Abstract
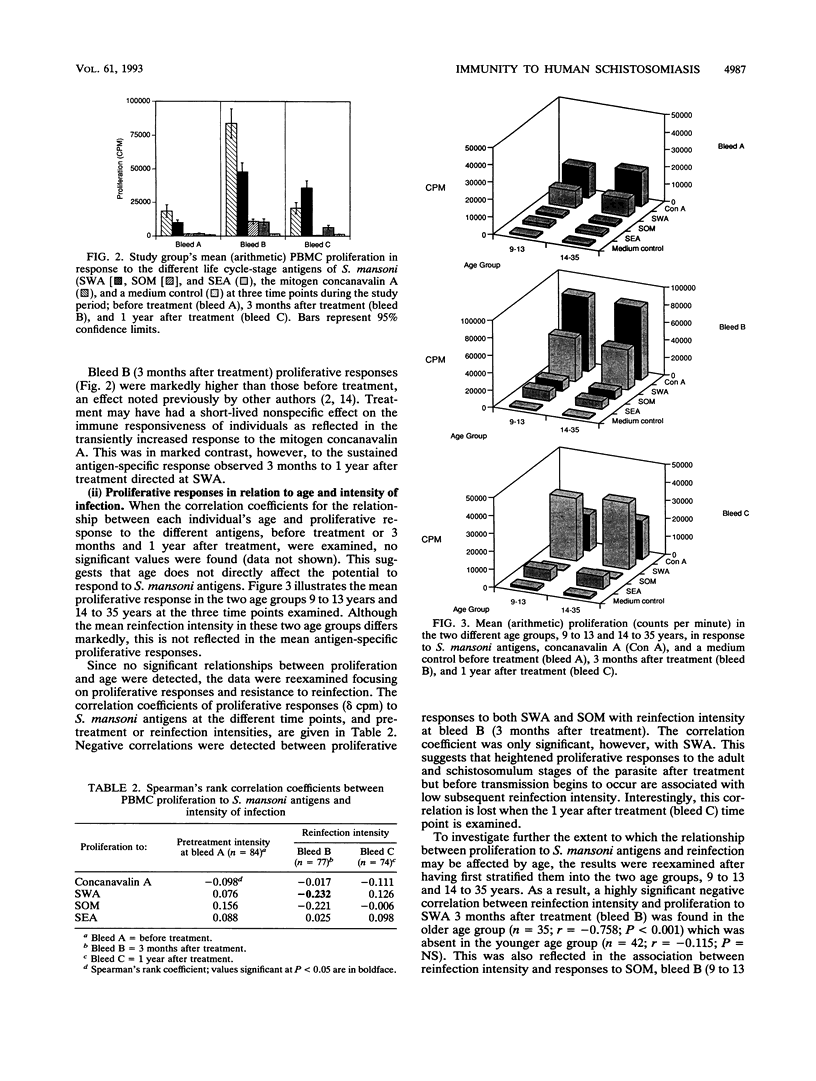
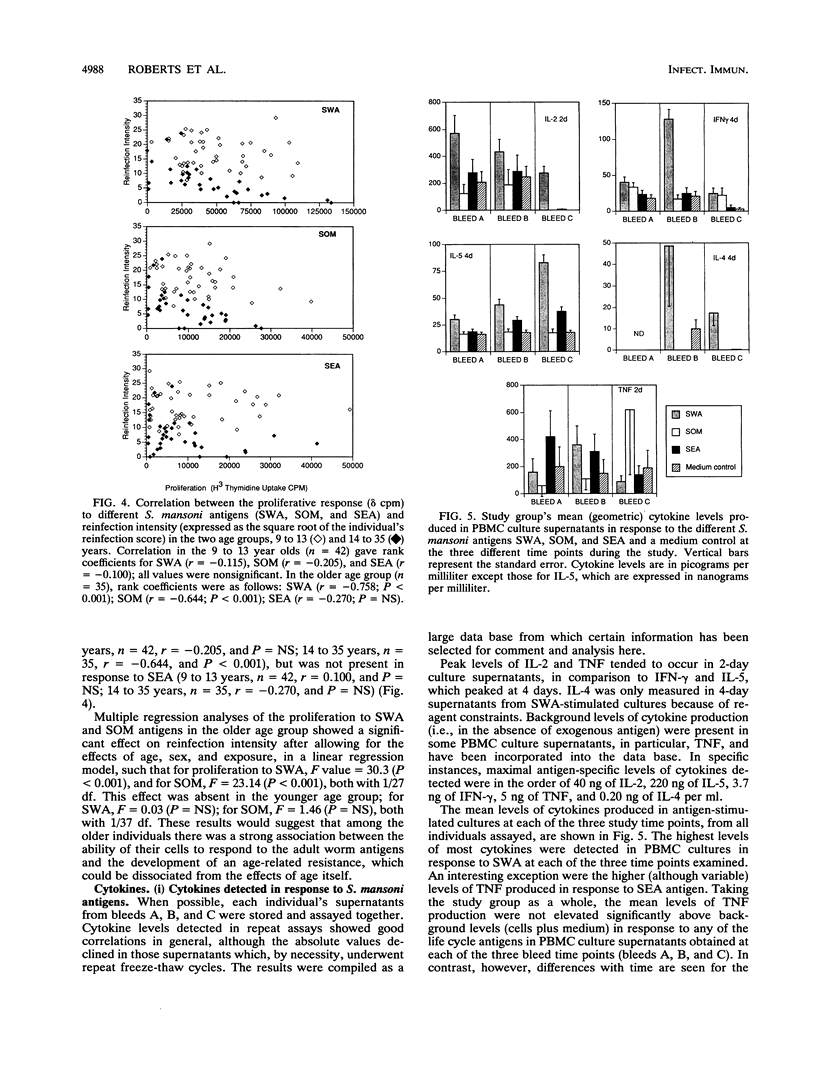
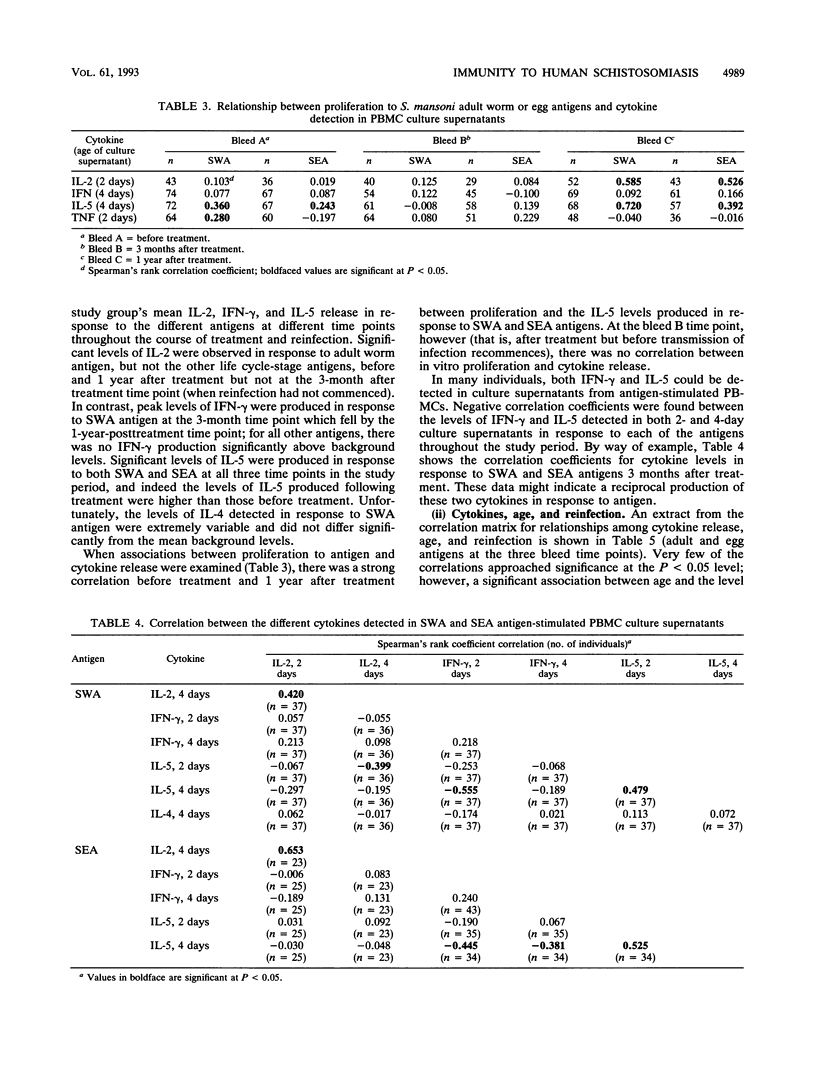
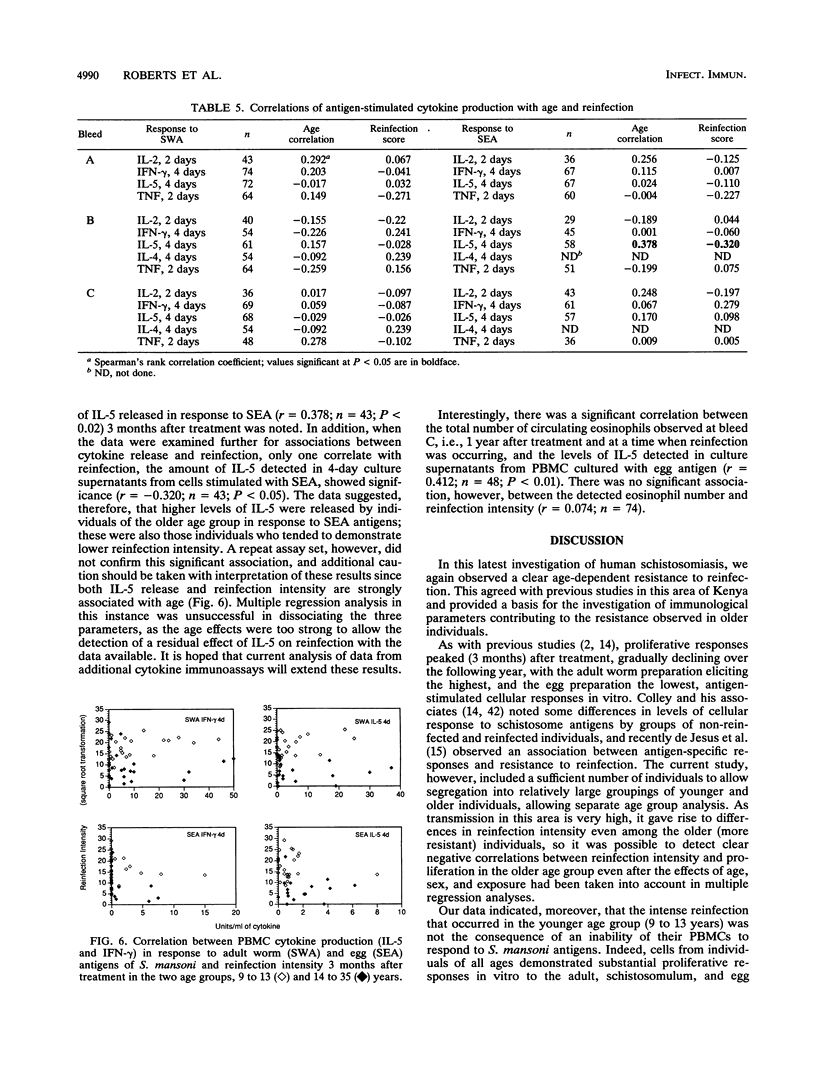
Previous studies have demonstrated the development of an age-dependent resistance to reinfection after chemotherapeutic cure of the helminthic parasite Schistosoma mansoni. Here we report on a longitudinal investigation of cell-mediated responses in infected individuals before and after treatment which was designed to outline those parameters important in mediating a protective response. A well-defined study group of 89 individuals with an age range of 9 to 35 years was selected from an area of high S. mansoni transmission in the Machakos district of Kenya. Peripheral blood mononuclear cell proliferation and cytokine production (interleukin-2 [IL-2], gamma interferon IL-5, IL-4, and tumor necrosis factor) in response to different crude life cycle-stage antigens of S. mansoni were assessed longitudinally in vitro before, 3 months after, and 1 year after treatment. Detailed statistical analyses of the results from this study have indicated a clear negative association between the proliferative responses to adult- and schistosomulum-stage antigens and subsequent reinfection intensity in older individuals (14 to 35 years) which was not present in the younger individuals (9 to 13 years). This association was significant even after the effects of age, sex, and exposure had been accounted for in multiple regression analyses. Cytokines were detected predominantly in response to adult worm and egg antigen extracts. An inverse association between the two cytokines gamma interferon and IL-5 was detected in response to all antigens at the three time points investigated, indicating cross-regulation in the production of these two mediators. Differences in antigen-specific cytokine levels between the two age groups were detected, with significantly higher IL-5 levels detected in the older (more resistant) age group. An inverse correlation between this cytokine and reinfection was detected but could not be dissociated from the effects of age and exposure in multiple regression analysis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiri P., Locksley R. M., Parslow T. G., Sadick M., Rector E., Ritter D., McKerrow J. H. