Abstract
Hematopoietic stem cells (HSC) are responsible for the life-long production of the blood system and are pivotal cells in hematologic transplantation therapies. During mouse and human development, the first HSCs are produced in the aorta-gonad-mesonephros region. Subsequent to this emergence, HSCs are found in other anatomical sites of the mouse conceptus. While the mouse placenta contains abundant HSCs at midgestation, little is known concerning whether HSCs or hematopoietic progenitors are present and supported in the human placenta during development. In this study we show, over a range of developmental times including term, that the human placenta contains hematopoietic progenitors and HSCs. Moreover, stromal cell lines generated from human placenta at several developmental time points are pericyte-like cells and support human hematopoiesis. Immunostaining of placenta sections during development localizes hematopoietic cells in close contact with pericytes/perivascular cells. Thus, the human placenta is a potent hematopoietic niche throughout development.
Keywords: human placenta, hematopoietic stem cells, NOD-SCID, Rag γC−/− pericytes, development
Introduction
Hematopoiesis in the human conceptus progresses in a wave-like manner in several different embryonic sites: the yolk sac (YS), the splanchnopleura/aorta-gonad-mesonephros (AGM) region, the liver and the bone marrow (BM) (Tavian and Peault, 2005; Zambidis et al., 2006). Blood generation begins at day 16 of development in the YS with the production of primitive erythroid cells. At day 19 the intra-embryonic splanchnopleura becomes hematopoietic. The emergence of multipotent progenitors and HSCs, organised in clusters of cells closely adherent to the ventral wall of the dorsal aorta, starts at day 27 in the developing splanchnopleura/AGM region (Tavian et al., 1996; Tavian et al., 1999; Tavian et al., 2001). Starting at day 30 the first erythroid progenitors (BFU-E, burst forming unit-erythroid) are found in the liver, with multilineage hematopoietic progenitors (CFU-Mix or -GEMM; colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte) appearing in this tissue at week 13 (Hann et al., 1983). Hematopoietic progenitors and long term culture initiating cells have been found in the human placenta at 8–17 weeks in gestation (Barcena et al., 2009; Zhang et al., 2004). Thereafter, the BM becomes hematopoietic. This sequence of hematopoietic events closely parallels that found in the mouse conceptus, in which the spatial/temporal appearance and the quantitative/qualitative characteristics of hematopoietic progenitor and stem cells have been carefully mapped (Ferkowicz et al., 2003; Kumaravelu et al., 2002; Medvinsky and Dzierzak, 1996; Palis et al., 1999). Importantly, the developing hematopoietic cells in the conceptus are increasing in their complexity (multilineage and higher proliferative potentials) and culminate with the generation of adult-type HSCs that sustain hematopoiesis throughout adult life (Dzierzak and Speck, 2008). While the YS generates the transient embryonic erythroid cells, the AGM is the first tissue to generate more complex hematopoietic progenitors and stem cells (Cumano et al., 1996; Medvinsky and Dzierzak, 1996). The liver and the BM are thought to be colonized by these cells and provide a potent supportive microenvironment for the growth of the fetal and life long blood system.
In addition to the AGM (Cumano et al., 1996; de Bruijn et al., 2000; Medvinsky and Dzierzak, 1996), the chorio-allantoic placenta of the mouse conceptus generates and supports hematopoietic cells at early developmental stages (Alvarez-Silva et al., 2003; Corbel et al., 2007; Gekas et al., 2005; Ottersbach and Dzierzak, 2005; Rhodes et al., 2008; Zeigler et al., 2006). Quantitatively, the midgestation mouse placenta contains more hematopoietic progenitors and HSCs than the AGM region and the YS, indicating that the placenta provides a potent supportive microenvironment for HSC amplification and may be, with the liver, a predominant source of adult BM HSCs (Alvarez-Silva et al., 2003; Gekas et al., 2005; Kumaravelu et al., 2002; Ottersbach and Dzierzak, 2005). In contrast to the mouse, there is little information concerning the hematopoietic potential of the human placenta (Bailo et al., 2004; Barcena et al., 2009; Challier et al., 2005; Zhang et al., 2004). Human studies have focused on umbilical cord blood (UCB), revealing that it is an important and easily accessible source of potent hematopoietic progenitors and HSCs for clinical transplantation procedures (Tse et al., 2008). However the HSC dose limitation in UCB samples and the increasing transplantation needs for treating hematologic disorders has stimulated the search for additional sources of potent HSCs and/or improved methods of ex vivo amplification of HSCs prior transplantation.
Generally, the human placenta has been thought to function as a facilitator of nutrient and waste exchange between the mother and fetus, a provider of immunoprotection for the fetus, and a producer of important factors and hormones for fetal growth (Gude et al., 2004). In this report, we present data showing that the human placenta beginning from gestation week 6 onwards contains fetal-derived immature hematopoietic progenitors and stem cells, differentially expressing CD34 through ontogeny. Furthermore, mesenchymal stromal cells, isolated from human placenta throughout development that we identify as pericyte-like cells, can support the in vitro maintenance of human cord blood hematopoietic progenitors. Together, our results show that the human placenta is a potent hematopoietic niche and a potentially useful source of cells at term for regenerative medicine.
Results
Human placenta contains hematopoietic progenitor cells throughout gestation
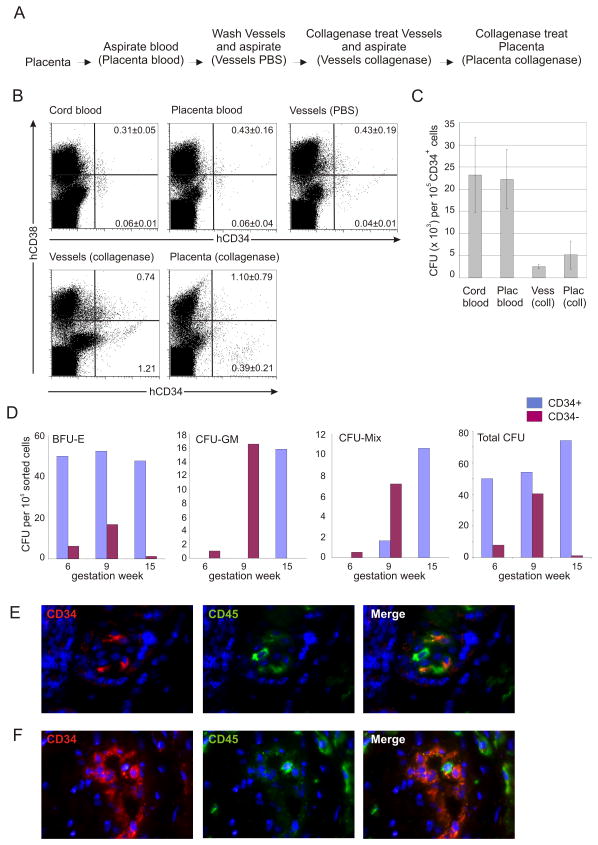
The human term placenta is comprised of the highly vascular fetal-derived chorionic plate and villi, and maternally-derived blood components that circulate in the intervillous space. We examined whether the human placenta obtained at the time of delivery contains hematopoietic progenitors. Blood from inside the placenta was collected (Placenta blood). The remaining cells inside the vasculature were collected in wash steps (Vessels PBS) and following collagenase treatment (Vessels collagenase). Finally, the placenta was dissociated after enzymatic treatment (Placenta collagenase) (Fig 1A).
