SUMMARY
Anthracyclines are used to treat childhood acute lymphoblastic leukaemia (ALL) but non-randomised studies suggest that cardiotoxicity may be a problem.
Individual patient data from trials in childhood ALL which randomized anthracyclines or methods of reducing cardiotoxicity were analysed by standard meta-analysis methods. Results were grouped and combined according to: addition of an anthracycline to standard therapy, type of anthracycline, mode of administration, and the use of a cardioprotectant.
Data from 958 patients in 4 trials, recruiting between 1972 and 1984, showed that addition of an anthracycline reduced bone marrow relapse and, non-significantly, non-bone marrow relapse, resulting in increased relapse free interval. However there was a non-significant increase in induction failures, and in deaths in first remission. Event free survival at 5 years was 56.7% with anthracycline versus 52.8% without (OR=0.91; 95% CI=0.76-1.10; p=0.3). There were no significant differences found in other treatment comparisons.
The limited data from trials did not demonstrate differences in clinically evident cardiotoxicity. Anthracyclines are effective against bone marrow relapse but have not been shown to significantly increase event free survival in childhood ALL. The evidence on type of anthracycline, method of administration or use of cardioprotectant is insufficient to be able to rule out important differences.
Keywords: Anthracycline, leukaemia, childhood ALL, meta-analysis, randomized
INTRODUCTION
There has been steady improvement in the outcome for children with acute lymphoblastic leukaemia (ALL), due to the gradual development of chemotherapy treatment protocols. One class of drug believed to play an important part in this is the anthracyclines, which were first introduced into randomized trials in the 1960s. Now that the great majority of children are cured it is important to try to make every effort to minimize any adverse long term effects of treatment. The most serious known adverse effect of anthracyclines is their cardiotoxicity (Elliott, 2006; Lipshultz, 2006). The emergence of this problem has led to the development of strategies aimed at reducing cardiac adverse effects while maintaining efficacy against the disease: including the use of cardioprotective therapies during anthracycline treatment, use of different derivatives of anthracyclines, and use of different infusion schedules during administration. A further concern long term with chemotherapy is the possible risk of second malignancies.
Despite the prominence of anthracycline treatment in childhood ALL there have been few studies of anthracycline safety specific to ALL in children. Also, only published data have been available for meta-analysis (Bryant et al, 2007; van Dalen et al, 2005; van Dalen et al, 2006a; van Dalen et al, 2006b). This review uses data on each individual patient to look at the total evidence available on the effectiveness of anthracyclines, and of the different methods aimed at reducing the long term cardiotoxicity, in treating childhood ALL. As useful data on cardiotoxicity were not available from the trials, a literature review was performed to identify evidence on cardiotoxicity from randomised controlled trials. As cardiotoxicity was not anticipated to be specific to ALL, evidence from all diseases was considered relevant. A Cochrane systematic review was found and, as this is the most reliable source when only published data are available, relevant results from this were included in the results section.
MATERIALS AND METHODS
Trials Included
Individual patient data were sought from all properly randomized trials, commencing before 2000, involving unconfounded treatment comparisons of anthracycline therapy for newly diagnosed childhood acute lymphoblastic leukaemia. Trials were included if at least 50% of patients were up to 21 years of age. The types of anthracycline therapy considered were: addition or not of an anthracycline to standard therapy; type of anthracycline; mode of administration of anthracycline; and the presence or not of a cardioprotectant.
Trials were identified following detailed searching of electronic clinical trial databases including MEDLINE and EMBASE. Additional hand searching was undertaken of content lists of major cancer and general medical journals, of review articles, of meeting abstracts and of reference lists of published trials. Members of the Childhood ALL Collaborative Group and other experts were consulted to ensure the completeness of the resulting list of trials.
Trial protocol details collected included period of recruitment, eligibility criteria, randomized treatment doses and timing, and any anthracycline treatment given in addition to that randomized.
Data Checking
For all trials the following information was sought on each patient aged 21 years or younger at random assignment to treatment: sex, white blood count (WBC) at diagnosis, immunophenotype, treatment allocation and site of first relapse; dates of birth, diagnosis, random assignment to treatment, first remission, relapse, and death or last contact; and the date and type of any secondary tumour.
Data were checked for internal consistency, balance between treatment groups by initial features, randomization dates, and length of follow-up, and consistency with publications on the trials. Data were amended only through correspondence with the principal trial investigators, who, additionally, received relevant summary tables for verification.
Events Analysed
Primary outcome measures were event free survival and overall survival (from date of randomization) with an event defined as any relapse or death. As these trials did not reliably record non-fatal secondary tumours, only fatal ones were included. Secondary outcome measures were no remission (defined as death without remission achievement), bone marrow (BM) relapse (including combined relapses with BM involvement), non-BM relapse, death in remission (including death due to secondary tumour), and relapse free interval which was defined as time to any relapse. In analyses of relapse, patients who died without achieving remission were excluded and deaths in remission were censored. Data were obtained only for first relapse, so analyses of a particular type of relapse are censored at relapse of any other type.
Grouping of trials and subgroup analyses
Trials were grouped and the results combined according to the type of treatment they compared. Trials addressing the addition of anthracycline treatment were subgrouped by the type of anthracycline and whether it was given in induction or maintenance. Comparisons by type of anthracycline were only combined if more than one trial addressed the same two types. Anthracycline administration trials comparing long duration infusion versus shorter administration were subgrouped by length of infusion time. Pre-specified subgroup analyses were by gender, age group (<10, ≥10), white blood count (WBC) (<10, 10-19, 20-49, 50-99, ≥100 × 109/l) and immunophenotype (B-lineage, T-lineage).
