Abstract
Background & aims
Goblet cells (GC) facilitate mucosal protection and epithelial barrier repair, yet the innate immune mechanisms that selectively drive GC functions have not been defined. The aim of this study was to determine whether TLR2 and modulation of GC-derived TFF3 are functionally linked in the intestine.
Methods
GC modulation was assessed using qRT-PCR, western blotting and confocal microscopy. DSS colitis was induced in wild-type, TFF3−/− and TLR2−/− mice. Recombinant TLR2 ligand or TFF3 peptide were orally administered after DSS termination. Caco-2 overexpressing full-length TLR2 or mutant TLR2-R753Q were tested for TFF3 synthesis and functional-related effects in a wounding-assay.
Results
Data from in-vitro (Ls174T) and ex-vivo models of murine and human GC reveal that TLR2 activation selectively induces synthesis of TFF3. In-vivo studies using TFF3−/− or TLR2−/− mice demonstrate the ability for oral treatment with a TLR2 agonist to confer anti-apoptotic protection of the intestinal mucosa against inflammatory stress-induced damage through TFF3. Recombinant TFF3 rescues TLR2-deficient mice from increased morbidity and mortality during acute colonic injury. Severe ulcerative colitis has recently been found to be associated with the R753Q polymorphism of the TLR2 gene. The relevance of the observed functional effect of TLR2 in regulating GC is confirmed by the finding that the UC-associated TLR2-R753Q variant is functionally deficient in the ability to induce TFF3 synthesis, thus leading to impaired wound healing.
Conclusions
These data demonstrate a novel function of TLR2 in intestinal GC that links products of commensal bacteria to innate immune protection of the host via TFF3.
Introduction
The intestinal epithelium is covered by a protective mucus layer that is in continuous intimate contact with myriad commensal bacteria. The mucus layer is composed predominantly of mucin glycoproteins and TFF3 that are synthesized and secreted by goblet cells (GC) throughout the small and large intestines. TFF3 plays a major role in wound healing and repair of the intestinal mucosa 1. TFF3−/− mice are highly susceptible to chemical, hypoxia or radiation stress-induced colonic injuries and fail to mount an effective repair response 2–4. TFF3 does not exhibit intrinsic activity in regulating cell proliferation, but promotes essential migration during epithelial restitution 5. Commensals may drive GC functions by modulating synthesis of mucus layer components, thus maintaining mucosal homeostasis in the intestine 6. Alterations of the intestinal mucus composition may contribute to imbalanced activation of immune responses in inflammatory bowel diseases (IBD) 7. However, the innate immune mechanisms of beneficial commensal-host interactions 8 that specifically affect GC dynamics have not been elucidated yet.
Toll-like receptor 2 (TLR2), one member of the TLR family, recognizes conserved molecular patterns associated with both Gram-negative and Gram-positive bacteria, including lipopeptides, such as synthetic Pam3CysSK4 (PCSK) 9. TLR2 has been shown to be functionally expressed in three out of the four intestinal epithelial cell lineages: enterocytes 10, Paneth cells and enteroendocrine cells 11, but not in GC so far. Recently, progress has been made in defining TLR2-dependent defence mechanisms that help maintain functional TJ-barrier integrity of the intestinal epithelial layer. TLR2 directly enhances transepithelial resistance via PKCα/δ of the enterocyte barrier in vitro. Treatment with the TLR2 ligand PCSK protects TJ-associated integrity and decreases intestinal permeability, leading to significant amelioration of acute DSS-induced colonic inflammation during the recovery phase 12, 13. Mice deficient in TLR2 exhibit delayed or diminished tissue repair responses 13, 14. Absence of TLR2 leads to deficient anti-apoptotic protection of the intestinal mucosa against toxic stress-induced injury, which further compromises TJ-associated barrier integrity and perpetuates intestinal inflammation 13. However, the molecular and cellular mechanisms of TLR2-mediated anti-apoptosis in mucosal inflammation of the intestine have not yet been further defined.
In this study, we provide evidence of an essential molecular link between innate immunity and host-protective GC function. We show that the benefit of commensal-host interaction in the intestine is through TLR2-mediated induction of the GC-product TFF3, which critically confers anti-apoptotic protection of the intestinal mucosa against inflammatory stress-induced damage. Of note, patients affected with ulcerative colitis (UC) can develop extensive colonic disease, a condition characterized by mucosal inflammation and ulceration. This severe phenotype has recently been associated with innate immune dysfunction through the R753Q polymorphism of the TLR2 gene 15, but the underlying pathophysiology remained so far unresolved. The relevance of our findings is confirmed by showing that the R753Q mutant of TLR2 resulted in reduced TFF3 and impaired healing, thus establishing the mechanistic link to disease pathogenesis. These findings provide a new strategy for developing therapeutic approaches in intestinal injuries.
Materials and Methods
Reagents and antibodies
Synthetic lipopeptide Pam3Cys-SKKKKx3HCl (PCSK; Lot #L08/02) was obtained from EMC Microcollections GmbH 12. Recombinant TFF3 (rTFF3) peptide was kindly provided by The GI Company, Framingham, MA. Rabbit polyclonal and mouse monoclonal antisera generated against rat TFF3 have recently been described 16. Polyclonal antibody (pab) to murine MUC2 and monoclonal antibody (mab) to pan-cytokeratin were purchased from Santa Cruz and pabs to cleaved/total caspases 7, 8 and 9 were obtained from Cell Signaling. ZO-1 pab was from Zymed-Invitrogen. Horseradish-peroxidase-conjugated anti-rabbit and anti-mouse antibodies were from Amersham. All other reagents were obtained from Sigma-Aldrich, unless otherwise specified.
Cells
Caco-2, IEC-6 and Ls174T cells (ATCC) were cultured as previously described 12 or as recommended by the manufacturer, respectively.
Mice
TLR2−/− (Tlr2tm1Kir;>F10 [C57BL6/J]) with WT (TLR2+/+) controls [C57BL6/J] and TFF3−/− (Tff3tm1Dkpy;>F7 [129S2/SvPaf]) with WT (TFF3+/+) controls [129S2/SvPaf] have previously been described 2, 17. Further details can be obtained online from The Jackson Laboratory. Representative allele-specific genotyping is provided in Suppl. Fig. 1. Mice were housed under strict SPF conditions (Helicobacter-species-, MNV-free) at the Central Animal Facility, University Hospital of Essen, Germany. Protocols were in compliance with German law for use of live animals and approved by the Institutional Animal Care and Use Committee at the University Hospital of Essen and the responsible district government. For more information, please see supplemental materials online.