Figure 1.
Human placenta contains hematopoietic progenitors throughout development. (A) Procedure for the isolation of cell populations from the human placenta. (B) Flow cytometric analyses of term blood and placenta. Cord blood cells, placental blood cells, cells recovered after extensive washes of the placental vasculature (Vessels PBS), from collagenase treatment of the placental vessels and subsequent collagenase treatment of the remaining placenta tissue were stained with anti-human (h)CD34 and CD38 antibodies and viable cells were analysed. Mean percentage ± SD (n=1–4) of relevant populations are indicated. (C) Clonogenic progenitors in term placenta were analysed in methylcellulose cultures. Frequency of total hematopoietic progenitors (CFU=colony forming unit) in the CD34+ cell fraction sorted from the different tissues. Sort purity for cord blood >96%, placenta blood >98%, vessel collagenase >93% and placenta collagenase >81%. Error bars display SEM (n=5). (D) Clonogenic progenitors in the sorted CD34+ (92–94% purity) and CD34− (98–100% purity) cell fractions of early stage placentas were analysed in methylcellulose cultures. Frequencies of the different hematopoietic progenitor types (BFU-E, CFU-GM, CFU-Mix and the sum of these, Total CFU) in both CD34+ and CD34− cell fractions sorted from placentas of gestational week 6, 9 and 15 are displayed. (E) Villus and (F) vasculature from 16 human placenta cryosections: CD34 (red), CD45 (green) and merged fluorescence are shown.
Flow cytometric analysis for CD34 and CD38 markers was performed on human placenta cell populations and UCB (Fig 1B). CD34+CD38+ cells (mature hematopoietic progenitors) and CD34+CD38− (immature hematopoietic progenitors/HSCs) were found in the vessel PBS wash, vessel collagenase and placenta collagenase preparations. Compared to UCB and placenta blood, the percentages of CD34+CD38− cells were increased (about 6 to 10-fold) and an extra population of cells, CD34++CD38− was found in the vessel collagenase and placenta collagenase cell preparations. Some of these cells coexpress CD31 but not CD45, and represent a population of endothelial cells (Suppl Fig 1).
Hematopoietic progenitor activity in term placental cell preparations was tested in the colony forming unit (CFU) assay. Colonies with typical morphology representing all hematopoietic lineages were found in both the vessel and placenta preparations - BFU-E, CFU-G, CFU-M, CFU-GM, CFU-Mix (Suppl Fig 2). The combined number of CFU-Cs in the placenta vessels and tissue obtained at the time of delivery (38 weeks) was found to be 8000 per 105 CD34+ cells (Fig 1C) and is a lower frequency than that found in UCB (23,000 per 105 CD34+ cells) or placental blood. This is a slight underestimate of placenta progenitor frequency since the CD34++CD38− population contains a proportion of endothelial cells, 19% for placenta vessels and 37% for tissue (Suppl Fig 1).
Clonogenic hematopoietic assays were also performed on placentas obtained from the first and second gestational trimesters. Colonies of all erythro-myeloid lineages were found beginning at gestational week 6, the earliest stage placenta tested (Fig 1D), and were in both the CD34+ and CD34− cell fractions. While the frequency of BFU-E remained similar between placentas obtained at gestational week 6, 9 and 15, abundant increases (up to 10-fold) of CFU-GM and CFU-Mix were found beginning at week 9. Until week 9, CFU-GM and CFU-Mix are mainly in the CD34− placenta fraction. Genotyping of CFU-Mix colonies from CD34+ and CD34− placenta cells (gestation week 9) revealed that the hematopoietic cells were fetal-derived (not shown). By week 15 (and 38; term) these progenitors are in the CD34+ fraction, suggesting a developmental regulation in the appearance, phenotype and frequency of more complex hematopoietic progenitors in the developing placenta. CD34+CD45+ hematopoietic cells are localized in the placenta villi (Fig 1E) and vasculature (Fig 1F) as shown by immunostaining of week 16 placenta sections.
Early stage human placenta contains hematopoietic stem cells
Hematopoietic engraftment of NOD-SCID (or Rag γC−/−) immunodeficient mice is considered as the gold-standard functional assay for detection of human HSCs (hereafter called hu-SRC; human-SCID repopulating cells) (Coulombel, 2004). Published results from developmental studies show that the mouse placenta contains a high number of HSCs during midgestation (Gekas et al., 2005; Ottersbach and Dzierzak, 2005). Since the analogous developmental period in the human begins at approximately week 6 in gestation, first and second trimester human placentas (total=17) were examined for hu-SRCs. Placenta cells were injected into 47 NOD-SCID recipients (Table 1) and multilineage engraftment was measured by flow cytometry and PCR.
Table 1.
Summary of NOD-SCID recipient repopulation with fetal-derived cells from first and second trimester human placenta cells
| Gestation week | Number of placentas | Cell number injected | Number repopulated/Number injected | ||
|---|---|---|---|---|---|
| Male | Female | Total | |||
| 19 | 1 | 1 | 2 | 1–3×106 | 3*/4 |
| 18 | 2 | 2 | 1.5–2.1×106 | 0/2 | |
| 17 | 3 | 3 | 1–3×106 | 0/7 | |
| 13 | 1 | 1 | 0.8–3×106 | 0/2 | |
| 11 | 1 | 1 | 1.3×106 | 1/1 | |
| 9 | 3 | 3 | 1–6×106 | 6/16 | |
| 8 | 1 | 1 | 2 | 3×106 | 3*/5 |
| 7 | 2 | 2 | 1–3×106 | 0/9 | |
| 6 | 1 | 1 | 1.5×106 | 1/1 | |
| TOTAL | 15 | 2 | 17 | 14/47 (30%) | |
First and second trimester human placenta cells were prepared and various cell doses were injected into NOD-SCID recipients. All 47 recipients were tested for donor cell engraftment by AMEL PCR for placental cells from a male conceptus and STR PCR for placenta cells from a female conceptus. Recipients were considered positive for repopulation if at least one hematopoietic tissue at the time of sacrifice (5–10 weeks post-injection) showed AMELY signal or an STR profile that matched that of the embryo. All PCR results were verified 2–3 times.
