Abstract
Elevated levels of survivin, telomerase catalytic subunit (TERT), integrin-linked kinase (ILK), cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS) and the regulatory factors c-MYB and Tcf-4 are often found in human cancers including colorectal cancer (CRC) and have been implicated in the development and progression of tumorigenesis. The aim of this study was to determine the expression of these genes in mouse models of sporadic and colitis-associated CRC. To address these issues, we used qRT-PCR approach to determine changes in gene expression patterns of neoplastic cells (high-grade dysplasia/intramucosal carcinoma) and surrounding normal epithelial cells in A/J and ICR mouse strains using laser microdissection. Both strains were injected with azoxymethane and ICR mice were also given drinking water that contained 2% dextran sodium sulphate. In both sporadic (A/J mice) and colitis-associated (ICR mice) models of CRC, the levels of TERT mRNA, COX-2 mRNA and Tcf-4 mRNA were higher in neoplastic cells than in surrounding normal epithelial cells. In contrast, survivin mRNA was upregulated only in neoplastic cells from A/J mice and ILK mRNA was upregulated only in neoplastic cells from ICR mice. However, the expression of iNOS mRNA was similar in normal and neoplastic cells in both models and c-MYB mRNA was actually downregulated in neoplastic cells compared with normal cells in both models. These findings suggest that the genetic background and/or the molecular mechanisms of tumorigenesis associated with genotoxic insults and colonic inflammation influence the gene expression of mTERT, COX-2, Tcf-4, c-MYB, ILK and survivin in colon epithelial neoplasia.
Keywords: colon cancer, gene expression, mouse
Colorectal cancer (CRC) is one of the major causes of cancer death throughout the world and is also one of the serious complications of chronic inflammatory bowel diseases such as ulcerative colitis. Colon tumorigenesis depends on the accumulation of alterations in a number of cancer-related genes, and several lines of evidence suggest that these changes affect the expression of many genes involved in proliferation, cell cycle, apoptosis and angiogenesis (Itzkowitz & Yio 2004; Reya & Clevers 2005; Hussain & Harris 2007). Recent investigations of the molecular biology of various human cancers, including malignancies of the gastrointestinal tract, have revealed changes in many genes and their products, including the Ras and Myc families of oncogenes, members of the inhibitor of apoptosis protein (IAP) family, elements of the Wnt/β-catenin pathway, metalloproteinases, telomerase and many others. In particular, the expression of transcription factor c-MYB is increased substantially in colon tumours (Ramsay et al. 1992), and, conversely, its downregulation is associated with colon cell differentiation and apoptosis (Thompson et al. 1998). Similarly, survivin, a member of the IAP family, was shown to be increased in colon cancer relative to adjacent normal mucosa (Endo et al. 2004), and the expression of integrin-linked kinase (ILK), a key regulator of cellular response to integrin and growth-factor signalling (Dedhar et al. 1999), correlates positively with colonic tumour progression (Marotta et al. 2003; Bravou et al. 2006). Some data also indicate that β-catenin and Tcf-4, factors involved in the Wnt signalling pathway, play a crucial role not only in many developmental processes in the intestinal epithelium but also in colorectal carcinogenesis (Barker et al. 2000). Similarly, human CRC shows increased telomerase activity, increased the mRNA abundance of the telomerase catalytic subunit (TERT) (Engelhardt et al. 1997; Yoshida et al. 1997) and expression of various inflammation-regulated genes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), which are well established pathogenic factors in colon carcinogenesis (Eberhart et al. 1994; Sano et al. 1995; Ambs et al. 1998).
Although the expression of the above-mentioned protumorigenic markers has been studied thoroughly in human colon cancer, there are only a few reports about their expression in rodent models of CRC (Narisawa et al. 1999; Kishimoto et al. 2002; Tanaka et al. 2003; Mori et al. 2007). In addition, the presence of stroma cells in cancer tissues might obscure their expression profiles. In the present study, we therefore employed laser microdissection and a quantitative RT-PCR approach to determine tissue-specific alteration in the proinflammatory genes (iNOS, COX-2) and proproliferative and antiapoptotic genes (c-MYB, Tcf-4, ILK, mTERT, survivin) in mouse models of sporadic (Bissahoyo et al. 2005) and colitis-associated tumorigenesis (Tanaka et al. 2003) that recapitulate many aspects of human CRC.