3D-human intestinal mucosa-like culture model of biopsies
Tissue samples from healthy patients undergoing complete colonoscopy for regular colon cancer screening examinations and/or polypectomy at the Endoscopy Unit (Head: M. Rünzi, M.D.), Kliniken Essen-Süd. Informed consent was obtained from all patients before the procedure and the protocol was approved by the Human Studies Committee – Kliniken Essen-Süd, Essen, Germany. For processing of biopsies, please see supplemental materials online.
Organ culture of murine small intestine
Organ culture of murine small intestine was performed as previously described 13.
Induction of colitis and treatment
Please see supplemental materials online.
Histological and morphometric analysis
Please see supplemental materials online.
Immunohistochemistry
Please see supplemental materials online.
Confocal immunofluorescence microscopy
Please see supplemental materials online.
Analysis of apoptosis in colonic specimens
Please see supplemental materials online.
Protein analysis by immunoblotting and cytokine array
Please see supplemental materials online.
RNA extraction and real-time PCR analysis
Please see supplemental materials online.
Plasmid constructs and cell transfection
Please see supplemental materials online.
Restitution (migration) in an in vitro model of wounding
Please see supplemental materials online.
Statistical analysis
Differences between means were calculated using the two-tailed, unpaired t-test (GraphPad Prism vers 4.03, GraphPad Software). P values of <0.05 were considered as significant. All data are expressed as the means ± SEM (n≥3 independent experiments or as indicated).
Results
TLR2 stimulates TFF3 synthesis in intestinal GC
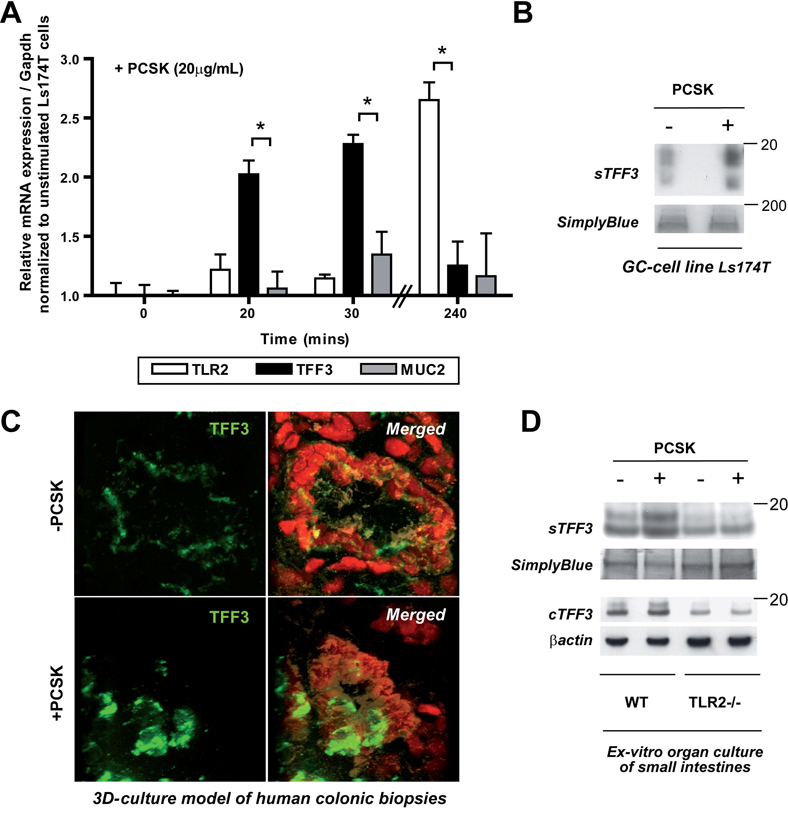
We first studied the effects of TLR2 in regulating intestinal GC function. We initially focussed on the human GC-like cell line Ls174T and found that it constitutively expressed TLR2 mRNA which was enhanced by stimulation with the synthetic TLR2 ligand PCSK at 4 hours (Fig. 1a). We then examined whether the major GC products, TFF3 and/or MUC2, were transcriptional and translational targets of TLR2. Stimulation of Ls174T cells with PCSK yielded increased steady-state TFF3 mRNA within 20 minutes (Fig. 1a), and maximal activation after 30 minutes with an EC50 (Suppl. Fig. 2) comparable to dose response values recently reported in enterocytes 12 or splenocytes 18. These results are consistent with previous findings that TFF3 acts as an “immediate-early” gene 19. Increase of TFF3 mRNA in Ls174T cells in response to PCSK was followed by induction of TFF3 dimer secretion into the culture supernatant (Fig. 1b) – with similar kinetics as previously described for other stimuli of the TFF3 peptide 20.
Figure 1. TLR2 induces TFF3 production in murine and human GCs in vitro and in vivo.
(A) Increase of TLR2 mRNA (240 mins) and TFF3 mRNA (30 mins) expression, but not MUC2 mRNA, in GC-like Ls174T cells by PCSK stimulation (20µg/ml), as determined by real-time RT-PCR analysis. Results are shown in relation to mRNA expression for the housekeeping gene Gapdh and normalized to unstimulated Ls174T cells. Data are presented as means ± SEM (n=2–3 independent experiments): *, p<0.05; +, p>0.05. (B) Induction of TFF3 peptide secretion in Ls147T cells by PCSK (20µg/ml) after 24 hours of stimulation, as determined by western blotting. (C) Up-regulation of TFF3 peptide (FITC: green) in PCSK-treated human primary GC in an ex-vivo culture model of colonic pinch biopsies, as assessed by confocal immunofluorescence (63x/1.3, oil, scan zoom 2.0). 71 separate images (0.29µM spacing) were collected through the region of interest and 3D-reconstruction was performed. Nuclei were counterstained with DAPI (red) and IEC with anti-cytokeratin (beige). (D) Increase of intracellular and secreted TFF3 peptide production after 24 hours of ex-vivo culture of murine WT [C57BL6/J], but not TLR2−/−, small intestines in the presence of PCSK (20µg/ml), as determined by western blotting.