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992 Apr 16;356(6370):604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- Barsoum I. S., Gamil F. M., Al-Khafif M. A., Ramzy R. M., El Alamy M. A., Colley D. G. Immune responses and immunoregulation in relation to human schistosomiasis in Egypt. I. Effect of treatment on in vitro cellular responsiveness. Am J Trop Med Hyg. 1982 Nov;31(6):1181–1187. doi: 10.4269/ajtmh.1982.31.1181. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Bensted-Smith R., Capron A., Capron M., Dalton P. R., Dunne D. W., Grzych J. M., Kariuki H. C., Khalife J., Koech D. Immunity in human schistosomiasis mansoni: prevention by blocking antibodies of the expression of immunity in young children. Parasitology. 1987 Apr;94(Pt 2):281–300. doi: 10.1017/s0031182000053956. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Capron M., Cordingley J. S., Dalton P. R., Dunne D. W., Kariuki H. C., Kimani G., Koech D., Mugambi M., Ouma J. H. Immunity after treatment of human schistosomiasis mansoni. II. Identification of resistant individuals, and analysis of their immune responses. Trans R Soc Trop Med Hyg. 1985;79(3):393–408. doi: 10.1016/0035-9203(85)90391-8. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Dalton P. R., Dunne D. W., Mugambi M., Ouma J. H., Richardson B. A., Siongok T. K., Sturrock R. F. Immunity after treatment of human schistosomiasis mansoni. I. Study design, pretreatment observations and the results of treatment. Trans R Soc Trop Med Hyg. 1984;78(1):108–123. doi: 10.1016/0035-9203(84)90190-1. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Fulford A. J., Dunne D. W., Ouma J. H., Sturrock R. F. Longitudinal studies on human schistosomiasis. Philos Trans R Soc Lond B Biol Sci. 1988 Oct 31;321(1207):495–511. doi: 10.1098/rstb.1988.0105. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Hagan P. Immunity in human schistosomiasis. Parasitol Today. 1987 Jan;3(1):11–16. doi: 10.1016/0169-4758(87)90091-3. [DOI] [PubMed] [Google Scholar]