Samples for which STR profiles were established. One recipient injected with 19 week female placenta and 3 recipients injected with 8 week female placenta were profiled.
Figure 2A shows PCR results of hematopoietic tissue DNA from recipients receiving male 6, 9 and 19 week placenta cells. The male Y chromosome specific AMELY fragment was found in the blood, spleen and BM, demonstrating that engraftment was due to placenta cells from the fetal part of the placenta. Flow cytometric analysis of the recipient injected with week 9 placenta (TCB) cells shows multilineage hematopoietic engraftment (Fig 2B). Human (h)CD45+ cells were found in the blood, BM and spleen and were of the myeloid (CD15+) and B lymphoid (CD19+) lineages. A small population of hCD34+CD38− cells, indicative of immature hematopoietic progenitors, was found in the recipient BM. BM cells from this repopulated mouse produced colonies of all myelo-erythroid lineages (Fig 2C), including the most immature multilineage colonies, CFU-Mix. PCR analysis of DNA prepared from individual CFU and pooled CFU verified that these human progenitors were fetal-derived (Fig 2D). Thus, early gestational stage human placenta cells home to the BM and provide multilineage hematopoietic repopulation of NOD-SCID mice.
Figure 2.
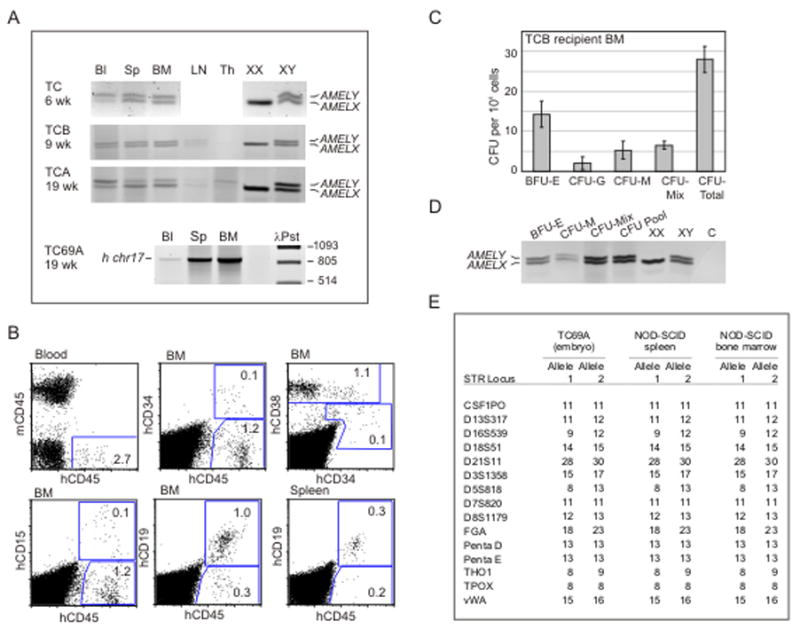
Long-term multilineage NOD-SCID hematopoietic repopulating cells are present in placenta throughout gestation. Human placenta cell engraftment was examined by (A) PCR for the human amelogenin gene (AMEL) or for the human chromosome 17 alpha-satellite sequence (h chr17) in blood (Bl), spleen (Sp), bone marrow (BM) and/or thymus (Th) and lymph node (LN) DNA isolated from cells of NOD-SCID mice transplanted with collagenase/dispase/pancreatin treated placenta tissue cells from the 6, 9 and 19 week (wk) gestation stages. 1.5×106 of TC, 3×106 of TCB, 3×106 of TCA and 3×106 of TC69A placenta cells were injected per mouse. TC, TCB, and TCA placentas were from male conceptuses and TC69A was from a female conceptus. TC, TCB, TCA and TC69A recipients were analysed respectively at 6, 10, 11 and 7 weeks post-transplantation. (B) Flow cytometric multilineage analyses of blood, bone marrow (BM) and spleen cells isolated from NOD-SCID mice 10 weeks after injection of 3×106 cells from collagenase/dispase/pancreatin treated TCB placenta tissue. Cells were stained with anti-mouse (m) CD45 and anti-human (h) CD34, CD38, CD45, CD19 and CD15 antibodies and analysed in the viable population. Number of events analysed were 3×105 for blood and 9×104 for BM and spleen. Percentages of gated populations are indicated. (C) Frequencies of the different hematopoietic progenitor types (BFU-E, CFU-G, CFU-M, CFU-Mix) present in the total BM isolated from the TCB reconstituted NOD-SCID recipient shown in panels A and B. (D) PCR analysis for the amelogenin gene was performed on each colony type and on a pool of colonies (CFU pool) harvested from the culture experiments in panel C. The presence of AMELY fragment reveals their fetal origin. (E) STR profiling of DNA from the spleen and BM of the NOD-SCID recipient transplanted with TC69A (female) placenta tissue cells. TC69A embryo DNA (female) served as the control for fetal-derived cells. STR alleles are designated as numbers of polymorphic repeats.
Engraftment of NOD-SCID mice with placenta cells from female conceptuses was tested by PCR for human chromosome 17 (hChr 17) alpha-satellite DNA (Fig 2A, TC69A), followed by a forensic PCR to discriminate fetal- from maternal-derived engraftment. 15 highly polymorphic short tandem repeat (STR) loci normally used for human identity testing were measured, and the STR profiles of recipient NOD-SCID hematopoietic tissue DNAs were compared to embryo STR profiles. As shown for the TC69A placenta (Fig 2E), the STR profiles of the spleen and BM cells of the NOD-SCID recipient were identical to the profile of the embryo, thus demonstrating exclusive engraftment from fetal-derived female placenta cells. In summary, of the 17 placentas transplanted into a total of 47 recipients, 14 recipients (30%) were repopulated by hu-SRCs of fetal placenta-origin (Table 1).
To further characterize the placenta-derived hu-SRCs, transplantation experiments into NOD-SCID and RAG γC−/− mice were performed with placenta cells sorted on the basis of expression of the CD34 marker. As shown in Table 2, HSCs are both in CD34+ and CD34− fractions in 6 week old placenta, the earliest time point tested. By 16 to 18 weeks, the HSC population appears to be enriched in the CD34+ fraction. An example of human multilineage analyses in a RAG γC−/− recipient is shown (Suppl Fig 3).