To validate the results obtained from the immortalized cell line in primary human GC, we established a novel intestinal mucosa-like culture model of human colonic pinch biopsies in the presence or absence of PCSK. As assessed by confocal immunofluorescence using 3D-reconstruction, TFF3 peptide expression was markedly enhanced in PCSK-treated human primary GC, but attenuated in untreated (Fig. 1c). To confirm the functional dependence between TLR2 signalling and PCSK-induced TFF3 synthesis, we cultured small intestines from WT and TLR2−/− mice with or without PCSK ex-vivo and assessed levels of cellular and secreted TFF3 peptide. As shown in Fig. 1d, murine primary small intestinal WT-GC produced substantially more TFF3 after PCSK stimulation compared to untreated GC. In contrast, small intestinal TLR2−/− GC expressed significantly less TFF3 which was not influenced by PCSK, suggesting that PCSK-induced TFF3 synthesis directly requires TLR2.
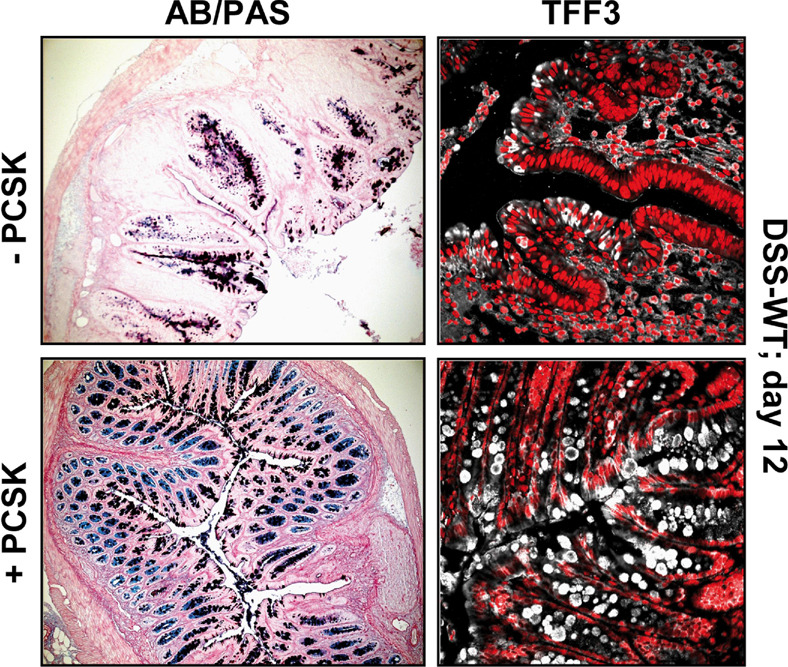
Next, we determined whether in-vivo TLR2 activation may modulate GC function in the intestine. Oral PCSK treatment of healthy mice induced GC proliferation and increased GC size in small intestines (data not shown) and colons via TLR2 (Suppl. Table 1a), correlating with enhanced TFF3 peptide production (data not shown). To investigate whether TLR2 may also induce TFF3 under conditions of inflammatory stress-induced damage, we used DSS colitis, a well-established model of acute intestinal mucosal injury. Indeed, PCSK treatment led to rapid colonic GC regeneration (Fig. 2, left; Suppl. Table 1b), as evidenced by enhanced TFF3 peptide production (Fig. 2, right) during the post-DSS healing phase, which correlated with inhibition of inflammatory destruction of the intestinal mucosa 13. Yet, expression levels of TFF3 mRNA were elevated in all DSS-mice during the recovery phase (day 12), regardless of PCSK administration (Suppl. Fig. 3a), possibly reflecting TLR2-mediated differences in mRNA/protein stability or post-transcriptional regulation of TFF3 during GC regeneration. In contrast, TLR2 stimulation did not induce MUC2 gene transcription or protein expression in vitro and in vivo (Fig. 1a, Suppl. Fig. 3a,b).
Figure 2. Oral treatment with TLR2 agonist induces GC regeneration and increases TFF3 peptide expression in acute DSS colitis.
DSS-colitis was induced in WT mice [C57BL6/J] that received 2.7% DSS for 6 days, followed by oral treatment with 150µg/ml PCSK or water for 6 subsequent days. Mice were sacrificed on day 12. Representative histology of distal DSS-colon with or without PCSK treatment is shown: left: alcian blue (AB)/periodic acid-schiff (PAS); right: TFF3 (CY5: white) - immunofluorescence assessed by confocal laser microscopy (40x/1.3, oil, scan zoom 0.7).
Anti-apoptotic efficacy of treatment with TLR2 ligand in colitis depends on TFF3 induction
Induction of anti-apoptosis has been reported to contribute to TLR2-induced accelerated mucosal recovery after acute inflammatory injury 13, yet the underlying cellular mechanism has not been resolved so far. TFF3 has been shown to block apoptosis, thus accelerating wound healing and repair of the intestinal mucosa 2–4, 16, 21, 22. Given these properties, we investigated whether TLR2-mediated therapeutic effects in acute colonic inflammation may depend functionally on induction of anti-apoptotic TFF3 in GC. Consistent with previous findings 2, TFF3−/− mice were highly susceptible to DSS-colitis with an overall mortality rate of 40% (versus 0% in the DSS-TFF3+/+ groups). While PCSK treatment ameliorated all colitis-associated signs in TFF3+/+ mice, colitis in TFF3−/− mice was not influenced during the early phase by the TLR2 ligand (Fig. 3a–c). Treatment with PCSK abolished mucosal apoptosis in DSS-TFF3+/+ during the acute phase of colitis by day 8. Yet, absence of TFF3 led to complete inhibition of TLR2-mediated anti-apoptosis in acute mucosal inflammation (Fig. 3a), resulting in significantly delayed mucosal healing of inflammatory stress-induced injury, as assessed by any of the several parameters (including body weight, histology (Fig. 3b,c), and colon length (Suppl. Fig. 4a)). Increased cellular apoptosis was found throughout the lamina propria and submucosa as well as surface intestinal epithelium, leading to recruitment of prominent leukocyte infiltrations with transmural involvement, which persisted in DSS-TFF3−/− up to day 12, irrespective of PCSK therapy. These findings indicate that TLR2 critically suppresses mucosal apoptosis in acute colitis via TFF3 in GC. However, compared with untreated DSS-TFF3−/−, PCSK-treated DSS-TFF3−/− mice showed significantly less rectal bleeding and demonstrated improved restoration of ZO-1-associated barrier integrity in differentiated enterocytes during the recovery phase, - even in close proximity to inflammatory infiltrates (Fig. 3d; Suppl. Fig. 4b,c), suggesting that TLR2 stabilizes colitis-induced TJ-associated barrier disassembly in the intestinal epithelial cell layer independent of TFF3.