Materials and methods
Animals and treatment
The study was performed on two mouse models of CRC – sporadic and colitis-associated CRC. Sporadic CRC was induced in 20-week-old male A/J mice (Jackson Laboratories, Bar Harbor, ME, USA; weight: 25 g) by giving intraperitoneal (i.p.) injections with azoxymethane (AOM; Sigma, St. Louis, MO, USA) dissolved in saline at a dose of 10 mg AOM per kg body weight once a week for 6 weeks (Bissahoyo et al. 2005). Colitis-associated CRC was induced in 20-week-old male ICR mice (Charles River, Sultfeld, Germany; weight: 35 g) by a single i.p. injection of AOM (10 mg/kg) followed 1 week later by consumption of 2% dextran sodium sulphate (DSS; MP Biomedicals, Irvine, CA, USA) in water for 7 days and distilled water for the following 14 days. This DSS administration cycle was repeated six times (Tanaka et al. 2003; Bissahoyo et al. 2005). Eight animals were included in each experimental group. All mice were killed 6 months after the first i.p. administration of AOM, and the colons were flushed with saline, split open longitudinally and macroscopically inspected for the presence of tumours.
Tissue preparation for histopathology and laser microdissection
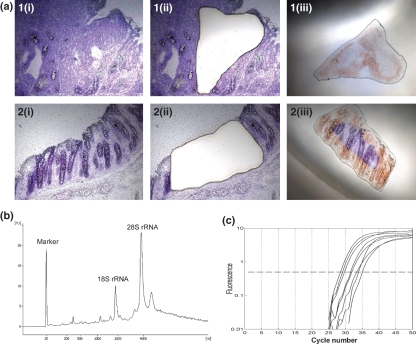
Mouse colon samples were embedded in OCT medium (Sakura Finetek, Torrance, CA, USA), snap-frozen in 2-methylbutane and the blocks were stored at −80 °C until required. All samples were cut in a cryostat (Leica CM 3000) at −23 °C. For histopathology, 4 μm serial frozen sections were fixed in 10% buffered formaldehyde; the slides were stained with haematoxylin and eosin and histopatological evaluation was carried out using a Nikon Eclipse E400 microscope (Nikon Instruments, Amstelveen, the Netherlands). For laser microdissection of neoplastic cells and the surrounding normal-looking epithelial cells, 8 μm serial sections were cut, transferred to Leica polyethylene-naphtalate membrane slides and immediately frozen on dry ice. After fixation in 95% ethanol, the sections were stained with cresyl violet acetate, dehydrated using a graded series of ethanol washes, treated with xylene and air dried according to the LCM Staining Kit protocol (Applied Biosystems/Ambion, Austin, TX, USA). Immediately after staining, tissue sections were microdissected using the Leica LMD6000 Laser Microdissection System (Leica Microsystems, Wetzlar, Germany). The foci of high-grade dysplasia/intramucosal carcinoma were microdissected from polyps greater than 1 mm in diameter and the efficiency of microdissection was evaluated by examining the tissue before and after harvesting the specimens (Figure 1a). The microcentrifuge tubes with collected material were vortexed for 30 s in lysis buffer and stored at −80 °C until RNA isolation.
Figure 1.
Laser microdissection and quantitative real-time PCR of neoplastic tissue. (a) Microdissection of cresyl violet stained sections of neoplastic (1) and normal colonic mucosa (2) before microdissection (1i, 2i), after microdissection (1ii, 2ii) and dissected tissue areas in lysis buffer (1iii, 2iii); original magnification: (×100). (b) Electropherogram of RNA isolated from a section stained with cresyl violet acetate (RIN = 8.0). The y-axis represents fluorescence unit (FU) and the x-axis represents the nucleotide length of the RNA (nt). The peaks of the 18S and 28S rRNA fragments are clearly visible. (c) Typical TaqMan qPCR amplification plots for (from left to right) c-MYB, Tcf-4, survivin, TBP, iNOS, ILK, TERT, COX-2. Ct was determined as a cycle number at which amplification curve intersected the threshold level (0.5).