Table 2.
Summary of NOD-SCID and Rag γC−/− recipient repopulation with CD34+ and CD34− sorted cells from first and second trimester human placenta
| Recipients | Gestation week | Cell number injected | Number repopulated/Number injected | ||
|---|---|---|---|---|---|
| CD34+ | CD34− | CD34+ | CD34− | ||
| NOD-SCID | 6 | 0.6×106 | 1.9×106 | 2/2 | 1/1 |
| Rag γC−/− | 16–17 | 0.6–1.0×106 | 2×106 | 1/3 | 0/4 |
| NOD-SCID | 16–18 | 0.06–0.6×106 | 0.75–1.6×106 | 1/2 | 1/2 |
| NOD-SCID | 19–20 | 0.5×106 | 5–10×106 | 1/1 | 0/2 |
Tissue DNA from all recipients injected with human placenta cells was tested for human engraftment by AMEL PCR. NOD-SCID adult recipients were irradiated with 3 Gy, injected intravenously with the indicated numbers of sorted placenta cells (and 1 × 105 NOD-SCID spleen cells) and analysed for human cell engraftment at 5 weeks post-injection. Rag γC−/− 5 day old recipients were irradiated with 3 Gy, injected in the liver with the indicated numbers of sorted placenta cells (10 μl volume), and at 11 weeks post-injection analyzed for human cell engraftment.
Human full term placenta contains NOD-SCID hematopoietic repopulating cells
Previously it was shown that HSC were almost undetectable in the mouse placenta at term (E18) (Gekas et al., 2005). To examine if this was also the case for the human placenta, cells from term human placenta vessels and tissues were prepared and injected into NOD-SCID mice. Recipients were analysed at 5 months post-transplantation for human hematopoietic cell engraftment by flow cytometric analysis and PCR..
Three experiments (3 male placentas) resulted in human hematopoietic repopulation of NOD-SCID mice (Fig 3 and Table 3). hCD45+ cells (Fig 3A) were detected in the blood of a NOD-SCID recipient receiving 20×106 placenta tissue cells from male term placenta (tP2). The BM and spleen contained high percentages of hCD45+cells (51% and 22% respectively) of which many were B lymphoid cells, with a few myeloid cells. Control transplantation of 20×106 human UCB cells showed similar levels of NOD-SCID BM engraftment (66% hCD45+, 49% hCD19+, 6% hCD15+cells) that were comparable to published cord blood NOD-SCID transplantation data (Bhatia et al., 1997). Collagenase treated placenta vessel cells (7×106 cells injected) from tP2 (Fig 3B) also resulted in similar engraftment, with high percentages of hCD45+ cells in BM and spleen, including B lymphoid and myeloid cells. Interestingly, injection of 7×106 vessel cells was sufficient to highly repopulate a NOD-SCID recipient, while injections of 5–6×106 tissue cells from tP3 and tP1 were not (Table 3). These data suggest that placental hu-SRCs are concentrated inside the placental labyrinth, possibly attached to the vascular endothelium.
Figure 3.
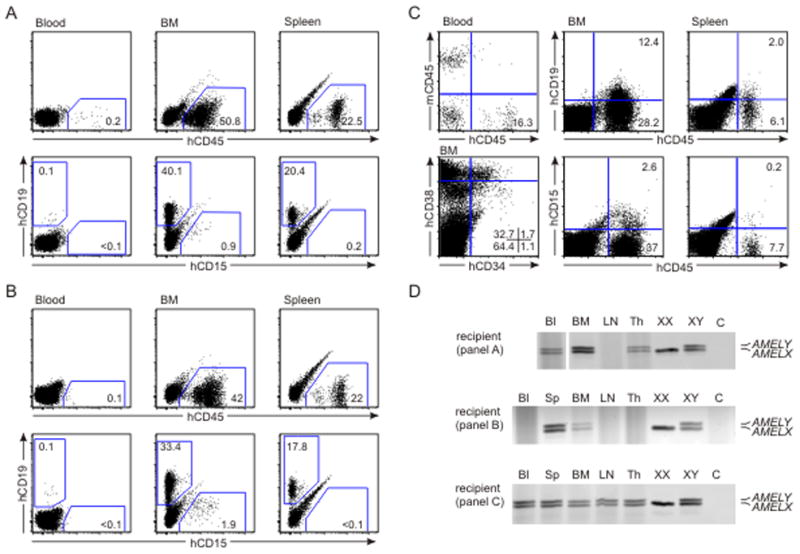
Long-term multilineage NOD-SCID hematopoietic repopulating potential of full term placenta cells. Flow cytometric multilineage analyses of blood, bone marrow (BM) and spleen cells isolated from NOD-SCID mice repopulated 5 months after injection of term placenta cells. (A) 20×106 cells from collagenase treated placenta tissue (tP2); (B) 7×106 cells from collagenase treated placental vessels (tP2); and (C) 10×106 cells from collagenase/dispase/pancreatin treated placenta tissue (tP3). Cells are stained with anti-mouse (m) CD45 and/or anti-human (h) CD45, CD19, CD15, CD34 and CD38 antibodies and analysed in the viable population. Number of events analysed was 3×104 for all tissues in panels A and B, and 2×105 for blood and 1×105 for BM and spleen in panel C. Percentages of gated and quadrant populations are indicated. (D) To verify the fetal (male) origin of the engraftment, PCR for the amelogenin gene was performed on blood (Bl), spleen (Sp), bone marrow (BM), thymus (Th) and lymph node (LN) DNA isolated from cells of the reconstituted recipients described in panels A, B and C. Control female (XX) cell DNA produces a single product (AMELX at 106 bp), whereas control male (XY) DNA produces two products (AMELY at 112 bp and AMELX).
Table 3.