Figure 3. Therapeutic efficacy of TLR2 agonist in acute inflammatory injury is dependent on anti-apoptotic TFF3.
(A–D) TFF3+/+ or TFF3−/− mice [129S2/SvPaf] (n=10 per group) received 2.5% DSS for 5 days followed by treatment with PCSK 150µg/ml or water for 7 subsequent days. Mice were sacrificed on days 8 or 12. Data are presented as means ± SEM. (−PCSK) vs. (+PCSK): *p<0.05; **p<0.01; ***p<0.001; +p>0.05. (A) TUNEL (FITC: green) assay of colonic tissues using confocal immunofluorescence on day 12 after DSS exposure with or without subsequent PCSK therapy in TFF3−/− mice. Cells were counterstained with PI for nuclei (rhodamine: red) and anti-pancytokeratin (CY5: white) specific for IEC (40x/1.3, oil, scan zoom 0.7). Representative immunofluorescent images are shown; green arrows indicate apoptotic cells. Evolution of (B) body weight changes and (C) histology scores during DSS colitis and subsequent treatment with or without PCSK. (D) Preservation of ZO-1 (CY5: white) – associated IEC barrier integrity after PCSK treatment in DSS-TFF3−/− on day 12, as assessed by confocal immunofluorescence analysis (40x/1.3 oil, scan zoom 1.0). Arrow indicates ZO-1 staining and star subepithelial infiltrate, respectively. Cells were counterstained with DAPI for nuclei (blue) and anti-pancytokeratin (FITC: green), as presented in inserts.
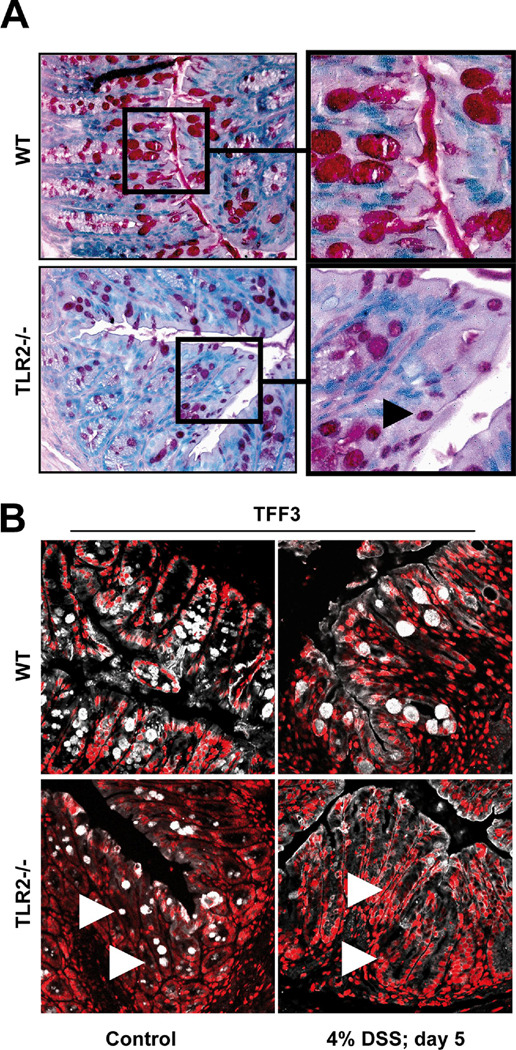
TLR2−/− mice exhibit a selective defect in TFF3 during intestinal GC maturation
To further substantiate the direct molecular link between TLR2 and TFF3 in the intestine, we next examined GC morphology and related product expression in the absence of TLR2. Intestinal GC in healthy TLR2−/− mice were hypotrophic along the upper half of the crypt (Fig. 4a), apparently lacking the ability to transform from pre-GC to mature GC during migration from mid-crypt to villus-surface. Quantative morphometry revealed almost a one-third decrease in GC size in TLR2−/− colons, but no reduction in total GC number, when compared to TLR2+/+ colons (Suppl. Table 1a). Colonic TFF3 mRNA levels were decreased by almost 50% in healthy TLR2−/− (Suppl. Fig. 5a) and TFF3 peptide expression was significantly diminished in the upper portions of the crypt epithelium of healthy TLR2−/− (Fig. 4b). Furthermore, during the acute phase of DSS-induced inflammation, expression of TFF3 was almost completely lost on mRNA and peptide levels in the absence, but not in the presence, of TLR2 (Fig. 4b; Suppl. Fig. 5a). In contrast, MUC2 mRNA expression in TLR2−/− mice was comparable to control WT and MUC2 protein was abundantly expressed in GC along the crypt-villus axis in both healthy and inflamed TLR2−/− and WT colons (Suppl. Fig. 5a,b). There were no microscopic signs of inflammation and no evidence of infection, essentially excluding secondary causes of GC hypoplasia and TFF3 deficiency.
Figure 4. Intestinal TLR2-deficient GC are hypotrophic due to TFF3-deficiency.
(A) Representative PAS-histology of the distal colon of healthy WT [C57BL6/J] or TLR2−/− mice (10x or 40x (insert)). Black arrow indicates example of hypotrophic GC. (B) Representative TFF3 (white) / PI (red) – immunofluorescence of the distal WT or TLR2-deficient colon with or without DSS exposure (4% for 5 days), as assessed by confocal laser microscopy (40x/1.3, oil, scan zoom 0.7). White arrows indicate examples of TFF3-deficient GC.