RNA isolation and quality control
Total RNA was extracted from microdissected tissue areas from three serial cryosections using the RNeasy Micro Kit (Qiagen, Hilden, Germany) including poly-A carrier. To remove the traces of genomic DNA, on-column DNase I treatment was performed at room temperature for 15 min and the isolated RNA was eluted with 20 μl of RNase-free water. RNA quality was assessed in each sample using the RNA 6000 Pico LabChip Kit on the Agilent 2100 Bioanalyser (Agilent Technologies, Waldbronn, Germany). The samples were shown to reach RNA Integrity Number (RIN) 7.2 ± 0.2 (mean ± SEM). An example of Agilent analysis is shown in Figure. 1b.
cDNA synthesis and quantitative PCR
First-strand cDNA was synthesized at 37 °C for 2 h using Sensiscript Reverse Transcriptase (Qiagen), 10× Buffer RT, 5 mM dNTP Mix, 3 μg/μl random primers and 10 U/μl RNase-OUT (both from Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions in a 20 μl total reaction volume and stored at −20 °C.
Quantitative PCR was performed on the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using TaqMan Gene Expression Master Mix and Pre-Developed TaqMan Assays specific to mouse TERT (cat.no. Mm01352136_m1), survivin (cat.no. Mm00599749_m1), ILK (cat.no. Mm00439671_g1), COX-2 (cat.no. Mm00478374_m1), iNOS (cat.no. Mm00440485_m1), c-MYB (cat.no. Mm00501741_m1), Tcf-4 (cat.no. Mm00443198_m1), and for the housekeeping gene TATA box-binding protein, TBP (cat.no. Mm00446973_m1). PCR was performed with TaqMan MGB probes labelled with FAM reporter dye in a final reaction volume of 20 μl. The amplification conditions consisted of uracil-DNA glycosylase (UDG) incubation at 50 °C for 2 min and initial DNA polymerase enzyme activation at 95 °C for 10 min, followed by 50 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. An example of the amplification plot is given in Figure 1c. The probes were validated to ensure the amplification efficiency and linearity in preliminary experiments using colon-specific RNA from tumour and surrounding tissue for each quantitative PCR assay individually using standard curve method.
Expression values were obtained from Ct numbers detected by the Applied Biosystems analysis software. The target gene levels were expressed as the N-fold difference in the target gene expression relative to TBP expression (ΔCt), where ΔCt was determined in each sample by subtracting the average Ct value of the target gene from the average Ct value of the TBP; in addition, the relative gene expression was calculated as 2−ΔCt.
Statistical analyses
Data are presented as the median, the 25th and 75th percentile and the minimum–maximum range. Comparison of mRNA levels between cancer cells and surrounding normal epithelial cells was made with the Mann–Whitney test using statistica 6.1. software (StatSoft Inc., Tulsa, OK, USA). A P-value smaller than 0.05 was considered statistically significant.
Results
Mouse model of colitis-associated carcinogenesis
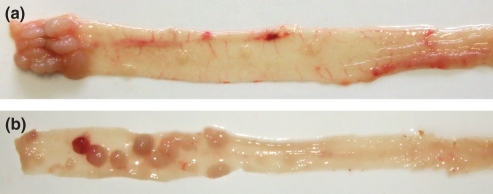
Macroscopically, colonic mucosa was smooth with scattered flat prominences that microscopically corresponded with focal hyperplasia of colonic lymphatic tissue. Multiple nodular to polypoid tumours were evident in the distal 1.5 cm of mouse colon in all treated animals (Figure 2a). Microscopically, these tumours displayed characteristics of intraepithelial neoplasias with changes ranging from low-grade dysplasia, usually located at the periphery of polyps, next to non-dysplastic colonic epithelium, to high-grade dysplasia/intramucosal carcinoma, infiltrating to the submucosa and predominantly forming the central parts of the tumours (Figure 3a–d). The quantitative changes in mRNA expression of proinflammatory, proproliferative and antiapoptotic genes during tumorigenesis associated with colitis were assessed in 23 samples of microdissected foci of high-grade dysplasia/intramucosal carcinoma and 18 samples of adjacent normal epithelial cells isolated from ICR mice.
Figure 2.