Summary of NOD-SCID recipient repopulation by fetal-derived cells from term placenta
| Term Placenta (Male) | Cell preparation | Number of mice injected | Cell number injected | Bleed I (2 months post-injection) | Bleed II (4.5–5 months post-injection) |
|---|---|---|---|---|---|
| tP2 | Vessel | 1* | 7×106 | +++ | +++ |
| Placenta fresh (c) | 1+ | 20×106 | +++ | +++ | |
| tP3 | Placenta fresh (cdp) | 2 | 1×106 | Negative | Negative |
| 2 | 5×106 | Negative | Negative | ||
| 1& | 10×106 | +++ | +++ | ||
| tP1 | Vessel | 1 | 21×106 | +++ | Dead |
| Placenta fresh (c) | 1 | 6×106 | +/− | Negative | |
| Placenta frozen (c) | 1# | 1.3×106 | +++ | +++ | |
Term (male) placentas were treated and made into cell suspensions as indicated, and injected at various cell doses into NOD-SCID recipients. Out of 10 injected mice, 6 were positive (by flow cytometry) at 2 months post-injection. Multilineage flow cytometric analysis performed (at 2 months post-injection) on the blood of the recipient repopulated with tP1 vessel cells (20×106). 67% hCD45+, 0.2% hCD15+, 49% CD19+, 67% hCD38+, 2.1% hCD34+ and 0.07% hCD34+38− cells were found and were similar to percentages obtained from a control recipient transplanted (in the same experiment) with 20×106 cord blood cells. The tP1 recipient transplanted with 6×106 fresh placenta cells was considered +/− at 2 months post-injection since only 0.35% hCD45+, 0.79% hCD38+ and 0.52% hCD19+ cells were found. cdp=collagenase, dispase and pancreatin treatment; c=collagenase treatment
Flow cytometric analysis of recipient shown in Figure 3B.
Flow cytometric analysis of recipient shown in Figure 3A.
Flow cytometric analysis of recipient shown in Figure 3C.
Flow cytometric analysis of recipient shown in Supplementary Figure 7.
A combination of three enzymes (collagenase, dispase and pancreatin) was used to further optimise placenta cell preparations. The improved digestion conditions resulted in a higher viable cell recovery (13-fold) and increased percentages of CD34+CD38+ and CD34+CD38− cells (Suppl Fig 4), as compared to single collagenase treatment (Fig 1B). After injection of 10×106 cells from male tP3 (prepared using this method) into a NOD-SCID recipient, high percentages of hCD45+ cells were found in the blood, spleen and BM (Fig 3C) and cells were of the B lymphoid and myeloid lineages. Moreover, the recipient mouse BM contained immature human CD34+CD38− cells, strongly suggesting that the term placenta contains bona fide hematopoietic progenitors/stem cells.
The human hematopoietic cells detected in the flow cytometric analysis of NOD-SCID recipients (Fig 3A, B, C) transplanted with term placenta cells were derived from the fetal (male) part of the placenta, as shown by AMEL PCR analysis (Fig 3D) of BM, blood, spleen, lymph node and thymus DNA. Thus, term human placenta contains fetal-derived hu-SRCs that home to the BM and provide robust long-term multilineage hematopoietic engraftment of recipients.
Human placenta derived cell lines support human hematopoietic progenitors and possess characteristics of pericytes/perivascular placenta cells
To examine whether the human placenta contains cells typical of a hematopoietic supportive microenvironment (i.e. mesenchymal stromal cells), cell lines were established at various developmental stages – 3, 6, 16, 18 and 38 weeks of gestation. All the cell lines showed a fibroblastic morphology and 2 cell lines from each developmental time point were analysed.
The growth rates of the placenta cell lines varied. Early stage (maternally-derived) and term placenta cell lines showed slower growth than cell lines from the first and second trimester tissues (Table 4). In agreement with the previously described cell surface phenotype of first trimester and term placenta stromal cells (Bhatia et al., 1997; Fukuchi et al., 2004; Li et al., 2005; Yen et al., 2005; Zhang et al., 2006; Zhang et al., 2004), our lines are CD13+, CD29+, CD44+, CD105+, HLA-DR−, CD14−, CD34−, CD45−, CD19−, CD2−, CD3−, CD4lo/−, CD8− and CD11blo/− (Suppl Fig 5A, Table 4). Also, in cultures allowing for osteogenic differentiation, our placenta lines (second trimester and term) were positive for alkaline phosphatase (Suppl Fig 5B) and mineralization and most cell lines also could be differentiated into adipocytes (Suppl Fig 5C). Interestingly, when three of these cell lines were tested (H93-6, H92-1 and H91-2) in matrigel, they formed tubules indicative of endothelial potential (Suppl Fig 5D, Table 4). Thus, the human placental cell lines have the same mesenchymal potential as reported previously in hematopoietic supportive AGM (Durand et al., 2006).
Table 4.
Phenotypic characteristics and functional properties of human placental stromal cell lines through development
| Line | Age (wk) | Doubling time (hrs) | Origin | Mesenchymal markers | Osteogenic potential | Adipogenic potential | Endothelial potential | Co-culture fold increase in CB CD34+ | Co-culture fold increase in CB CFU-GM | Co-culture fold increase in CB CFU-Mix |
|---|---|---|---|---|---|---|---|---|---|---|
| R19-a | 3 | 59 | M | + | − | − | ND | 3.9±1.6 | 80.4 | 7.9 |
| R19-3 | 3 | 50 | M | + | − | − | ND | 6.6±1.9 | 69.3 | 3.0 |
| R17-2 | 6 | 32 | F or M | ND | +++ | ND | ND | ND | ND | ND |
| R17-3 | 6 | 34 | F & M | ND | − | ND | ND | ND | ND | ND |
| H93-6 | 16 | 29 | F | + | +++ | +/− | + | 3.6±1.3 | 65.0 | 0 |
| H92-1 | 16 | 29 | F | + | +++ | ++ | + | 7.7±2.9 | 370.6 | 6.1 |
| H91-1 | 18 | 35 | F | + | +++ | ++ | + | 2.2±0.5 | 80.4 | 3.1 |
| H91-2 | 18 | 35 | F | + | +++ | +++ | ND | 3.8±0.3 | 102.7 | 0 |
| L13-1 | term | 41 | ND | ND | +++ | ND | ND | ND | ND | ND |
| L13-5 | term | 41 | ND | ND | +++ | ND | ND | ND | ND | ND |
Cell line origin was determined by STR profiling. The profile of R17-2 yielded only 2 alleles for each gene, while R17-3 gave a mix of alleles for many genes. ND=not done. M=maternal; F=fetal
Since a recent publication has highlighted pericytes/perivascular cells as the in vivo correlate/precursors to mesenchymal stromal/stem cells (Crisan et al., 2008), we examined our cell lines for pericyte characteristics. Flow cytometric analyses showed that cell line H92-1 expressed pericyte markers NG2 and CD146 (Fig 4A), as did H93-6 and H91-1 (not shown). To localize these cells in vivo, cryosections from week 16 human placenta were immunostained with three pericyte markers CD146, NG2 and α-SMA (smooth muscle actin) (Fig 4B and C). As previously shown (Crisan et al., 2009), the only cells expressing CD146, NG2 and α-SMA in situ are pericytes/perivascular cells closely associated to endothelial cells in microvessels (MV), capillaries (C) and large vessels (LV). These data demonstrate that placenta stromal cell lines at week 16 of gestation are pericyte-like cells, and together with data in Fig 1E and F, suggest that the perivascular/vascular microenvironment and the hematopoietic system develop in parallel in the placenta.