We considered whether perturbation of stem cell transcriptional factors or associated stromal mediators known to be involved in terminal GC differentiation may have contributed to the observed GC phenotype in TLR2−/−. We observed only a statistically significant increase in Gfi-1 mRNA levels in healthy TLR2−/− compared to WT colons, while the mRNA levels of other progenitor/postmeitotic GC regulators remained unchanged (Suppl. Table 2). However, baseline KGF was decreased, potentially contributing secondarily to aberrant GC-specific lineage differentiation in the absence of TLR2.
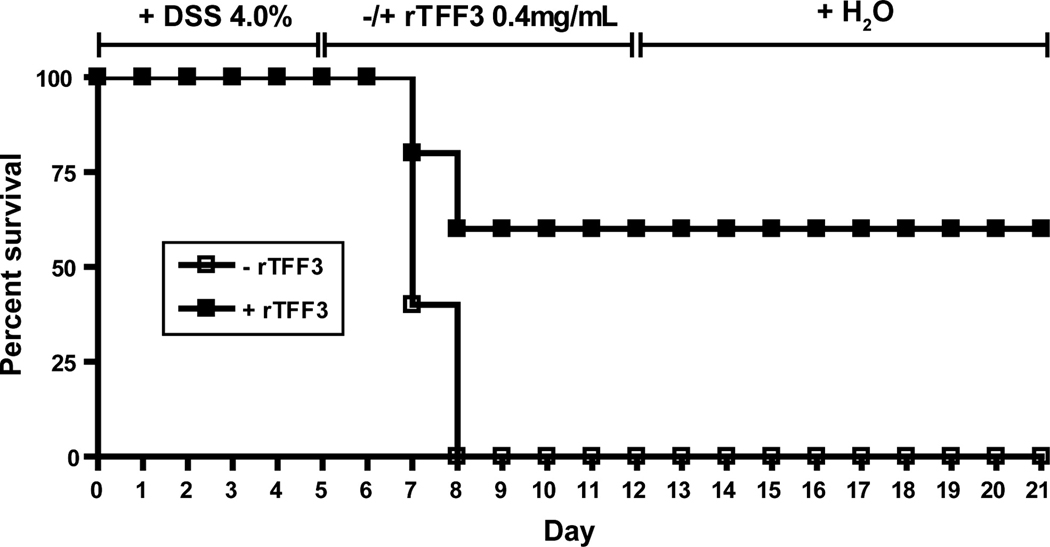
TFF3 supplementation rescues TLR2-deficient mice from tissue injury-induced lethality
We and others have recently demonstrated that mice deficient in TLR2 exhibit severe morbidity and mortality during the acute phase of DSS-colitis 13, 14. We hypothesized that increased tissue injury-induced lethality may be caused by lack of TFF3 in the absence of TLR2. We therefore assessed the effect of oral supplementation of recombinant TFF3 (rTFF3 [0.4mg/ml] 4) on the survival rate of TLR2−/− mice after DSS exposure (4%; 5 days). As shown in Fig. 5, overall mortality rate was 100% in DSS-TLR2−/− control mice vs. only 40% in DSS-TLR2−/− that received rTFF3, implying that TFF3 supplementation can rescue TLR2−/− from early colitis-induced wasting disease and subsequent death. Consistent with previous findings 13, mortality rate of TLR2+/+ controls that did not receive rTFF3 was less than 10% (data not shown).
Figure 5. TFF3 supplementation rescues TLR2-deficient mice from colitis-induced lethality.
Survival rate of TLR2−/− mice (n=5 per group) that first received 4.0% DSS for 5 days followed by rescue therapy with recombinant TFF3 (0.4mg/ml p.o.) or water for 6 subsequent days. Mice were then followed up to day 21 after DSS start.
rTFF3 ameliorates colitis in TLR2-deficient mice by suppressing mucosal apoptosis
We hypothesized that the selective lack in TFF3 production due to impaired GC maturation in TLR2−/− is an important contributing factor for impaired healing of the intestinal mucosa after inflammatory stress-induced damage. To further investigate the disease course during mucosal restitution, we lowered the concentration of DSS (2.0% for 6 days) to reduce its toxicity in TLR2−/− mice. DSS-alone TLR2−/− mice still showed marked colitis-associated signs, while administration of rTFF3 significantly ameliorated all signs of DSS-induced colonic inflammation (Fig. 6a–c; Suppl. Fig. 6a–c). Mucosal cells in DSS-TLR2−/− underwent mitochondrial-dependent apoptosis via initiation of the apoptosome-catalysed caspase-9→7-cascade (intrinsic cell death pathway) (Fig. 6c,d). Excessive apoptosis in DSS-TLR2−/− mice coincided with increased chemokine expression, peak migration and delayed clearance of leukocytes at the site of mucosal inflammation (Suppl. Table 3; Fig. 6b,c). Remarkably, TFF3 supplementation completely abolished ongoing caspase-dependent apoptosis in DSS-TLR2−/− mice and consequently reduced apoptosis-associated inflammatory recruitment of leukocytes.
Figure 6. Administration of rTFF3 ameliorates acute DSS colitis in TLR2-deficient mice by abolishing mucosal apoptosis and associated leukocyte recruitment.
TLR2−/− mice (n=8 per group) received 2.0% DSS for 6 days followed by treatment with rTFF3 (0.4mg/ml p.o.) or water for 6 subsequent days. Mice were sacrificed on days 8 or 12. Data are presented as means ± SEM. (−rTFF3) vs. (+rTFF3): *p<0.05; **p<0.01; ***p<0.001; +p>0.05. (A) Evolution of body weight during DSS colitis and subsequent supplementation of rTFF3. (B) Representative histology of the distal DSS colon with or without rTFF3 treatment on day 12 (hematoxylin-eosin staining on frozen sections, (2.5x)). Arrows indicate leukocyte infiltrates; star indicates ulcer. (C) TUNEL (FITC: green) assay of distal colonic tissues using confocal immunofluorescence on days 8 or 12 after DSS exposure with or without subsequent rTFF3 therapy in TLR2−/− mice. Cells were counterstained with PI for nuclei (rhodamine: red) and anti-pancytokeratin (CY5: white) specific for IEC (40x/1.3, oil, scan zoom 0.7). Representative immunofluorescent images (day 12) are shown; arrows indicate apoptotic cells. (D) Assessment of cleavage of caspases 9, 8 and 7 in whole distal colonic tissues of DSS-TLR2−/− with or without rTFF3 treatment on day 12 by western blotting.