Statistics
Within trial analyses were of time from randomization to event, with the observed minus expected (o-e) number of events and its variance (v) obtained by the log-rank method. These o-e values were then added over all trials to produce a total (T), with variance (V) equal to the sum of the separate variances. These were used to calculate an overall odds ratio (OR), or ratio of event rates, and its 95% confidence interval (CI) equal to exp(T/V±1.96/√V). Results are presented as forest plots with a square representing the point estimate of the OR and horizontal line showing the 99% confidence interval for each trial. The size of the square is proportional to the amount of information available, with larger squares representing trials or subgroups with a larger number of events. Overall estimates are shown by a diamond with the width representing the 95% confidence interval. All p-values given are two-sided. Heterogeneity between the effects in different trials or subgroups was tested with Χ2n−1 equal to S-T2/V, where S is the sum of (o-e)2/v from each of n trials or n subgroups (EBCTCG, 1990).
T and V obtained by summing o-e and v from log rank analyses restricted to each one year time period were used to estimate the log OR, b, for each year. The estimated overall event rate in each time period, r, equals the number of events divided by the number of person years, and the probability of surviving event free during that year is exp(-r). Descriptive survival curves were drawn from the separate probability estimates p+0.5p(p-1)b for one treatment group, and p-0.5p(p-1)b for the other treatment group (EBCTCG, 1990).
RESULTS
The only trials for which data were not available were two older trials (1968-1978) looking at the addition of an anthracycline or not to standard therapy (SWOG 690/691, and ALGB 6801)
Addition of an anthracycline
Eight trials were found, but two were excluded (one only included patients aged over 20 years (CALGB 7612) and one was in relapsed disease (POG 869)). Details of the included trials are shown in table I. One trial (DFCI 73001) randomized children between the addition of daunorubicin, doxorubicin, or neither to induction treatment. Doxorubicin was included in consolidation for all patients, whereas the other trials only included anthracyclines in treatment if randomized to them. Five trials randomized daunorubicin, one (ALGB 6801) for use during both induction and maintenance, two during induction (DCLSG-ALL-V/EORTC 99801 and MRC UKALLVIII) and two during maintenance therapy (DCSLG-ALL-1 and SWOG 690/691). Cumulative doses in these trials were all below 100 mg/m2 daunorubicin, 80 mg/m2 doxorubicin, or 60 mg/m2 daunorubicin plus 35 mg/m2 doxorubicin. In the DFCI 73001, DCLSG-ALL-V/EORTC 99801 and MRC UKALLVIII trial protocols all patients received cranial irradiation. It was randomized in SWOG 690/691, and not given in DCSLG-ALL-1 and ALGB 6801.
Table I.
Trial characteristics
| Trial Name | Trial Start Year |
Trial End Year |
Reference | Trial Entry Criteria | Treatment period of anthracycline randomisation |
Anthracycline Randomisation |
Other Anthracycline Therapy |
Cumulative Anthracycline dose (mg/m2) |
|---|---|---|---|---|---|---|---|---|
| Anthracycline vs. not | ||||||||
| ALGB 6801 induction / maintenance |
1968 | 1971 | Halazun et al, 1974 | Untreated, age<20 | Induction / Maintenance |
Dnr (total dose range 360-1260 mg/m2 iv bolus, first dose given at half dosage) vs.not |
None (all anthracycline is randomised) |
360-1260 |
| SWOG 690/691 / ALinC 9 |
1971 | 1973 | Komp et al, 1976 | Age<15 | Dnr given monthly around time of CNS therapy |
Dnr (monthly iv) vs. not Dose unclear |
None (all anthracycline is randomised) |
Not reported |
| DCLSG-ALL-I | 1972 | 1973 | van der Does-van den Berg et al, 1975 | Age<15, no med mass |
Maintenance | Rand if CR at day 42: Dnr (30mg/m2 iv ×2) vs. not |
None (all anthracycline is randomised) |
60 |
| DFCI 73001 | 1973 | 1974 | Sallan et al, 1977 | Untreated, age<20 | Induction | Dnr (60 mg/m2 iv, d1) vs. not; Dox (45 mg/m2 iv, d1) vs. not |
Dox (35 mg/m2 iv consolidation d22) |
80-95 |
| DCLSG-ALL-V / EORTC 99801 |
1979 | 1982 | van der Does-van den Berg et al, 1989 | SR only (age 0-15, WBC<50, no med mass &/or cerebreomeningeal leuk at diag) |
Induction | Dnr (25mg/m2/w iv over 4w) vs. not |
None (all anthracycline is randomised) |
100 |