Figure 4.
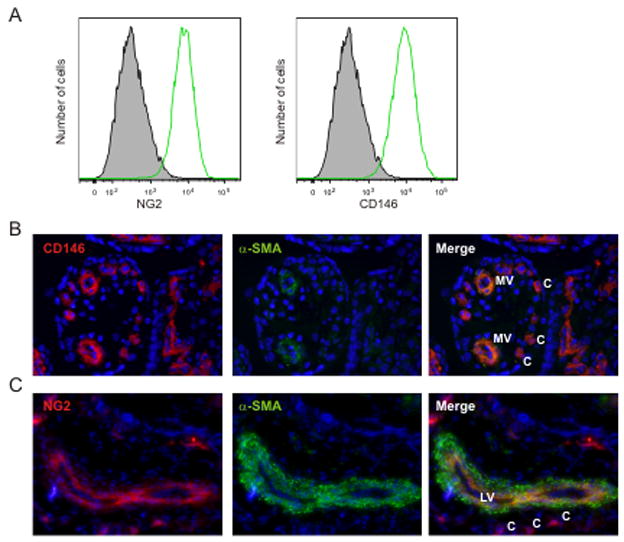
Pericyte marker expression on human placenta stromal cell lines and human placenta tissue. (A) Histogram of flow cytometric analysis for NG2 and CD146 expression on H92.1 placenta stromal cell line is shown. Immunostained cryosections from 16 week human placenta co-stained for (B) CD146 (red) and α-SMA (green) (20× lens) or (C) NG2 (red) and α-SMA (green) (10× lens). Single and merged fluorescence are shown. MV=microvessels; C=capillaries; LV=large vessel.
The hematopoietic supportive properties of placenta stromal cell lines were tested in co-cultures. Confluent monolayers of stromal cells (3, 16, 18 week stages) were overlayed with 5000 CD34+ UCB cells and cultured in factor-supplemented medium. After 12 days the number of CD34+ cells was increased 2 to 8-fold (Table 4). Clonogenic activity was also tested. As compared to the input number of CFU (in freshly sorted CB CD34+ cells), the placenta cell lines supported a 65–370-fold expansion of CFU-GM and up to 8-fold expansion of CFU-Mix (Table 4 and Suppl Fig 6). Thus, based on the results of the cell lines, the human placenta contains hematopoietic supportive pericytes/perivascular stromal cells.
Discussion
Prior to this study, only the presence of progenitors in the human placenta has been reported (Barcena et al., 2009). Here we confirm that the human placenta contains all types of hematopoietic progenitors but more importantly, we show that the human placenta also contains hu-SRCs. hu-SRCs are detected in the human placenta as early as week 6 in gestation, throughout fetal development and most surprisingly at term. This is an unexpected result since previous data in the mouse term placenta show almost no adult repopulating HSCs (Gekas et al., 2005). Given that human placenta cells throughout ontogeny provide long-term repopulation of the NOD-SCID hematopoietic system to the same levels and in the same hematopoietic lineages (B lymphoid and myeloid) as UCB cells, they are bona fide hu-SRCs (Cashman et al., 1997; Coulombel, 2004; Larochelle et al., 1996; Pflumio et al., 1996). Thus, the human placenta can now be acknowledged as a new territory of hu-SRCs and this routinely discarded tissue can now be used to provide further insight into cell-cell interactions and molecules relevant to human hematopoietic progenitor/stem cell growth.
Our data demonstrate that the temporal sequence of hematopoietic cell appearance in the human placenta is generally conserved as compared to the mouse placenta. In the mouse, adult repopulating HSCs appear in the AGM region, vitelline and umbilical arteries first and are thereafter found in the YS and placenta (de Bruijn et al., 2000; Dzierzak and Speck, 2008; Gekas et al., 2005; Ottersbach and Dzierzak, 2005). In the human conceptus, hematopoietic progenitor/stem cells are found at day 27 in the aorta, concomitant to the appearance of clusters of cells closely adherent to the aortic lumenal wall (Tavian et al., 1996; Tavian et al., 1999). Hematopoietic progenitors are found in the human YS but with a less robust hematopoietic potential (Tavian et al., 2001). Our results indicate that fetal-derived hu-SRCs are present in the human placenta already at gestational week 6. The presence of HSCs at earlier stages, particularly between gestational weeks 3 to 6, is still undetermined. Most placentas we analysed at these stages were variable quality. Considering that, in the mouse placenta, limiting numbers of fetal-derived HSCs are found at E11 and rapidly increase to the highest numbers at E12–13 (Gekas et al., 2005; Ottersbach and Dzierzak, 2005), our future analyses on the possible earlier appearance of HSCs in human placenta will depend on improved placenta isolation and more sensitive maternal/fetal genotyping.
Another important and timely result comes from our panel of placental stromal cell lines. For the first time these cells are identified as CD146 and NG2 expressing pericyte-like cells. These stromal cell lines support the expansion of cord blood CD34+ cells and immature hematopoietic progenitors in co-cultures. Interestingly such pericyte-like cells were found in situ in the developing human placenta, suggesting an in vivo role in hematopoietic support. At all gestational stages the placenta stromal cells express classical mesenchymal markers and after gestation week 6 possess mesenchymal lineage potentials (osteo- and adipogenic), in agreement with other reported placenta cell lines (Fukuchi et al., 2004; Igura et al., 2004; Li et al., 2005; Miao et al., 2006; Parolini et al., 2008; Portmann-Lanz et al., 2006; Wulf et al., 2004; Yen et al., 2005; Zhang et al., 2006). Since some mesenchymal cell lines constitute a suitable feeder layer for in vitro maintenance and/or expansion of primate and human ES cells (Kim et al., 2007; Miyamoto et al., 2004) and long term culture initiating cells (Zhang et al., 2004), it will be interesting to determine whether they are pericyte-like and are of maternal or fetal origin (In ’t Anker et al., 2004). Our cell lines from gestation week 3 placenta were found to be maternally derived (by STR profiling) and exhibited slow growth, as compared to week 6, 16 and 18 placenta cell lines. Nonetheless, these cells effectively support the growth of CD34+ cells in co-cultures yielding an 8-fold increase in CFU-Mix. Maternal stromal cells therefore may contribute at early stages to hematopoietic support and later in gestation, the more rapidly doubling fetal stromal cells predominate in the growth of the placenta as a highly vascular and hematopoietic territory.