- Butterworth A., Dunne D., Fulford A., Capron M., Khalife J., Capron A., Koech D., Ouma J., Sturrock R. Immunity in human schistosomiasis mansoni: cross-reactive IgM and IgG2 anti-carbohydrate antibodies block the expression of immunity. Biochimie. 1988 Aug;70(8):1053–1063. doi: 10.1016/0300-9084(88)90268-4. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Joseph M., Pestel J. Role of anaphylactic antibodies in immunity to schistosomes. Am J Trop Med Hyg. 1980 Sep;29(5):849–857. doi: 10.4269/ajtmh.1980.29.849. [DOI] [PubMed] [Google Scholar]
- Capron M., Capron A. Rats, mice and men - models for immune effector mechanisms against schistosomiasis. Parasitol Today. 1986 Mar;2(3):69–75. doi: 10.1016/0169-4758(86)90158-4. [DOI] [PubMed] [Google Scholar]
- Clutterbuck E. J., Hirst E. M., Sanderson C. J. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989 May 1;73(6):1504–1512. [PubMed] [Google Scholar]
- Colley D. G., Barsoum I. S., Dahawi H. S., Gamil F., Habib M., el Alamy M. A. Immune responses and immunoregulation in relation to human schistosomiasis in Egypt. III. Immunity and longitudinal studies of in vitro responsiveness after treatment. Trans R Soc Trop Med Hyg. 1986;80(6):952–957. doi: 10.1016/0035-9203(86)90268-3. [DOI] [PubMed] [Google Scholar]
- Doenhoff M., Long E. Factors affecting the acquisition of resistance against Schistosoma mansoni in the mouse. IV. The inability of T-cell-deprived mice to resist re-infection, and other in vivo studies on the mechanisms of resistance. Parasitology. 1979 Apr;78(2):171–183. doi: 10.1017/s0031182000049222. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Butterworth A. E., Fulford A. J., Kariuki H. C., Langley J. G., Ouma J. H., Capron A., Pierce R. J., Sturrock R. F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992 Jun;22(6):1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Grabowska A. M., Fulford A. J., Butterworth A. E., Sturrock R. F., Koech D., Ouma J. H. Human antibody responses to Schistosoma mansoni: the influence of epitopes shared between different life-cycle stages on the response to the schistosomulum. Eur J Immunol. 1988 Jan;18(1):123–131. doi: 10.1002/eji.1830180119. [DOI] [PubMed] [Google Scholar]