| MRC UKALL VIII | 1981 | 1984 | Eden et al, 1991 | Age<15 | Induction | Dnr (45 mg/m2 iv d1,d2) vs. not |
None (all anthracycline is randomised) |
90 |
| Type of anthracycline | ||||||||
| DFCI 73001 | 1973 | 1974 | Sallan et al, 1977 | Untreated, age<20 | Induction | Dnr (60mg/m2 iv, d1) vs. Dox (45 mg/m2 iv, d1) |
Dox (35 mg/m2 iv consolidation d22) |
80-95 |
| New Delhi 1989 | 1989* | 1998 | Bhutani et al, 2002 | Untreated, age 11- 70 |
Induction | Dox (30 mg/m2 iv d1,d8,d15,d22) vs. Epi (45 mg/m2 iv d1,d8,d15,d22) |
Dox (30mg/m2 iv early intensification d1,d8,d15,d22) |
240-300 |
| FRALLE 93 IR, ind |
1993 | 1999 | Leblanc et al, 1996 | IR** | Induction | Dnr (40 mg/m2 iv d8,d15, ±d22†) vs. Ida (8 mg/m2 iv d8,d15, ±d22†) |
Dox (25mg/m2 iv d1,d8,d15) |
91-195 |
| Anthracycline administration | ||||||||
| MSK-NY-II | 1986 | 1991 | Steinherz et al, 1993 | SR or HR (exclude if age 2-10 with WBC<10) |
Induction and maintenance |
Dnr push (60 mg/m2 iv bolus (d1, d2 or d3, d4) ×2, 20 mg/m2 iv bolus daily ×18 over 2yr) vs Dnr infusion (120 mg/m2 iv infusion (d1-d2 or d3-d4) ×1, 40 mg/m2 iv 48h infusion ×9 over 2 yr) |
None (all anthracycline is randomised) |
600 |
| DFCI ALL 91-001, high risk (Dox) |
1991 | 1995 | Silverman et al, 2001 | HR (at least one of: WBC>=20, 1<=age<2 or age>=9, leukaemic blasts in CSF, med mass, T-cell) |
Intensification | Dox (30mg/m2 iv bolus, every 3w ×10) vs. Dox (30 mg/m2 iv 48h infusion, every 3w ×10) |
Dox (30 mg/m2 induction d1, d2) |
360 |
| COALL-05-92 | 1992 | 1994 | Escherich et al, 2007 | LR (age 1-9, WBC<25, C or pre- B ALL) or HR (WBC>25 or age>=10 or T-cell or pre-pre-B ALL) |
Pre-phase | Dnr (36mg/m2 iv 1hr infusion d7) vs. Dnr (36mg/m2 iv 24 hr infusion d7) |
Dnr (36mg/m2 iv induction d1,d8,d15). Dox (30 mg/m2 iv reinduction d1,d8, (& d22 , d29 (HR only)) |
204-264 |
| Cardioprotectant | ||||||||
| DFCI ALL 95-001 | 1996 | 2000 | Barry et al, 2008 | Age 1-18 & HR (one of: WBC>=50, age>10, T-cell, med mass, CNS disease) or age<1 |
Induction (?) | Dzr (30 mg/m2 × 10) pre- Dox vs. not |
Dox (30 mg/m2 ×10) |
300 |
| POG-9404 | 1996 | 2001 | POG 9404 trial to be published | Age 1-21, T-cell | Induction and consolidation |
Dzr (300 mg/m2 iv ×12) pre-Dox vs. not |
Dox (30 mg/m2 iv induction ×3 over 6w). Dox (30 mg/m2 iv consolidation ×9 over 27w) |
360 |
published report states 1990 but first patient in dataset randomised in 1989
3rd dose given if marrow not blast free at day 21
Abbreviations: SR - Standard Risk, IR** - Intermediate Risk, HR - High Risk, Dnr - Daunorubicin, Dox - Doxorubicin, Dzr - Dexrazoxane, Epi - Epirubicin, Ida – Idarubicin
non B ALL: age 7-15, or if 1<age<15, tumour syndrome, or, 10<WBC<100×109/l, or Hb>10g/dl, or one myeloid marker, or testicular or CNS localisation, or abnormal karotype (exclude t(9;22) or t(4;11))
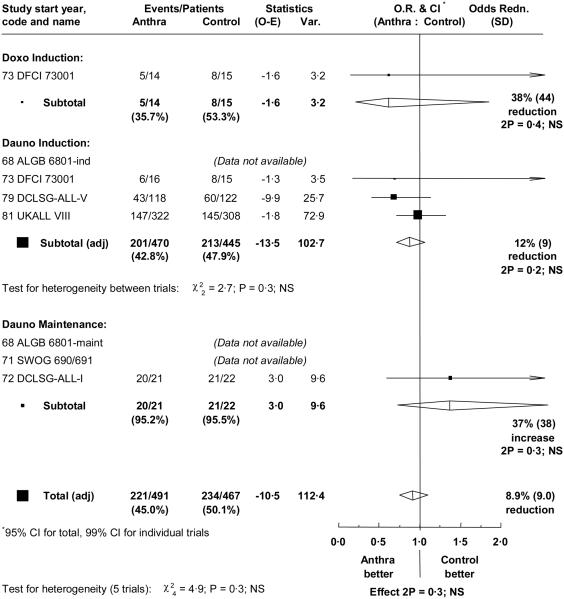
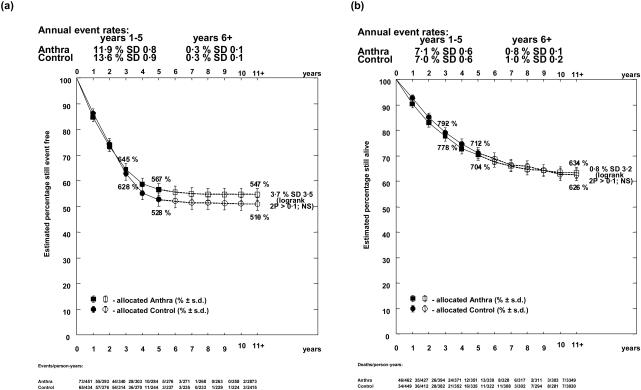
Individual patient data were available for four of the six eligible trials, involving a total of 455 events in 958 patients. Median follow-up for these 4 trials was long, at 13.6, 16.1, 22.5 and 24.1 years (table II). There was a reduction in bone marrow relapse (OR = 0.77; 95% CI = 0.60 to 1.00; p=0.05) with anthracycline, and a non-significant reduction in non-bone marrow relapse (OR=0.88; 95% CI = 0.63 to 1.25; p=0.5). This resulted in improved relapse free interval (OR = 0.81; 95% CI = 0.66 to 1.00; p=0.05). However, there was a non-significant increase in induction failures (21 versus 14; OR=1.44; 95% CI = 0.73 to 2.82; p=0.3), and in deaths in first remission (32 versus 21; OR = 1.45; 95% CI = 0.84 to 2.48; p=0.2) (table III). In these trials, only one patient who failed to achieve remission survived to the end of the follow-up period. Thus the addition of an anthracycline did not significantly affect event free interval, either overall (OR = 0.91; 95% CI = 0.76 to 1.10) (figure 1), or if the comparison was restricted to induction trials (OR = 0.88; 95% CI = 0.72 to 1.06). Within the induction failures, there was a non-significant increase in the number of early deaths in the anthracycline group (14 versus 6 within 40 days; p=0.08) but similar numbers of later deaths, likely to be due to resistant disease, in the two groups (7 versus 8). Event free survival at 5 years was 56.7% with anthracycline versus 52.8% without (figure 2), with a long term difference of 3.7% (95% CI = −3.2% to 10.6%).