Typical macroscopic features of tumours in colon of ICR (a) and A/J (b) mice 6-month after the first i.p. administration of AOM. Numerous polypoid tumours developed in all treated mice with distinct localization patterns; they appeared randomly dispersed in the distal half of the colon of A/J mice, but were restricted to the most distal 1.5 cm segment in ICR mice.
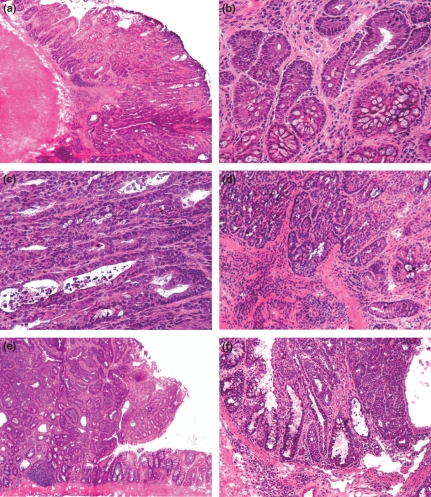
Figure 3.
Representative histopathology of neoplastic changes in the ICR mouse model of colitis-associated carcinogenesis (a–d) and the A/J mouse model of sporadic colon carcinogenesis (e,f). In both models, the epithelial neoplasia exhibited morphological changes ranging from low-grade (b) to high-grade dysplasia (c) and intramucosal carcinoma (f) predominantly forming the central parts of the tumours. In some tumours of the ICR model, the invasive growth was found initially (d). Haematoxylin and eosin stained cryosections, original magnification: a, e, f (×40), d (×100), b,c (×200).
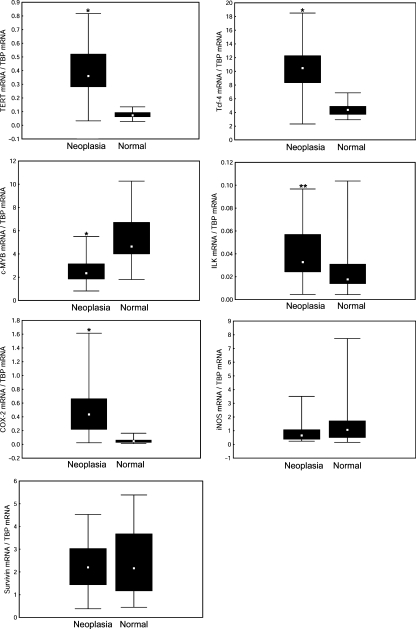
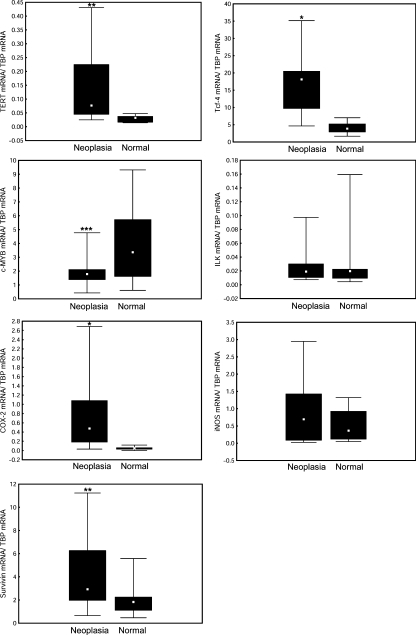
As shown in Figure 4, all microdissected samples revealed detectable transcripts not only for COX-2 and iNOS but also for survivin, ILK, mTERT, Tcf-4 and c-MYB. Compared with adjacent normal epithelial cells, the neoplastic cells revealed significantly increased mRNA levels for TERT, COX-2, Tcf-4 and ILK. Median expression of TERT mRNA was increased 5.1-fold and COX-2 mRNA 9.1-fold, whereas Tcf-4 mRNA and ILK mRNA were 2.4-fold and 1.9-fold higher respectively. In contrast, the qRT-PCR analysis of c-MYB revealed a significantly decreased expression of this gene (1.9-fold). No significant changes were observed in the levels of iNOS mRNA or survivin mRNA. These observations suggest that neoplastic transformation is associated with changed gene expression of COX-2, but not iNOS, even though upregulation of iNOS was observed in colonic tumours of ICR mice treated with DSS and AOM using both immunohistochemical and Western blot analysis (Tanaka et al. 2003; Kim et al. 2008).