- Else K. J., Hültner L., Grencis R. K. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology. 1992 Feb;75(2):232–237. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Urban J. F., Jr Cytokines: making the right choice. Parasitol Today. 1992 Sep;8(9):311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Ford M. J., Bickle Q. D., Taylor M. G. Immunity to Schistosoma mansoni in congenitally athymic, irradiated and mast cell-depleted rats. Parasitology. 1987 Apr;94(Pt 2):313–326. doi: 10.1017/s003118200005397x. [DOI] [PubMed] [Google Scholar]
- Fulford A. J., Mbugua G. G., Ouma J. H., Kariuki H. C., Sturrock R. F., Butterworth A. E. Differences in the rate of hepatosplenomegaly due to Schistosoma mansoni infection between two areas in Machakos District, Kenya. Trans R Soc Trop Med Hyg. 1991 Jul-Aug;85(4):481–488. doi: 10.1016/0035-9203(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Hagan P., Blumenthal U. J., Dunn D., Simpson A. J., Wilkins H. A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991 Jan 17;349(6306):243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- Hagan P., Wilkins H. A., Blumenthal U. J., Hayes R. J., Greenwood B. M. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 1985 Nov;7(6):625–632. doi: 10.1111/j.1365-3024.1985.tb00106.x. [DOI] [PubMed] [Google Scholar]
- James S. L., Natovitz P. C., Farrar W. L., Leonard E. J. Macrophages as effector cells of protective immunity in murine schistosomiasis: macrophage activation in mice vaccinated with radiation-attenuated cercariae. Infect Immun. 1984 Jun;44(3):569–575. doi: 10.1128/iai.44.3.569-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Sher A. Cell-mediated immune response to schistosomiasis. Curr Top Microbiol Immunol. 1990;155:21–31. doi: 10.1007/978-3-642-74983-4_2. [DOI] [PubMed] [Google Scholar]