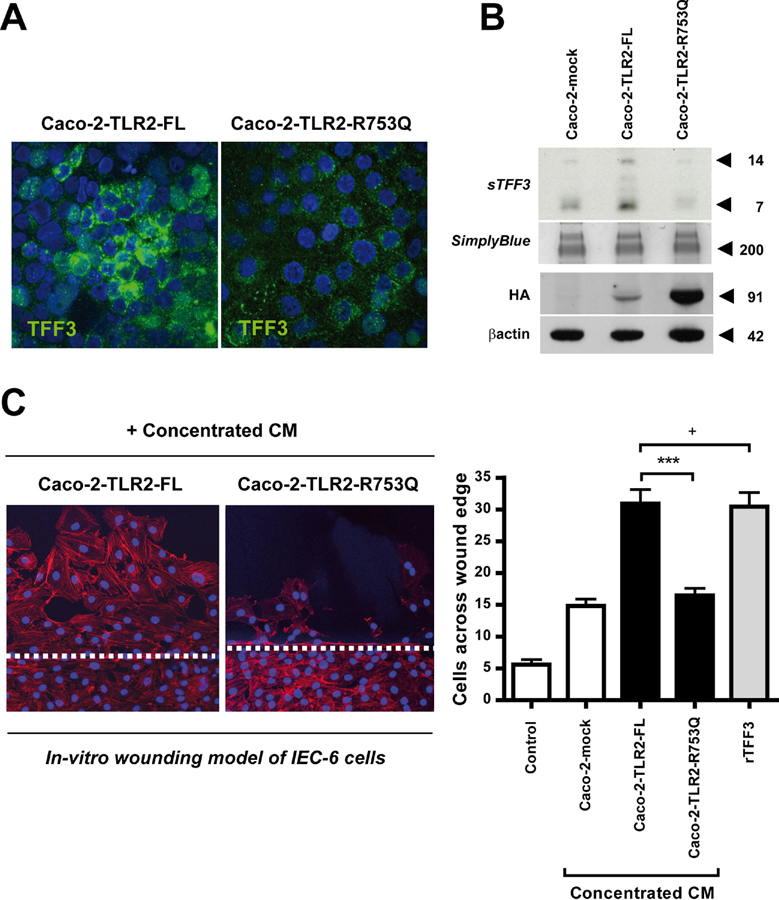
UC-associated R753Q variant of the TLR2 gene impairs wound healing due to TFF3 deficiency
Up to 40 percent of UC patients develop extensive colonic disease, called pancolitis 7, which still represents a therapeutic challenge for the clinician. This more severe phenotype has recently been associated with the heterozygous TLR2-R753Q polymorphism 15, but the underlying pathogenesis remained so far unresolved. TFF3 promotes essential migration during epithelial restitution 5. We therefore hypothesized that this UC-associated TLR2 variant delays wound repair by failing to induce TFF3 synthesis, thus leading to disease exacerbation. To address this, we stably-transfected the intestinal epithelial cell line Caco-2 (which inducibly express TFF3 23) with self-active 24 HA-tagged plasmids of TLR2 full-length (TLR2-FL) or TLR2 mutant (TLR2-R753Q) and assessed basal production of TFF3 and its functional effects on epithelial cell migration in an in-vitro wound healing model of IEC-6 monolayers. Overexpression of Caco-2 cells with TLR2-FL led to significant induction of cellular synthesis (Fig. 7a) and constitutive secretion of TFF3 peptide into the culture supernatant (Fig. 7b) which markedly promoted restitution (Fig. 7c). Other factors present in the conditioned media of Caco-2-TLR2-FL may have secondarily contributed to increased IEC-6 restitution, but the degree of migration was comparable to that observed of rTFF3 5 using the same protein concentration. In contrast, Caco-2 cells overexpressing the TLR2-R753Q mutant did not lead to intracellular production (Fig. 7a) or secretion of TFF3 into the supernatants (Fig. 7b) which impaired epithelial cell migration (Fig. 7c), comparable to untransfected Caco-2 cells (mock). Although stable protein expression levels of HA-tagged full-length were lower than mutant TLR2, transfection efficiency was sufficient to initiate robust TFF3 production in Caco-2-TLR2-FL cells which was neither evident in Caco-2-mock nor in Caco-2-TLR2-R753Q cells.
Figure 7. UC-associated R753Q variant of the TLR2 gene impairs restitution in a wound assay due to TFF3 deficiency.
Increase of intracellular production (A) and secretion (B) of TFF3 peptide in Caco-2-TLR2-FL, but not in Caco-2-TLR2-R753Q or Caco-2-mock (untransfected), as assessed by confocal immunofluorescence (FITC: TFF3 (green); DAPI: nuclei (blue); 63x/1.3, oil, scan zoom 1.0) or western blotting, respectively. Lysates were blotted with anti-HA antiserum confirming efficiency of stable transfection. (C) Effects of conditioned media (CM) from Caco-2-mock, Caco-2-TLR2-FL, or Caco-2-TLR2-R753Q, on restitution. Wounded IEC-6 monolayers were cultured for 20 h in fresh serum-deprived medium (control) with or without proteins (50µg/ml): concentrated CM [from Caco-2-mock (untransfected), Caco-2-TLR2-FL, or Caco-2-TLR2-R753Q] and rTFF3 as positive control. IEC-6 monolayers were counterstained with phalloidin-647 (red) and DAPI (blue). Migration was assessed by blinded counting of the number of cells observed across the wound border using confocal immunofluorescence (20x; scan zoom 1.0). Representative images are shown (white dashed line marks wound margin); data are presented as means ± SEM (n=3 independent experiments): ***, p<0.001; +, p>0.05.
Discussion
Commensals serve as an important stimulus for diverse innate immune functions that protect intestinal mucosal homeostasis. TLRs are involved in host recognition and response to commensals and play a key role in innate immunity of the gastrointestinal tract. Here, we identify a previously unappreciated TLR2 regulated pathway necessary for induction of TFF3 synthesis during terminal GC maturation, thus critically balancing mucosal homeostasis against pro-inflammatory apoptosis. These studies provide a molecular link between the innate immune system and commensal-mediated modulation of GC-derived TFF3 in the intestine.