Figure 4.
Gene expression in intraepithelial neoplasia (high-grade dysplasia/intramucosal carcinoma cells) and adjacent normal epithelium in the colons of ICR mice that received one injection of azoxymethane and six cycles of dextran sodium sulphate consumption. Expression levels of inducible NO-synthase (iNOS), cyclooxygenase-2 (COX-2), integrin-linked kinase (ILK), telomerase catalytic subunit (TERT), survivin and the transcription factors c-MYB and Tcf-4 were evaluated in neoplastic cells and in surrounding normal epithelial cells by laser microdissection and qRT-PCR. Median values, 25th and 75th percentile and min.–max. ranges are denoted by small squares, boxes and bars respectively. Significant differences between neoplastic cells and surrounding normal epithelial cells: *P < 0.001; **P < 0.05.
To determine whether the expression of the iNOS gene is increased permanently in colonic mucosa of this mouse model, i.e. DSS exerts a sufficient promoting effect to maintain this upregulation during the remission phase of colitis, we compared the levels of iNOS mRNA and COX-2 mRNA in healthy untreated ICR mice and in the animals treated with DSS and AOM and found no significant changes in expression of either gene. The expression of iNOS mRNA [median (25–75% percentile)] in healthy controls was 1.36 (0.74–2.15) and in epithelial cells surrounding the tumour 1.06 (0.50–1.73); similarly, the expression of COX-2 mRNA was measured to be 0.06 (0.05–0.07) and 0.05 (0.03–0.06).
Mouse model of sporadic colon carcinogenesis
In the colon of A/J mice, repeated administration of AOM induced tumours that, in contrast to the colitis-associated model, were randomly dispersed in the distal half of the colon (Figure 2b). Microscopically, these tumours displayed structural characteristics of polypoid tubular adenomas with either low- or high-grade dysplasia. In some polyps, foci of intramucosal carcinoma were found (Figure 3e,f).
The quantitative changes in mRNA expression of selected genes during AOM induced tumorigenesis were assessed in 21 samples of microdissected foci of high-grade dysplasia/intramucosal carcinoma cells and 19 samples of adjacent normal epithelial cells. Similar to the colitis-associated model of tumorigenesis in ICR mice, the neoplastic cells in A/J mice showed significantly elevated transcript levels for TERT (median increased 2.4-fold), COX-2 (9.7-fold) and Tcf-4 (4.7-fold) and a decreased level of c-MYB mRNA (1.9-fold) (Figure 5). Expression of ILK mRNA was not changed. In contrast to ICR mice, the neoplastic cells of A/J mice also showed upregulation of survivin mRNA (1.6-fold). Consistent with ICR mice, RT-PCR analysis did not show any changes in expression of iNOS between neoplastic and normal epithelial cells of AOM treated mice (Figure 5) and between the colonic mucosa of healthy controls [0.56 (0.36–1.25)] and normal epithelial cells of AOM treated mice [0.36 (0.12–0.93)].
Figure 5.
Gene expression in intraepithelial neoplasia (high-grade dysplasia/intramucosal carcinoma cells) and adjacent normal epithelium in colons of A/J mice treated repeatedly with azoxymethane. For further details see Figure 4. *P < 0.001; **P < 0.01; ***P < 0.05.
Discussion
The main goal of this study was to analyse the expression profile of protumorigenic genes that have been implicated in human colorectal carcinogenesis in mouse models of sporadic and colitis-associated tumorigenesis. A limiting factor in the analysis of genes that are altered in neoplastic cells is the difficulty of separating these cells from the compartments of the stroma or surrounding normal epithelial cells. In the present study, we used laser microdissection to isolate populations of high-grade dysplasia/intramucosal carcinoma cells and corresponding adjacent normal epithelium. We showed for the first time differences in the expression of selective proproliferative and antiapoptotic genes between these neoplastic cells and the surrounding epithelial compartment in mouse models of sporadic and colitis-associated CRC.