Table II.
Patient characteristics
| Gender | Age Group | WBC | Immunophenotype | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial Name | Total patients |
Median follow up (yrs) |
Male | Female | 0-1 | 2-9 | ≥10 | <20 | 20- 49 |
≥50 | B- lineage |
T | Other / Unknown |
| Anthracycline vs. not | |||||||||||||
| DCLSG-ALL-I | 43 | 13.6 | 22 51% |
21 49% |
4 9% |
32 74% |
7 16% |
29 67% |
9 21% |
5 12% |
0 0% |
0 0% |
43 100% |
| DCLSG-ALL-V / EORTC 99801 | 240 | 16.1 | 119 50% |
121 50% |
28 12% |
170 71% |
42 18% |
208* 86% |
28 12% |
0 0% |
132 55% |
2 1% |
106 44% |
| MRC UKALL VIII | 630 | 22.5 | 337 53% |
293 47% |
61* 10% |
471 75% |
97 15% |
443 70% |
91 14% |
96 15% |
394 63% |
46 7% |
190 30% |
| Type of anthracycline | |||||||||||||
| DFCI 73001 (also addressed anthracycline vs. not) |
45 | 24.1 | 28 62% |
17 38% |
2 4% |
36 80% |
7 16% |
34 76% |
7 16% |
4 9% |
0 0% |
0 0% |
45 100% |
| New Delhi 1989 | 42 | 6.0 | 36 86% |
6 14% |
0 0% |
0 0% |
42 100% |
23 55% |
9 21% |
10 24% |
0 0% |
0 0% |
42 100% |
| FRALLE 93 IR, ind | 532 | 3.2 | 287 54% |
245 46% |
33 6% |
445 84% |
54 10% |
368 69% |
126 24% |
38 7% |
530 100% |
0 0% |
2 0% |
| Anthracycline administration | |||||||||||||
| MSK-NY-II | 44 | 15.4 | 30 68% |
14 32% |
5 11% |
20 45% |
19 43% |
27 61% |
4 9% |
13 30% |
35 80% |
8 18% |
1 2% |
| DFCI ALL 91-001, high risk (Dox) | 204 | 8.9 | 116 57% |
88 43% |
34 17% |
111 54% |
59 29% |
95 47% |
49 24% |
60 29% |
178 87% |
21 10% |
5 2% |
| COALL-05-92 | 178 | 10.0 | 104 58% |
74 42% |
14 8% |
129 72% |
35 20% |
103 58% |
40 22% |
35 20% |
159 89% |
19 11% |
0 0% |
| Cardioprotectant | |||||||||||||
| DFCI ALL 95-001 | 205 | 5.7 | 120 59% |
85 41% |
31 15% |
90 44% |
84 41% |
88 43% |
26 13% |
91 44% |
155 76% |
49 24% |
1 0% |
| POG-9404 | 363 | 6.2 | 267 74% |
96 26% |
11 3% |
187 52% |
165 45% |
89 25% |
66 18% |
208 57% |
0 0% |
363 100% |
0 0% |
1, 4 missing values for age, WBC, respectively
Table III.
Effects of treatment on different endpoints.
| Numbers with outcome by randomised treatment allocation |
Log rank OR |
95% LCI |
95% UCI |
2p | ||
|---|---|---|---|---|---|---|
| A. Anthracycline vs. not | ||||||
| Anthra n=491 |
Control n=467 |
|||||
|
|
||||||
| No remission | 21 | 14 | 1.44 | 0.73 | 2.82 | 0.3 |
| Any BM relapse | 106 | 130 | 0.77 | 0.60 | 1.00 | 0.05 |
| Non BM relapse | 62 | 69 | 0.88 | 0.63 | 1.25 | 0.5 |
| Death in first remission | 32 | 21 | 1.45 | 0.84 | 2.48 | 0.2 |
| Any event | 221 | 234 | 0.91 | 0.76 | 1.10 | 0.3 |
| Any death | 182 | 182 | 1.00 | 0.81 | 1.23 | 0.9 |
| B. Type of anthracycline | ||||||
| Dnr vs. Ida (FRALLE 93) | ||||||
| Dnr n=262 |
Ida n=270 |
|||||
|
|
||||||
| Any BM relapse | 18 | 22 | 0.83 | 0.45 | 1.55 | 0.6 |
| Non BM relapse | 6 | 6 | 1.00 | 0.32 | 3.11 | 1.0 |
| Death in first remission | 3 | 2 | 1.58 | 0.27 | 9.11 | 0.6 |
| Any event | 27 | 30 | 0.92 | 0.55 | 1.54 | 0.7 |
| Any death | 14 | 13 | 1.11 | 0.52 | 2.36 | 0.8 |
| Dnr vs. Dox (DFCI 73001) | ||||||
| Dnr n=16 |
Dox n=14 |
|||||
|
|
||||||
| Any BM relapse | 5 | 5 | 0.88 | 0.25 | 3.06 | 0.8 |
| Non BM relapse | 0 | 0 | - | - | - | - |
| Death in first remission | 1 | 0 | - | - | - | - |
| Any event | 6 | 5 | 1.05 | 0.32 | 3.45 | 0.9 |
| Any death | 5 | 4 | 1.13 | 0.31 | 4.20 | 0.9 |
| Epi vs. Dox (New Delhi 1989) | ||||||
| Epi n=20 |
Dox n=22 |
|||||
|
|
||||||
| No remission | 1 | 3 | - | - | - | - |
| Any BM relapse | 8 | 8 | 1.03 | 0.38 | 2.74 | 1.0 |
| Non BM relapse | 0 | 1 | - | - | - | - |
| Death in first remission | 1 | 0 | - | - | - | - |
| Any event | 10 | 12 | 0.86 | 0.37 | 1.98 | 0.7 |
| Any death | 10 | 12 | 0.85 | 0.37 | 1.98 | 0.7 |
| C. Anthracycline administration | ||||||
| 48 hr infusion vs. bolus | ||||||
| 48 hr inf n=102 |
Bolus n=102 |
|||||
|
|
||||||
| Any BM relapse | 15 | 13 | 1.08 | 0.51 | 2.27 | 0.8 |
| Non BM relapse | 0 | 3 | 0.13 | 0.01 | 1.22 | 0.07 |
| Death in first remission | 2 | 6 | 0.36 | 0.09 | 1.43 | 0.1 |
| Any event | 17 | 22 | 0.73 | 0.39 | 1.37 | 0.3 |
| Any death | 14 | 18 | 0.75 | 0.37 | 1.49 | 0.4 |
| 24 hr infusion vs. 1 hr infusion | ||||||
| 24 hr inf n=93 |
1 hr inf n=84 |
|||||
|
|
||||||
| Any BM relapse | 15 | 14 | 0.96 | 0.46 | 1.98 | 0.9 |
| Non BM relapse | 3 | 3 | 0.87 | 0.18 | 4.36 | 0.9 |
| Death in first remission | 5 | 4 | 1.14 | 0.31 | 4.20 | 0.8 |
| Any event | 23 | 21 | 0.98 | 0.54 | 1.77 | 0.9 |
| Any death | 21 | 15 | 1.27 | 0.66 | 2.45 | 0.5 |
| Infusion vs. push | ||||||
| Infusion n=23 |