Our data from in-vitro and ex-vivo studies indicate that stimulation of TLR2 with PCSK promptly induces sustained TFF3 synthesis in murine and human GC. TFF3 transcriptional activation occurs through Ras/MEK/MAPK and PI3K/Akt -pathways 19, 25 which are both shared by TLR2 signaling 10, 13. Downstream, TFF3 transcription is regulated through induction of the goblet cell-specific transcription factor GCSI-BP 26. Future studies will need to determine whether TLR2-induced signalling modules may recruit GCSI-BP or other regulatory elements that may distinctly drive the expression of this key GC product. TLR2 did not modulate MUC2 gene transcription in vitro and in vivo, suggesting a selective and direct GC-regulatory effect on TFF3 synthesis by TLR2 in the intestine.
Furthermore, our findings demonstrate that TLR2 activation distinctly modulates GC function in vivo. A significant increase in TFF3-producing GC via TLR2 was detected in PCSK-treated intestine. The specific and anti-inflammatory effects of PCSK via TLR2 have previously been shown 13, yet the full array of cell-specific protective mechanisms of TLR2 remains to be resolved. We now demonstrate that PCSK-mediated inhibition of inflammatory destruction of the intestinal mucosa correlated with rapid induction of GC regeneration and increased TFF3 expression. TLR2-induced suppression of mucosal apoptosis in acute DSS-colitis was essentially regulated through TFF3 in GC. Lack of TFF3 led to abrogation of TLR2-mediated anti-apoptosis and significantly delayed mucosal healing of acute stress-induced injury of the intestine. However, independent of TFF3, the TLR2 ligand PCSK efficiently maintained TJ-associated barrier integrity in differentiated enterocytes and thus accelerated wound repair in DSS-TFF3−/− during the late recovery phase. These findings imply that TLR2 exerts diverse mucosa-protective properties in different epithelial cell types, critically suppressing mucosal apoptosis in acute colitis via TFF3 in GC. However as TLR2 is expressed by many cell types within intestinal mucosa, the contribution of additional anti-inflammatory responses mediated by TLR2 on lamina propria mononuclear cells cannot be excluded 27.
TFF3 expression correlates with terminal GC differentiation 28. TLR2−/− GC seen in the small intestine and colon were immature due to impairment of commensal-mediated TFF3 production. These cells most likely represent pre-goblet cells, as the total GC number was similar and MUC2 expression remained unchanged in TLR2−/−, when compared to WT controls, suggesting that the GC differentiation program is not completely ablated, - rather the terminal part of the GC differentiation pathway essential for TFF3 synthesis is disturbed due to lack of TLR2 stimulation. Degree of intestinal TFF3 expression may be programmed in pluripotent stem cells under the direct or indirect influence of TLR2. However, only Gfi1 was significantly elevated in TLR2−/− colons. Transcription and other factors with well-defined roles in intestinal epithelial stem cell control, such as KLF4 29 or Math1 30, were largely unaffected by loss of TLR2. Gfi1 is essential for normal lineage allocation in the intestine and controls TFF3 expression during GC migration 31. Therefore increase of Gfi1 may reflect an innate compensatory mechanism of secretory lineage progenitor cells in the attempt to enforce TFF3+ GC maturation in the absence of TLR2 stimulation. Aberrant mesenchymal-epithelial interactions have been shown to impair colonic epithelial progenitor responses in TLR/MyD88−/− mice 32. Of note, we observed a decrease in KGF in TLR2−/− colons, reflecting proliferative alteration in the pericryptal mesenchyme. KGF is an endogenous paracrine effector synthesized by stromal fibroblasts in the colonic stem cell niche 33. KGF regulates mouse TFF3 transcription through the GCSI element, which is essential for goblet cell-specific expression of TFF3 26. Reduction of homeostatic KGF in the TLR2-deficient intestinal mucosa may have contributed secondarily to the defect in GC differentiation. The IL-6/Gp130/STAT-pathway has also been linked to TFF3 production 34, but we were also not able to detect any modulation of baseline IL-6 in healthy TLR2−/− intestines.
Our findings indicate that GC-dependent injury responses require functional TLR2 in order to abolish excessive mucosal apoptosis through TFF3 induction. Lack of TFF3 contributed to increased morbidity and mortaliy in TLR2−/− mice in stress-induced damage. This defect was reversed by rescue with topical TFF3. The pro-apoptotic phenotype of DSS-exposed TLR2−/− was highly similar to the intestinal mucosal phenotype exhibited by mice deficient in TFF3 after DSS challenge. TFF3 has been shown to block the induction of both p53-dependent and p53-independent apoptosis 21, 22. Remarkably, TFF3 supplementation in DSS-TLR2−/− mice completely abolished ongoing caspase-dependent apoptosis which rapidly limited the inflammatory recruitment of leukocytes to the inflamed mucosa. The precise mechanisms responsible for (direct or indirect) attenuation of apoptosis remain to be resolved in future studies. TFF3 may induce XIAP which binds tightly to caspase-9 in the apoptosome complex, resulting in abrogation of caspase-7 processing 35. TFF3 also exerts anti-apoptotic effects through an EGFR-dependent mechanism 21. Collectively, these studies reveal that TFF3 represents an important anti-apoptotic checkpoint in protective innate immune signalling in intestinal GC via TLR2.
We have previously shown that human TLR2 is not upregulated in inflamed epithelium from UC patients 36. Novel risk variants in the TLR2 gene have been associated with a more severe disease phenotype in UC patients 15. Our study demonstrates that the UC-associated variant TLR2-R753Q fails to induce TFF3 synthesis which impairs restitution during wound healing. Dysfunction in GC-TLR2 by the R753Q mutant could be responsible for the typical features seen in UC, including reduction of expression of TFF3 and enhanced apoptosis 7, thus leading to more extensive disease due to impaired innate immune host defense in a subgroup of IBD patients.
