111/j.1365-2559.2005.02286.x. [DOI] [PubMed] [Google Scholar]
- Xia SJ, Barr FG. Chromosome translocations in sarcomas and the emergence of oncogenic transcription factors. Eur. J. Cancer. 2005;41:2513–2527. doi: 10.1016/j.ejca.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Xie Y, Xu K, Dai B, et al. The 44 kDa PIM-1 kinase directly interacts with tyrosine kinase EtklBMX and protects human prostate cancer cells from apoptosis induced by chemotherapeutic drugs. Oncogene. 2006;25:70–78. doi: 10.1038/sj.onc.1209058. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang T, Tang H, et al. Overexpression of Pim-1 is a potential biomarker in prostate carcinoma. J. Surg. Oncol. 2005;92:326–330. doi: 10.1002/jso.20325. [DOI] [PubMed] [Google Scholar]
- Yan B, Zemskova M, Holder S, et al. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J. Biol. Chem. 2003;278:45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S. Identification of Flk-1 target genes in vasculogenesis: PIM1 is required for endothelial and mural cell differentiation in vitro. Blood. 2004;103:4536–4544. doi: 10.1182/blood-2003-11-3827. [DOI] [PubMed] [Google Scholar]