Push n=21 |
|||||
|
|
||||||
| Any BM relapse | 4 | 1 | 3.10 | 0.52 | 18.43 | 0.2 |
| Non BM relapse | 1 | 0 | 10.75 | 0.20 | 587.47 | 0.2 |
| Death in first remission | 1 | 1 | 1.20 | 0.07 | 19.14 | 0.9 |
| Any event | 7 | 4 | 1.90 | 0.57 | 6.29 | 0.3 |
| Any death | 5 | 4 | 1.38 | 0.37 | 5.15 | 0.6 |
| F. Cardioprotectant | ||||||
| Yes n=292 |
No n=276 |
|||||
|
|
||||||
| Any BM relapse | 33 | 27 | 1.15 | 0.69 | 1.90 | 0.6 |
| Non BM relapse | 13 | 16 | 0.76 | 0.36 | 1.57 | 0.5 |
| Death in first remission | 9 | 4 | 2.01 | 0.68 | 5.97 | 0.2 |
| Any event | 67 | 63 | 1.02 | 0.72 | 1.44 | 0.9 |
| Any death | 54 | 47 | 1.11 | 0.75 | 1.63 | 0.6 |
Figure 1.
Effect of the addition of an anthracycline on overall event rate.
O.R. = Odds ratio. CI = confidence interval. The result for each trial and its 99% confidence interval is represented by a black square, whose size is proportional to the amount of information available, and a horizontal line. Overall results for each subgroup and overall are represented by diamonds whose widths indicate 95% confidence intervals. Total numbers of events and patients are adjusted for trials contributing more than one comparison so that patients are only counted once.
Figure 2.
Descriptive curve showing the effect of the addition of an anthracycline on (a) event free survival and (b) overall survival.
There was no significant heterogeneity between trials, and no evidence of a different effect in any age, sex, WBC or immunophenotype subgroup. However immunophenotype data were very limited in these trials, which all began in or before 1981 (table II). Additionally, NCI risk subgroups were examined (High risk: WBC≥50 or age ≥10; Standard risk: all other) but did not reveal any heterogeneity. The only trial which used doxorubicin (DFCI 73001) was too small to enable indirect comparisons of the effects of different types of anthracycline.
Type of Anthracycline
Four trials comparing different anthracyclines were found, but one was excluded as it was for relapsed patients (CCG 1884). One small trial (DFCI 73001) compared daunorubicin with doxorubicin on day 1 of induction, with all patients receiving one dose of doxorubicin in consolidation. Another small trial (New Delhi 1989) compared 4 doses of epirubicin with 4 doses of doxorubicin in induction, with all patients receiving 4 doses of doxorubicin in intensification. The numbers of patients involved are too small to draw conclusions on clinical outcomes, but, for completeness, numbers of events are given in table III.
One trial (FRALLE 93) randomized 532 children between 2 doses of daunorubicin or 2 doses of idarubicin in induction. Patients received a further dose of randomised anthracycline if their marrow was not blast free at day 21. All patients received 3 doses of doxorubicin in intensification.
Cumulative doses in these trials were: 60 mg/m2 daunorubicin plus 35 mg/m2 doxorubin or 80 mg/m2 doxorubicin (DFCI 73001); 80 (or 120) mg/m2 daunorubicin plus 75 mg/m2 doxorubicin or 16 (or 24) mg/m2 idarubicin plus 75 mg/m2 doxorubicin (FRALLE 93); and 240 mg/m2 doxorubicin or 120 mg/m2 doxorubicin plus 180 mg/m2 epirubicin (New Delhi 1989).
No significant differences in any outcome measure were found (table III), but median follow-up available for FRALLE 93 is only just over 3 years at present.
There were insufficient data to make subgroup analyses meaningful.
A Cochrane systematic review suggested that for adults the rate of clinical heart failure (CHF) might be lower with epirubicin compared with doxorubicin(van Dalen et al, 2006a). In adult patients with solid tumours there were 3 cases of CHF among 521 patients randomised to epirubicin, and 12 cases among 515 patients randomised to doxorubicin. However the difference was not statistically significant even with over 1000 patients randomised, as the number of events was small (RR = 0.35, 95% CI 0.12 to 1.11; p = 0.07). Two trials (Batist et al, 2001; Harris et al, 2002) comparing liposomal-encapsulated versus conventional doxorubicin appeared to indicate that the former reduced CHF in adult patients with breast cancer. There were 2 cases of CHF in 250 patients randomised to liposomal-encapsulated and 14 cases in 271 patients randomised to conventional doxorubicin (RR = 0.20; 95% CI 0.05 to 0.75; p=0.02). This Cochrane review found no published results for children or for leukaemia, and data on cardiotoxicity was not collected for the trials in our review.