In summary, these studies provide first evidence that a specific TLR, namely TLR2, acts to control terminal GC differentiation by selectively regulating TFF3 expression in the intestine, thus conferring anti-apoptotic protection of the intestinal mucosa. They suggest that TLR2 deficiency results in an innate immune defect of GC-derived TFF3, contributing to exacerbation of mucosal apoptosis and associated leukocyte influx during acute inflammatory stress-induced damage of the intestine, which can be reversed by supplementation with recombinant TFF3 peptide. They also demonstrate that cell-type specific functional differences in mucosa-protective effects via TLR2 exist within the intestinal epithelial lineages. Finally, the more severe disease phenotype seen in UC patients with TLR2-mutant haplotype is pathogenetically linked to intestinal TFF3 reduction. As recognition grows for TLRs to play a major role in IBD pathogenesis 7, significant efforts have begun to find a cellular approach of therapeutic TLR-manipulation in the gastrointestinal tract 37. Our results suggest that specifically targeting TLR2 in intestinal GC could help in the design of an adjuvant therapeutic means by enhancing cell survival through TFF3 induction, thus protecting the inflamed mucosa during acute gastrointestinal injuries, such as IBD, but potentially also other causes of damage, including e.g. radiation- or chemotherapy-induced mucositis. In conclusion, these data demonstrate a novel function of TLR2 in intestinal GC that links products of commensal bacteria to innate immune protection of the host.
Supplementary Material
Acknowledgments
We thank The GI Company (Framingham, MA) for the gift of recombinant TFF3, Dr. Michael Rünzi (Kliniken Essen-Süd) for kindly providing human colonic biopsies, Kathryn L. Devaney (MGH-GI Unit) for breeding and genotyping the TFF3-knockout mice, and Yvonne Schwafertz (Univ Hosp Essen) for technical assistance.
Grant Support:
CCFA grant SRA-1790, DFG grant Ca226/4-2, IFORES program (to E.C.) and NIH grants DK60049, DK43351 (to D.K.P).
Nonstandard abbreviations used in this paper
- CM
conditioned media
- GC
goblet cell
- IBD
inflammatory bowel diseases
- PCSK
Pam3Cys-SK4
- PAS
periodic acid-schiff
- PI
propidium iodide
- TFF3
trefoil factor 3 (intestinal)
- TJ
tight junction
- UC
ulcerative colitis
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure:
D.K.P. is a founder and holds equity in The GI Company which has licensed rights for commercial development of TFF3.
References
- 1.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 2.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 3.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology. 2004;126:796–808. doi: 10.1053/j.gastro.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 7.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 8.Cario E. Innate immune signalling at intestinal mucosal surfaces: a fine line between host protection and destruction. Curr Opin Gastroenterol. 2008;24:725–732. doi: 10.1097/MOG.0b013e32830c4341. [DOI] [PubMed] [Google Scholar]
- 9.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 11.Palazzo M, Balsari A, Rossini A, Selleri S, Calcaterra C, Gariboldi S, Zanobbio L, Arnaboldi F, Shirai YF, Serrao G, Rumio C. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol. 2007;178:4296–4303. doi: 10.4049/jimmunol.178.7.4296. [DOI] [PubMed] [Google Scholar]
- 12.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, Vermeire S. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- 16.Itoh H, Beck PL, Inoue N, Xavier R, Podolsky DK. A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest. 1999;104:1539–1547. doi: 10.1172/JCI6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 18.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem. 2006;281:9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 19.Taupin D, Wu DC, Jeon WK, Devaney K, Wang TC, Podolsky DK. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J Clin Invest. 1999;103:R31–R38. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen FP, Woon CW, Varoga D, Jansen A, Garreis F, Jager K, Amm M, Podolsky DK, Steven P, Barker NP, Sel S. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. J Biol Chem. 2008;283:13418–13427. doi: 10.1074/jbc.M800177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita K, Taupin DR, Itoh H, Podolsky DK. Distinct pathways of cell migration and antiapoptotic response to epithelial injury: structure-function analysis of human intestinal trefoil factor. Mol Cell Biol. 2000;20:4680–4690. doi: 10.1128/mcb.20.13.4680-4690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson AC, Kupershmidt I, Edlundh-Rose E, Greco G, Serafino A, Krasnowska EK, Lundeberg T, Bracci-Laudiero L, Romano MC, Parasassi T, Lundeberg J. Global gene expression analysis in time series following N-acetyl L-cysteine induced epithelial differentiation of human normal and cancer cells in vitro. BMC Cancer. 2005;5:75. doi: 10.1186/1471-2407-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465–1477. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- 25.Durual S, Blanchard C, Estienne M, Jacquier MF, Cuber JC, Perrot V, Laboisse C, Cuber JC. Expression of human TFF3 in relation to growth of HT-29 cell subpopulations: involvement of PI3-K but not STAT6. Differentiation. 2005;73:36–44. doi: 10.1111/j.1432-0436.2005.07301006.x. [DOI] [PubMed] [Google Scholar]
- 26.Iwakiri D, Podolsky DK. A silencer inhibitor confers specific expression of intestinal trefoil factor in gobletlike cell lines. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1114–G1123. doi: 10.1152/ajpgi.2001.280.6.G1114. [DOI] [PubMed] [Google Scholar]
- 27.Cario E. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 2008;1 Suppl 1:S62–S66. doi: 10.1038/mi.2008.47. [DOI] [PubMed] [Google Scholar]
- 28.Iwakiri D, Podolsky DK. Keratinocyte growth factor promotes goblet cell differentiation through regulation of goblet cell silencer inhibitor. Gastroenterology. 2001;120:1372–1380. doi: 10.1053/gast.2001.24029. [DOI] [PubMed] [Google Scholar]
- 29.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 31.Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Housley RM, Morris CF, Boyle W, Ring B, Biltz R, Tarpley JE, Aukerman SL, Devine PL, Whitehead RH, Pierce GF. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, Heath JK, Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 35.Twiddy D, Cohen GM, Macfarlane M, Cain K. Caspase-7 is directly activated by the approximately 700-kDa apoptosome complex and is released as a stable XIAP-caspase-7 approximately 200-kDa complex. J Biol Chem. 2006;281:3876–3888. doi: 10.1074/jbc.M507393200. [DOI] [PubMed] [Google Scholar]
- 36.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.