In both colitis-associated and sporadic mouse models of CRC, higher gene expression levels for TERT, COX-2 and Tcf-4 were observed in neoplastic cells than in adjacent normal epithelium. Telomerase is an enzyme whose activation is thought to play an important role in cell growth and immortality and in some human cancers TERT mRNA levels were found to be correlated with telomerase activity (Arinaga et al. 2000; Yan et al. 2001; Nowak et al. 2003). Similar to the results obtained by Ohno et al. (2001) in urethane-induced lung tumour in A/J mice, we found upregulation of mouse TERT mRNA in both models of CRC. However, the question is how telomerase activation contributes to tumour development, because telomerase activation does not seem to be essential for tumour development in mice, as tumours have also been found in telomerase-deficient mice (Rudolph et al. 1999). In this regard, it is noteworthy that telomerase not only compensates for natural telomere erosion (reverse transcriptase function) and thus contributes to permanent cell growth but that TERT is also able to activate progenitor cells by controlling Myc- and Wnt-related transcription pathways (Choi et al. 2008). This idea is supported by the finding that c-Myc is a known target of the Wnt pathway and is a downstream signal controlling intestinal proliferation (Sansom et al. 2007). Similarly, we observed in both cancer models a large upregulation of Tcf-4, a transcription factor that has been implicated as a player in the activation of Wnt/β-catenin/TCF signalling pathways and in tumorigenesis (Duval et al. 2000). In addition, an increased level of Tcf-4 mRNA was found in human cancer tissues (Barker et al. 2000). Our data suggest that both sporadic and colitis-associated models of CRC are associated with activation of the Wnt pathway and constitutive upregulation of the Tcf-4 transcript.
Consistent with the fact that survivin is a target gene of the Wnt/β-catenin signalling pathway and that Tcf-4 is one of the major transcription factors involved in the regulation of survivin promoter activity (Zhang et al. 2001), we observed upregulation of survivin mRNA expression in the model of sporadic CRC. These findings are in line with the mouse model of colon carcinogenesis induced by 1,2-dimethylhydrazine (Mori et al. 2007), a metabolite of AOM, and with most human tumours (Altieri 2003). Our ICR data, however, seem to contradict these observations. Using qRT-PCR, we did not find any upregulation of survivin mRNA. The opposite situation was observed with expression of ILK, which has been shown to be upregulated in malignant transformations of human epithelial cells (Marotta et al. 2003; Bravou et al. 2006). The level of ILK mRNA was increased in ICR mice treated with AOM and DSS, but not in A/J mice treated only with AOM. These findings indicate that ILK might play a role in colon carcinogenesis that is associated with successive rounds of colonic inflammation induced by DSS following an initial genotoxic insult. Notably in this regard, ILK intestinal knockout mice treated with AOM and DSS showed tumour growth retardation, probably as a result of reduced proliferation and enhanced apoptosis (Assi et al. 2008). However, the association of ILK with colon carcinogenesis is based primarily on clinical samples and some recent data indicate that elevated levels of ILK are associated with cellular differentiation in many high-turnover tissues and not generally with a malignant phenotype (Haase et al. 2008).
Cytodifferentiation of colon epithelial cells is associated with reduced expression of c-MYB. Conversely, c-MYB is frequently overexpressed in human colon cancer (Ramsay et al. 1992). In addition, potential binding sites for c-MYB were identified within mouse and human COX-2 promotors (Ramsay et al. 2000). To establish whether a similar pattern of alterations may also be present in a mouse tumorigenesis, analysis of c-MYB mRNA levels was undertaken in colon neoplastic cells and adjacent normal colonic epithelium. A quite different pattern of alterations was found in mouse, suggesting that colon tumorigenesis in ICR and A/J mice may occur without increased expression of c-MYB, but with large elevation of COX-2 mRNA in both mouse models. These findings indicate that COX-2 upregulation during tumorigenesis may be driven by a distinct pathophysiological mechanism.