At early developmental time points (week 6 and 9), hematopoietic progenitors are in both the CD34+ and CD34− fractions. CFU-GM and CFU-Mix are restricted initially to the CD34− fraction of week 6 placenta and switch to the CD34+ fraction by week 15 suggesting developmental regulation of this marker on progenitors. Similarly, hu-SRCs are found in both CD34+ and CD34− fractions at week 6. Later at week 16–20, hu-SRCs are in both fractions but appear to be more enriched in the CD34+ fraction. This is in agreement with the published data showing a subset of hu-SRCs in the CD34− fraction of UCB (Bhatia et al., 1998; Wang et al., 2003). Interestingly, immunostainings of week 16 placenta sections show CD34+CD45+ hematopoietic cells within placental villi stroma and CD45high expressing cells that appear to be budding from the vasculature. Moreover, high percentages of 34+38− cells and hu-SRCs were found in the collagenase treated vessel cell preparations (after extensive pre-washing of the placenta to remove circulating blood) and in collagenase treated placenta tissue. Since previous studies demonstrated the hematopoietic potential of human YS, embryonic liver and fetal BM vascular endothelium (Oberlin et al., 2002) and also was suggested in the early mouse placenta (Corbel et al., 2007; Ottersbach and Dzierzak, 2005; Zeigler et al., 2006: Gekas et al. 2005), our data support the notion that hu-SRCs are generated, harboured and/or amplified in vascular labyrinth placenta niche.
Finally, in addition to revealing the fundamental aspects of human placenta HSC development, our results have implications for the human placenta as source of HSCs alongside UCB for banking and potential clinical use. From our data, a conservative estimate of the HSC content of a human placenta (using the 3 enzyme treatment) is about 10% of the published number of HSCs contained in one unit of UCB (Bhatia et al., 1997; Wang et al., 1997). As a 13-fold increase was already achieved through the implementation of 3 enzymes versus collagenase only, further increases in placenta hu-SRC harvest are expected. Importantly, if placental cells are to be a source of clinically useful HSCs, they must withstand storage procedures. In preliminary experiments, we found that the percentage of CD34+ placenta cells increased 1.4-fold and that hu-SRC potential was retained and enriched after storage in liquid nitrogen. Remarkably, only 1.3×106 thawed unsorted cells from placenta tP1 were required for robust NOD-SCID multilineage hematopoietic engraftment (Suppl Fig 7) as compared to the low engraftment yielded with 6 × 106 freshly prepared collagenase treated tP1 cells (Table 3). Taken together, the human placenta is a highly hematopoietic tissue throughout development, containing potent hu-SRCs. As a rest tissue normally discarded in the birthing process, the human placenta can be considered as potential source for additional hematopoietic progenitor/stem cells useful for hematologic clinical applications and human regenerative medicine.
Experimental Procedures
Tissues and cell preparation
Human fetal tissues were obtained from elective abortions (CASA clinics, Leiden and Rotterdam) and were contingent on informed consent. Umbilical cord blood and term placentas were obtained from vaginal deliveries or by cesarean section. The use of fetal tissues was approved by the Medical Ethical Committee of the Erasmus Medical Center (MEC-2006-202). Gestational age was determined by ultrasonic fetal measurements. Placenta cells were isolated directly or after overnight storage at 4°C. Umbilical cord was cut and removed, along with the amniotic sac and deciduas, from the placenta under sterile conditions. The outside of the placenta was washed with cold PBS/EDTA/PS (Phosphate-buffered saline added with EDTA, penicillin (100 U/ml) and streptomycin (100 μg/ml)). The blood remaining inside placenta was aspirated and collected (placenta blood) and the placental vasculature was flushed extensively with PBS/EDTA (up to 10 times) via the umbilical vein and arteries to eliminate residual blood within the placental vascular labyrinth.
Collagenase (0.125% w/v type I collagenase (Sigma) in PBS/10 % fetal calf serum (FCS)/PS) was injected inside the placental vascular labyrinth. After 1 hour of incubation at 37°C, intra-vascular cells detached by the collagenase treatment were aspirated and collected (vessels collagenase).
Placenta tissue was minced and washed thoroughly in cold PBS/FCS/PS, and treated with 0.125% w/v type I collagenase in PBS/FCS/PS for 1 – 1.5 hours under agitation. 5 g of placenta tissue per 200 ml of buffer was found to be optimal for enzymatic digestion. Placenta was treated, in some cases, with Collagenase, Pancreatin (Sigma, 0.3%) and Dispase I (neutral protease grade I, Roche, 0.33 mg/ml) (3 enzyme treatment). All enzymatic treatments were performed in presence of DNase (Sigma). Tissue was dissociated by repeated pipetting, passed through cotton gauze to eliminate non-digested tissue clumps, and the filtered cell suspension was washed twice. Mononuclear cells were recovered by Ficoll density gradient fractionation (Density 1.077 g/ml, Lymphoprep, Axis-Shield PoC AS), washed twice and filtered through a 40 μm nylon cell strainer. Umbilical cord blood was diluted (1/2) into PBS/FCS/PS and mononuclear cells were collected after Ficoll. Cells were washed, counted and kept at 4°C for further utilisation.
Enzyme stock solution preparation
Pancreatin (2.5%) was prepared with pancreatin powder from porcine pancreas dissolved in 0.5% PVP solution (polyvinylpyrolidone K30, Fluka). DNAse and dispase I (5 mg/ml) were prepared in sterile MilliQ water. Collagenase (2.5%) was prepared with collagenase powder dissolved in sterile PBS.
Mouse transplantations and post-transplantation tissue collection
Cells from placenta preparations or placenta cells sorted on the basis of CD34 expression (along with 1–2 × 105 helper spleen cells) were intravenously injected into irradiated (3–3.5 Gy) NOD-SCID (adult) or intrahepatically into Rag γC−/− (5 day old) mice. 4 weeks to 5 months later, hematopoietic tissues were collected. Peripheral blood was diluted and mononuclear cells were isolated using Ficoll or lysing solution. Spleen, lymph nodes and thymus were crushed separately on a 40 μm nylon cell strainer. BM cells were flushed from the femurs and tibias of recipient mice. For all tissues collected, cells were kept for further DNA analyses.