Methods of administration
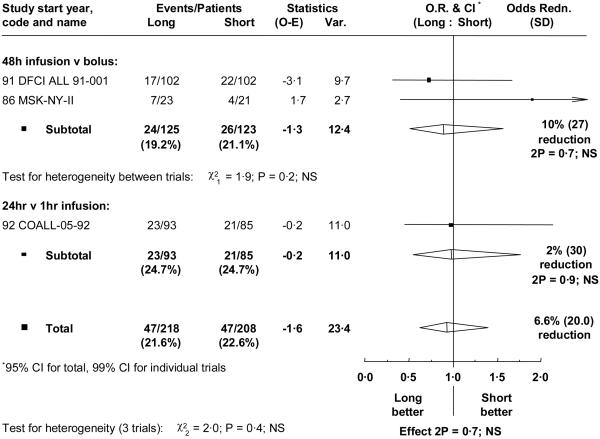
Three trials including 437 children compared slow infusion, over 24 or 48 hours, with short infusion over 1 hour or bolus injection. In the two trials giving anthracycline by 48 hour infusions, either no (MSK-NY-II), or just 2 doses (DFCI ALL 91-001), of anthracycline were given apart from the randomized treatment. However, in the third trial (COALL-05-92) the randomized comparison only applied to one dose in the pre-phase period and all patients had further anthracycline doses by one hour infusion (three in induction, and two in reinduction, plus two more if high risk). Cumulative doses were 600 mg/m2 daunorubicin, 330 mg/m2 doxorubicin, and 60 or 120 mg/m2 doxorubicin plus 144 mg/m2 daunorubicin, respectively. Median follow-up was over 8 years for all these trials.
No significant differences in outcome were found (figure 3, table III), nor evidence of any different effect in any subgroup. All those who failed to achieve remission died.
Figure 3.
Effect of the method of anthracycline administration on overall event rate. Abbreviations and symbols as in figure 1.
A Cochrane systematic review shows that there is evidence that giving anthracyclines to adult cancer patients by an infusion of 6 or more hours reduces cardiotoxicity (van Dalen et al, 2006b). Outcomes examined in the review were CHF and subclinical heart failure (SHF), defined as ≥10% decrease in left ventricular ejection fraction (LVEF). Two trials were for childhood ALL, but relevant data were not available from the publications so these could not be included in the analyses. One trial, DFCI ALL91-001, reported echocardiogram measurements for 121 children and concluded that both regimens used were associated with progressive subclinical cardiotoxicity (Lipshultz et al, 2002). The other, MSK-NY-II, reported on 36 patients monitored with serial echocardiograms (Steinherz et al, 1993). Four children, all on the bolus arm, had a clinically significant decrease in cardiac function, but this difference was not statistically significant (p = 0.10). The small numbers randomised mean that there is a lack of information with respect to longer duration infusions in children for both cardiotoxicity and disease recurrence.
Cardioprotectant
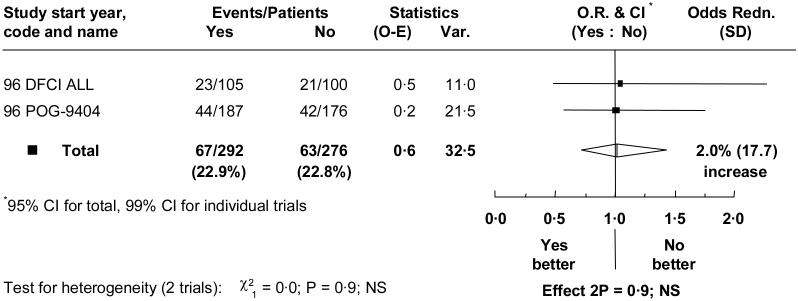
Two trials involving 568 children compared anthracycline with the addition of cardioprotectant to the same anthracycline treatment. Median follow-up was 6 years. The cumulative anthracycline doses given in these trials, which both used doxorubicin, were 300 mg/m2 and 360mg/m2. Median follow-up is over 5 years.
There were no significant differences seen for any endpoint (table III). For event free survival the OR = 1.02 (95% CI = 0.72 to 1.44) (figure 4). Event free survival at 5 years was 77.0% with, and 77.5% without, cardioprotectant, a difference of −0.5% (95% CI = −7.7% to 6.8%). There was no evidence of any different effect within subgroups.
Figure 4.
Effect of cardioprotectant on overall event rate.
Abbreviations and symbols as in figure 1.
A Cochrane systematic review of cardioprotectants showed that there were insufficient data for any conclusions on any drug except for dexrazoxane (van Dalen et al, 2005). In meta-analysis of 5 trials of dexrazoxane which included mainly adults, but also some children, with solid tumours, a reduction in the rate of CHF was demonstrated (RR = 0.18; 95% CI 0.10 to 0.35; p<0.00001), with 10 cases of CHF in 472 patients randomised to dexrazoxane and 59 in 503 control patients.
The randomised trials in the current meta-analysis have not reported on CHF and surrogate measures of heart failure have yielded little information. In DFCI ALL 95-001, there were no significant differences between treatments in echocardiogram measurements performed in a subgroup of children (Lipshultz et al, 2004). A recent review of clinical and cost-effectiveness of cardioprotection in children with cancer, which included one additional (non-randomised) study more than the current review, also reported that conclusions could not be drawn given the limited quality and quantity of the evidence (Bryant et al, 2007).
DISCUSSION
It is likely that anthracyclines are effective against ALL in childhood. Relapses were prevented with their use, but the increased incidence of treatment related deaths resulted in no significant effect on event free survival. However, the event free survival with modern protocols is generally about 20% higher than in the trials included in the review, so the average absolute benefit obtained by adding an anthracycline is likely to be smaller. Randomised evidence on cardiotoxicity comes from trials which mostly used higher cumulative doses of anthracyclines than are generally used for childhood ALL.