The significant protumorigenic effect of COX-2 on the development of colorectal tumours has been well documented not only in the sporadic (Oshima et al. 1996; Fukutake et al. 1998) but also in the colitis-associated model of CRC (Tanaka et al. 2003; Kim et al. 2008). Also, our data suggest a markedly increased level of COX-2 mRNA in neoplastic cells in both models of tumorigenesis. However, both these models illustrate the lack of correlation between COX-2 mRNA and iNOS mRNA, even though upregulation of both enzymes has been found in ICR mice treated with AOM and DSS using immunohistochemistry and Western blot assay (Tanaka et al. 2003; Kim et al. 2008). One possible explanation for this discrepancy might be that iNOS overexpression is at least partly localized to tumour stroma, especially to tumour-associated macrophages (Wei et al. 2003), and these cells were significantly reduced by the laser microdissection approach. It is also possible that there is no simple relationship between steady state transcript level and matured protein. Nevertheless, recent data from Seril et al. (2007) indicate that mouse iNOS is not essential for the development of colitis-associated tumorigenesis, even though iNOS inhibition attenuated tumorigenesis in mice treated with DSS (Kohno et al. 2007).
In conclusion, we demonstrated differences between the expression profiles of protumorigenic genes in colitis-associated colon tumorigenesis and sporadic tumorigenesis. Our findings suggest that the genetic background and/or molecular mechanisms of tumorigenesis associated with genotoxic insult and colonic inflammation significantly influence the expression of mTERT, COX-2, Tcf-4, c-MYB, ILK and survivin.
Acknowledgments
We thank Dr Michal Dvořák (Institute of Molecular Genetics, Czech Academy of Sciences) for providing us an opportunity to perform the pilot experiments of laser microdissection using the instrument installed in his laboratory. This study was supported by grants from the Ministry of Health (IGA 1A/8651-4 and NS 9982-4) and the Academy of Sciences (AVOZ 50110509).
References
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat. Rev. Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Ambs S, Miriam WG, Bennett WP, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- Arinaga M, Shimizu S, Gotoh K, et al. Expression of human telomerase subunit genes in primary lung cancer and its clinical significance. Ann. Thorac. Surg. 2000;70:401–405. doi: 10.1016/s0003-4975(00)01454-5. [DOI] [PubMed] [Google Scholar]
- Assi K, Mills J, Owen D, et al. Integrin-linked kinase regulates cell proliferation and tumour growth in murine colitis-associated carcinogenesis. Gut. 2008;57:931–940. doi: 10.1136/gut.2007.142778. [DOI] [PubMed] [Google Scholar]
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv. Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Bissahoyo A, Pearsall RS, Hanlon K, et al. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice: effects of dose, route, and diet. Toxicol. Sci. 2005;88:340–345. doi: 10.1093/toxsci/kfi313. [DOI] [PubMed] [Google Scholar]
- Bravou V, Klironomos G, Papadaki E, Taraviras S, Varakis J. ILK over-expression in human colon cancer progression correlates with activation of beta-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J. Pathol. 2006;208:91–99. doi: 10.1002/path.1860. [DOI] [PubMed] [Google Scholar]
- Choi J, Southworth LK, Sarin KY, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- Duval A, Rolland S, Tubacher E, Bui H, Thomas G, Hamelin R. The human T-cell transcription factor-4 gene: structure, extensive characterization of alternative splicings, and mutational analysis in colorectal cancer cell lines. Cancer Res. 2000;60:3872–3879. [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Endo T, Abe S, Seidlar HB, et al. Expression of IAP family proteins in colon cancers from patients with different age groups. Cancer Immunol. Immunother. 2004;53:770–776. doi: 10.1007/s00262-004-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt M, Drullinsky P, Guillem J, Moore MA. Telomerase and telomere length in the development and progression of premalignant lesions to colorectal cancer. Clin. Cancer Res. 1997;3:1931–1941. [PubMed] [Google Scholar]
- Fukutake M, Nakatsugi S, Isoi T, et al. Suppressive effects of nimesulide, a selective inhibitor of cyclooxygenase-2, on azoxymethane-induced colon carcinogenesis in mice. Carcinogenesis. 1998;19:1939–1942. doi: 10.1093/carcin/19.11.1939. [DOI] [PubMed] [Google Scholar]