- James S. L., Sher A., Lazdins J. K., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. II. Killing of newly transformed schistosomula in vitro by macrophages activated as a consequence of Schistosoma mansoni infection. J Immunol. 1982 Apr;128(4):1535–1540. [PubMed] [Google Scholar]
- Kelly E. A., Colley D. G. In vivo effects of monoclonal anti-L3T4 antibody on immune responsiveness of mice infected with Schistosoma mansoni. Reduction of irradiated cercariae-induced resistance. J Immunol. 1988 Apr 15;140(8):2737–2745. [PubMed] [Google Scholar]
- Kullberg M. C., Pearce E. J., Hieny S. E., Sher A., Berzofsky J. A. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992 May 15;148(10):3264–3270. [PubMed] [Google Scholar]
- Lebman D. A., Coffman R. L. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988 Sep 1;168(3):853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S., Abrams J. S., King C. L., Limaye A. P., Nutman T. B. Parallel regulation of IL-4 and IL-5 in human helminth infections. J Immunol. 1992 Jun 1;148(11):3567–3571. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Linette G. P., Doughty B. L., Byram J. E., Von Lichtenberg F. In vivo T cell depletion regulates resistance and morbidity in murine schistosomiasis. J Immunol. 1987 Aug 1;139(3):919–926. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Bonnefoy J. Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Rihet P., Demeure C. E., Bourgois A., Prata A., Dessein A. J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991 Nov;21(11):2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- Rihet P., Demeure C. E., Dessein A. J., Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol. 1992 Aug;22(8):2063–2070. doi: 10.1002/eji.1830220816. [DOI] [PubMed] [Google Scholar]
- Rolink A. G., Melchers F., Palacios R. Monoclonal antibodies reactive with the mouse interleukin 5 receptor. J Exp Med. 1989 May 1;169(5):1693–1701. doi: 10.1084/jem.169.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Sher A., Fiorentino D., Caspar P., Pearce E., Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991 Oct 15;147(8):2713–2716. [PubMed] [Google Scholar]