There are insufficient data comparing different types of anthracyclines to draw firm conclusions on differences in event rates, with only the daunorubicin versus idarubicin comparison including a significant number of patients. Even here there was only one trial (FRALLE 93), and an absolute difference in events of as much as 10% cannot be ruled out. Treatment with different anthracycline derivatives or formulations may affect cardiac adverse events. A Cochrane systematic review in adults with various cancers demonstrated significantly reduced rates of subclinical and clinical heart failure with liposomal encapsulated doxorubicin compared with standard doxorubicin (van Dalen et al, 2006a), with no evidence to suggest any differences in anti-tumour response rate or survival. The review also suggested a lower rate of clinical heart failure with epirubicin compared with doxorubicin treatment, although the difference was non-significant.
The limited evidence on giving anthracycline as continuous infusion over 24 or 48 hours did not suggest a difference in effectiveness, although in two of these trials, all patients also received some standard administration of i.v. anthracycline during induction, which may have weakened the comparison.
Previous cardiac studies have shown impairment in both adults and children with long-infusion, suggesting that it may not in any case be a way of preventing clinical cardiotoxicity. However, in some reports, different dosage schedules of anthracycline have been found to affect the incidence of cardiac damage. The meta-analysis of trials in adults showed a statistically significant lower rate of heart failure with an infusion duration of 6 or more hours compared with a duration of 1 hour or less, but data were not available for children. A lower incidence of subclinical cardiac damage was also observed with longer infusions, although this effect was not statistically significant (van Dalen et al, 2006b).
The use of dexrazone as a cardioprotectant seems more promising, with evidence that it has a beneficial effect on surrogate measures of cardiac damage. However, although the totality of the evidence does not show that it affects the activity of anthracycline against the disease, the small number of patients randomized means that an absolute difference in event free survival of as much as 7% cannot be ruled out. Event free survival in these trials was 77% at 5 years. Even if newer treatments were to increase this to 85%, applying the relative event rate estimates from the trial evidence would include a possible 5% detriment with cardioprotectant.
A recent systematic review of published data looking at the clinical and cost-effectiveness of cardioprotection found some limited evidence of protection against toxicity (Bryant et al, 2007). In the only two studies in children, dexrazoxane (Lipshultz et al, 2004) and coenzyme Q10 (Iarussi et al, 1994) were reported to protect cardiac function during anthracycline therapy. In another review in adult cancer patients a meta-analysis of 6 studies found that dexrazoxane showed a statistically significant protective benefit against development of heart failure (van Dalen et al, 2005).
No different treatment effects were found in age, sex, WBC or immunophenotype subgroups for any of the review questions. However, although this review used all available data in childhood ALL, the meta-analysis did not include sufficient numbers of patients in subgroups to rule out differences that may exist, particularly in immunophenotype, for which data were very limited in the early trials.
Primary outcome measures in the meta-analysis were event free survival and overall survival. Cardiotoxicity data were not generally collected in the trials, but in any case, the follow up times may have been too short to demonstrate any differences in cardiac damage. It is questionable whether a surrogate endpoint such as subclinical heart failure should be used rather than death.
Anthracycline therapy has been used in childhood ALL since the1960s, since when it may have contributed to the increase in the 5 year survival rate from 30% to its current level of over 70% (Gatta et al, 2002). However due to the risk of cardiac damage and heart failure there has been a tendency in some countries to drop the use of anthracyclines in their treatment protocols or to use them only for selected high risk cases.
Numerous retrospective studies have assessed the cardiac risk of anthracyclines in children. Prevalence of subclinical cardiac damage of up to 56% has been reported in children 6.4 years following anthracycline treatment for various cancers (Kremer et al, 2002b), with a risk of developing clinical heart failure of 2% at 2 years and 5% at 15 years following treatment (Kremer et al, 2001). Reported incidence of heart failure varies from 0% to 16% at 0.9-4.6 years following anthracycline treatment (Kremer et al, 2002a). The only independent risk factor yet identified is a cumulative anthracycline dose of 300 mg/m2. In a long term follow up study of 830 children this dosage increased the risk of heart failure to 9.8% at 20 years compared with 0.5% for patients receiving less than 300 mg/m2 (van Dalen et al, 2006c).
CONCLUSION
Anthracyclines significantly reduced bone marrow relapse when added to standard therapy but did not significantly increase event free survival. There were no significant differences in any relapse, event or death between any anthracycline derivatives, between 48 or 24 and 1 hour anthracycline infusions or between treatment with or without the cardioprotectant dexrazoxane. Keeping cumulative doses below 300mg/m2 appears to reduce cardiotoxicity but there is no clear evidence that other strategies have an effect. Future studies need to be larger, longer term, and to look at clinically important outcomes. With the high survival rates on current protocols and the possibility of long term cardioxicity even at lower doses, whether anthracyclines are necessary for at least some patients remains an important issue. This meta-analysis suggests that, since they appear to have a valuable anti-leukaemic effect but involve increased toxicity, especially cardiac, they should perhaps be reserved for higher risk patients.
Acknowledgments
The secretariat for this work was at CTSU, Oxford University. The work was funded by a grant from the Kay Kendall Leukaemia Fund (KKL293) with additional support from Cancer Research UK and Medical Research Council. Funders were not involved in the design, analysis or reporting.