- Haase M, Gmach CC, Eke I, Hehlgans S, Baretton GB, Cordes N. Expression of integrin-linked kinase is increased in differentiated cells. J. Histochem. Cytochem. 2008;56:819–829. doi: 10.1369/jhc.2008.951095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int. J. Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kundu JK, Le JS, Oh TY, Na HK, Surh YJ. Chemopreventive effects of the standardized extract (DA-9601) of Artemisia asiatica on azoxymethane-initiated and dextran sulfate sodium-promoted mouse colon carcinogenesis. Nutr. Cancer. 2008;60(Suppl. 1):90–97. doi: 10.1080/01635580802404170. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Morisawa T, Hosoda A, Shiota G, Kawasaki H, Hasegawa J. Molecular changes in the early stage of colon carcinogenesis in rats treated with azoxymethane. J. Exp. Clin. Cancer Res. 2002;21:203–211. [PubMed] [Google Scholar]
- Kohno H, Takahashi M, Yasui Y, et al. A specific inducible nitric oxide synthase inhibitor, ONO-1714 attenuates inflammation-related large bowel carcinogenesis in male Apc(Min/+) mice. Int. J. Cancer. 2007;121:506–513. doi: 10.1002/ijc.22736. [DOI] [PubMed] [Google Scholar]
- Marotta A, Parhar K, Owen D, Dedhar S, Salh B. Characterisation of integrin-linked kinase signalling in sporadic human colon cancer. Br. J. Cancer. 2003;88:1755–1762. doi: 10.1038/sj.bjc.6600939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Piro FR, Della Rocca C, et al. Survivin and cyclooxygenase-2 are co-expressed in human and mouse colon carcinoma and in terminally differentiated colonocytes. Histol. Histopathol. 2007;22:61–77. doi: 10.14670/HH-22.61. [DOI] [PubMed] [Google Scholar]
- Narisawa T, Fukaura Y, Terada K, Sekiguchi H. Inhibitory effects of ursodeoxycholic acid on N-methylnitrosourea-induced colon carcinogenesis and colonic mucosal telomerase activity in F344 rats. J. Exp. Clin. Cancer Res. 1999;18:259–266. [PubMed] [Google Scholar]
- Nowak J, Januszkiewicz D, Lewandowski K, et al. Activity and expression of human telomerase in normal and malignant cells in gastric and colon cancer patients. Eur. J. Gastroenterol. Hepatol. 2003;15:75–80. doi: 10.1097/00042737-200301000-00013. [DOI] [PubMed] [Google Scholar]
- Ohno J, Horio Y, Sekido Y, et al. Telomerase activation and p53 mutations in urethane-induced A/J mouse lung tumor development. Carcinogenesis. 2001;22:751–756. doi: 10.1093/carcin/22.5.751. [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Ramsay RG, Thompson MA, Hayman JA, Reid G, Gonda TJ, Whitehead RH. Myb expression is higher in malignant human colonic carcinoma and premalignant adenomatous polyps than in normal mucosa. Cell Growth Differ. 1992;3:723–730. [PubMed] [Google Scholar]
- Ramsay RG, Friend A, Vizantios Y, et al. Cyclooxygenase-2, a colorectal cancer nonsteroidal anti-inflammatory drug target, is regulated by c-MYB. Cancer Res. 2000;60:1805–1809. [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Sano H, Kawahito Y, Wilder RL, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- Seril DN, Liao J, Yang GY. Colorectal carcinoma development in inducible nitric oxide synthase-deficient mice with dextran sulfate sodium-induced ulcerative colitis. Mol. Carcinog. 2007;46:341–353. doi: 10.1002/mc.20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Rosenthal MA, Ellis SL, et al. c-Myb down-regulation is associated with human colon cell differentiation, apoptosis, and decreased Bcl-2 expression. Cancer Res. 1998;58:5168–5175. [PubMed] [Google Scholar]
- Wei D, Richardson EL, Zhu K, et al. Direct demonstration of negative regulation of tumor growth and metastasis by host-inducible nitric oxide synthase. Cancer Res. 2003;63:3855–3859. [PubMed] [Google Scholar]
- Yan P, Saraga EP, Bouzourene H, Bosman FT, Benhattar J. Expression of telomerase genes correlates with telomerase activity in human colorectal carcinogenesis. J. Pathol. 2001;193:21–26. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH728>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Sugino T, Goodison S, et al. Detection of telomerase activity in exfoliated cancer cells in colonic luminal washings and its related clinical implications. Br. J. Cancer. 1997;75:548–553. doi: 10.1038/bjc.1997.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Otevrel T, Gao Z, et al. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–8667. [PubMed] [Google Scholar]