- Sturrock R. F., Butterworth A. E., Houba V. Schistosoma mansoni in the baboon (Papio anubis): parasitological responses of Kenyan baboons to different exposures of a local parasite strain. Parasitology. 1976 Dec;73(3):239–252. doi: 10.1017/s003118200004693x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. F., Kimani R., Cottrell B. J., Butterworth A. E., Seitz H. M., Siongok T. K., Houba V. Observations on possible immunity to reinfection among Kenyan schoolchildren after treatment for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1983;77(3):363–371. doi: 10.1016/0035-9203(83)90166-9. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Mazza G. Eosinophilia, activated eosinophils and human schistosomiasis. J Cell Sci. 1991 Mar;98(Pt 3):265–270. doi: 10.1242/jcs.98.3.265. [DOI] [PubMed] [Google Scholar]
- Urban J. F., Jr, Madden K. B., Svetić A., Cheever A., Trotta P. P., Gause W. C., Katona I. M., Finkelman F. D. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992 Jun;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- Vella A. T., Hulsebosch M. D., Pearce E. J. Schistosoma mansoni eggs induce antigen-responsive CD44-hi T helper 2 cells and IL-4-secreting CD44-lo cells. Potential for T helper 2 subset differentiation is evident at the precursor level. J Immunol. 1992 Sep 1;149(5):1714–1722. [PubMed] [Google Scholar]
- Vignali D. A., Bickle Q. D., Taylor M. G. Immunity to Schistosoma mansoni in vivo: contradiction or clarification? Immunol Today. 1989 Dec;10(12):410–416. doi: 10.1016/0167-5699(89)90038-8. [DOI] [PubMed] [Google Scholar]
- Wilkins H. A., Blumenthal U. J., Hagan P., Hayes R. J., Tulloch S. Resistance to reinfection after treatment of urinary schistosomiasis. Trans R Soc Trop Med Hyg. 1987;81(1):29–35. doi: 10.1016/0035-9203(87)90273-2. [DOI] [PubMed] [Google Scholar]
- de Jesus A. M., Almeida R. P., Bacellar O., Araujo M. I., Demeure C., Bina J. C., Dessein A. J., Carvalho E. M. Correlation between cell-mediated immunity and degree of infection in subjects living in an endemic area of schistosomiasis. Eur J Immunol. 1993 Jan;23(1):152–158. doi: 10.1002/eji.1830230125. [DOI] [PubMed] [Google Scholar]