REFERENCES
- Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, Clavell LA, Larsen EC, Moghrabi A, Samson Y, Schorin MA, Cohen HJ, Lipshultz SE, Sallan SE, Silverman LB. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. Journal of Clinical Oncology. 2008;26:1106–11. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair MK, Hoelzer K, Tkaczuk K, Park YC, Lee LW. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. Journal of Clinical Oncology. 2001;19:1444–54. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- Bhutani M, Kumar L, Vora A, Bhardwaj N, Pathak AK, Singh R, Kochupillai V. Randomized study comparing 4′-epi-doxorubicin (epirubicin) versus doxorubicin as a part of induction treatment in adult acute lymphoblastic leukemia. American Journal of Hematology. 2002;71:241–7. doi: 10.1002/ajh.10211. [DOI] [PubMed] [Google Scholar]
- Bryant J, Picot J, Baxter L, Levitt G, Sullivan I, Clegg A. Clinical and cost-effectiveness of cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review. British Journal of Cancer. 2007;96:226–30. doi: 10.1038/sj.bjc.6603562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBCTCG . In: Treatment of early breast cancer: worldwide evidence 1985-1990. by Early Breast Cancer Trialists' Collaborative Group, editor. Oxford University Press; 1990. [Google Scholar]
- Eden OB, Lilleyman JS, Richards S, Shaw MP, Peto J. Results of Medical Research Council Childhood Leukaemia Trial UKALL VIII (report to the Medical Research Council on behalf of the Working Party on Leukaemia in Childhood) British Journal of Haematology. 1991;78:187–96. doi: 10.1111/j.1365-2141.1991.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Elliott P. Pathogenesis of cardiotoxicity induced by anthracyclines. Semin Oncol. 2006;33:S2–7. doi: 10.1053/j.seminoncol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Escherich G, Göbel U, Jorch N, Spaar HJ, Janka-Schaub GE. Daunorubicin-induced cell kill with 1-hour versus 24-hour infusions: a randomized comparison in children with newly diagnosed acute lymphoblastic leukemia. Klinische Padiatrie. 2007;219:134–8. doi: 10.1055/s-2007-973849. [DOI] [PubMed] [Google Scholar]
- Gatta G, Capocaccia R, Coleman MP, Ries LA, Berrino F. Childhood cancer survival in Europe and the United States. Cancer. 2002;95:1767–72. doi: 10.1002/cncr.10833. [DOI] [PubMed] [Google Scholar]
- Halazun JF, Wagner HR, Gaeta JF, Sinks LF. Proceedings: Daunorubicin cardiac toxicity in children with acute lymphocytic leukemia. Cancer. 1974;33:545–54. doi: 10.1002/1097-0142(197402)33:2<545::aid-cncr2820330233>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- Iarussi D, Auricchio U, Agretto A, Murano A, Giuliano M, Casale F, Indolfi P, Iacono A. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Molecular Aspects of Medicine. 1994;15(Suppl):s207–12. doi: 10.1016/0098-2997(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Komp DM, George SL, Falletta J, Land VJ, Starling KA, Humphrey GB, Lowman J. Cyclophosphamide-asparaginase- vincristine-prednisone induction therapy in childhood acute lymphocytic and nonlymphocytic leukemia. Cancer. 1976;37:1243–7. doi: 10.1002/1097-0142(197603)37:3<1243::aid-cncr2820370303>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. Journal of Clinical Oncology. 2001;19:191–6. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- Kremer LC, van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Annals of Oncology. 2002a;13:503–12. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Annals of Oncology. 2002b;13:819–29. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- Leblanc T, Auclerc MF, Cornu G, Tabone MD, Chastagner P, Stephan JL. Randomized study with idarubicine (IDR) versus daunorubicine (DNR) for induction treatment in intermediate risk acute lymphoblastic leukemia in childhood. Proceedings of the American Association for Cancer Research. 1996;37:171. a1177. [Google Scholar]
- Lipshultz SE. Exposure to anthracyclines during childhood causes cardiac injury. Seminars in Oncology. 2006;33:S8–14. doi: 10.1053/j.seminoncol.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Giantris AL, Lipsitz SR, Kimball Dalton V, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE, Colan SD. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. Journal of Clinical Oncology. 2002;20:1677–82. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. New England Journal of Medicine. 2004;351:145–53. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- POG 9404 trial to be published.
- Sallan SE, Camitta BM, Frei E, 3rd, Furman L, Leavitt P, Bishop Y, Jaffe N. Clinical and cytokinetic aspects of remission induction of childhood acute lymphoblastic leukemia (ALL): addition of an anthracycline to vincristine and prednisone. Medical and Pediatric Oncology. 1977;3:281–7. doi: 10.1002/mpo.2950030310. [DOI] [PubMed] [Google Scholar]
- Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Arkin S, Declerck L, Cohen HJ, Sallan SE. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–8. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- Steinherz PG, Redner A, Steinherz L, Meyers P, Tan C, Heller G. Development of a new intensive therapy for acute lymphoblastic leukemia in children at increased risk of early relapse. The Memorial Sloan-Kettering-New York-II protocol. Cancer. 1993;72:3120–30. doi: 10.1002/1097-0142(19931115)72:10<3120::aid-cncr2820721038>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews. 2005 doi: 10.1002/14651858.CD003917.pub2. CD003917. [DOI] [PubMed] [Google Scholar]
- van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database of Systematic Reviews. 2006a doi: 10.1002/14651858.CD005006.pub2. CD005006. [DOI] [PubMed] [Google Scholar]
- van Dalen EC, van der Pal HJ, Caron HN, Kremer LC. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews. 2006b doi: 10.1002/14651858.CD005008.pub2. CD005008. [DOI] [PubMed] [Google Scholar]
- van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. European Journal of Cancer. 2006c;42:3191–8. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- van der Does-van den Berg A, de Koning J, Reerink H, de Vries JA, van Zanen GE. Acute lymphatic leukemia in children in the Netherlands; study ALL I, 1972-1973; Dutch Childhood Leukemia Study Group. Nederlands Tijdschrift voor Geneeskunde. 1975;119:1445–51. [PubMed] [Google Scholar]
- van der Does-van den Berg A, van Wering ER, Suciu S, Solbu G, van 't Veer MB, Rammeloo JA, de Koning J, van Zanen GE. Effectiveness of rubidomycin in induction therapy with vincristine, prednisone, and L-asparaginase for standard risk childhood acute lymphocytic leukemia: results of a Dutch phase III study (ALL V). A report on behalf of the Dutch Childhood Leukemia Study Group (DCLSG) American Journal of Pediatric Hematology-Oncology. 1989;11:125–33. [PubMed] [Google Scholar]