Flow cytometry analysis
Placenta cells were stained with the following antibodies: purified CD16/CD32 (pre-block), fluorescein isothiocyanate (FITC) or APC-human (h)CD34, FITC -mouse (m)CD45, phycoerythrin (PE) or PE Cy7- hCD38, FITC-hCD31, PE or PerCP Cy5.5-hCD45. Cells from tissues of NOD-SCID or Rag γC−/− recipient mice were stained with the following antibodies: purified CD16/CD32, FITC or PE or PerCP-Cy5.5-hCD45, FITC or PE-mCD45, FITC-hCD15, PE-hCD19, FITC-hCD34, PE-hCD38, PE Cy7-hCD14, APC Cy7-HLA-DR. After 30 minutes of staining, cells were washed and stained with 7AAD (Molecular Probes, Leiden, NL) or Hoechst 33258 (1 μg/ml, Molecular Probes) for dead cell exclusion. Blood samples from a non-injected mouse and from informed consent individuals were used as controls. Stromal cell lines were stained with primary antibodies NG2 and CD146, followed by goat anti-mouse Alexa 488. All antibodies were from BD Pharmingen, Immunotech or Invitrogen and analyses performed on a FACScan or Aria (Becton Dickinson).
Immunohistochemistry
Cryosections (7 μm) of human placenta (1–2 cm pieces) were prepared and immunostained as described previously (Crisan et al., 2008). Primary antibodies used: CD146 (BD Pharmingen), NG2 (BD Pharmingen), and CD45 (eBioscience). α-SMA-FITC (Sigma) and biotinylated anti-CD34 (NOVUS Biologicals). Secondary goat anti-mouse antibody was biotinylated (Dako) or coupled to Alexa 488 (Invitrogen). Streptavidin-Cy3 (Sigma) was used with biotinylated antibodies. Sections were mounted with medium for fluorescence (Vector) containing DAPI. An isotype-matched negative control was performed for each immunostaining. Sections was observed on an epifluorescence microscope (Zeiss).
Hematopoietic colony assay
CD34+ and CD34− cells from human placentas at different stages of development or BM from NOD-SCID recipients tranplanted with human placenta cells were cultured in methylcellulose medium (Methocult H4434; Stem Cell Technologies Inc.) at 37°C. CFU-GM and Mix and BFU-E colonies were scored with an inverted microscope after day 21 and 28 of culture.
Generation of human placenta-stromal cell lines
Placenta tissues were dissected into small pieces and explant cultured on 0.1% gelatin coated 6-well plates at the air-medium interface in hu-LTCSM medium (50% H5100, Stem Cell Technologies; 15% heat-inactivated FCS, Gibco; 35% α-MEM, Gibco; 1% Pen/Strep, Gibco; 1% Glutamax-I (100×), Gibco; 10 μM β-mercaptoethanol, Merck) at 37 °C, 5% CO2. After several days, cells were harvested using trypsin-EDTA and were seeded on new pre-coated dishes supplemented with 20% filtered supernatant from the previous passage. Six lines per placenta at 3, 6, 16, and 18 weeks of gestation and 11 lines from term placentas were established. Lines were checked daily and split when sub-confluency was reached. Growth curves were established from passage 3 onwards.
Mesenchymal differentiation
Osteogenic differentiation was performed in Dulbecco’s Moified Eagle’s Medium (DMEM) (Gibco) containing 15% heat-inactivated FCS (Gibco), 1% PS (Gibco), 200 μM ascorbic acid (Sigma), 10 mM β-glycerophosphate (Sigma) and 10−7 M dexamethasone (Sigma). Cells were seeded in uncoated 6-well plates (500, 1000 and 2000 cells/cm2) and incubated at 37 °C. After 11 and 14 days alkaline phosphatase activity was determined (Sigma) and at 28 days Alazarin Red staining was performed (Sigma). Adipocyte differentiation of subconfluent cells was performed in DMEM (10% FCS), dexamethasone (1 μM), IBMX (500 μM), indomethacin (60 μM) and 5 μg/ml insulin for 7 days. Cells were stained with Oil red. Endothelial differentiation was performed as previously described (Chen et al., 2009) on Matrigel Matrix (BD Matrigel™ Basement Membrane Matrix, 354234) precoated 96 well dishes (50 μl/well) and incubated at 37° C for 40 min. Stromal cells (1 × 104) were seeded on top of matrigel, incubated at 37° C and observed up to 6 hours.
Hematopoietic supportive stromal co-cultures
Mononuclear cord blood cells were sorted on a FACSAria (Becton Dickinson) based on CD34 expression and Hoechst 33258 exclusion (Molecular Probes). 5000 CD34+ CB cells were co-cultured with a pre-established confluent irradiated layer (12 Gy) of human stromal cell lines in a 24-well plate using h-LTCSM medium, supplemented with hFLT3 (50 ng/ml), hSCF (100 ng/ml) and hTPO (20 ng/ml). After 12 days of co-culture, cells were harvested, counted, analyzed by flow cytometry and plated in hematopoietic colony assays.
Conventional PCR analysis
Embryo gender was determined by PCR amplification of the amelogenin locus (AMEL) (Sullivan et al., 1993) that differs in size on the X (106bp) and Y (112bp) chromosomes. PCR mixture contained AmpliTaq DNA polymerase PCR Buffer (15mM MgCl2; Roche, Applied Biosystems), 200μM of each dNTP, 400nM of each primer, 0,01U/μl of SuperTaq DNA polymerase (HT Biotechnology, Applied Biosystems) and products were separated on a 4% agarose gel. Human chromosome 17 α-satellite PCR (Chr 17) (Becker et al., 2002) mixture contained AmpliTaq DNA polymerase PCR Buffer (15mM MgCl2), 200μM of each dNTP, 250nM of each primer, MgCl2 2mM, 0,01U/μl of SuperTaq DNA polymerase and yields a PCR product of 850bp Conditions for all PCRs are in Supplementary Table 1. 0.5–3 μg DNA from hematopoietic tissues of recipient mice or in vitro cultures was used for AMEL PCR) and 0.25–0.5 μg DNA was used for human Chr 17 PCR.Limits of sensitivity of human AMEL PCR and Chr 17PCR were both 1 human cell in 105 mouse cells.
STR typing
Human embryo samples and recipient mouse tissue samples were profiled using the PowerPlex 16 System Kit (Promega) as usually applied to human identity testing in forensic DNA-analysis. PCR mixtures contained 1.3μl Gold Star 10× Buffer, 1.3μl PowerPlex16 10× Primer Pair Mix, 2U of AmpliTaq Gold DNA polymerase (Roche, Applied Biosystems) and 1ng of human genomic DNA or 50–200ng of DNA from mouse samples. PCR was done as described in Supplementary Table 1 using the PTC-200 Thermal Cycler from MJ Research (Biorad). 1μl of PCR product was mixed with 9,6 μl of Hi-Di Formamide (Applied Biosystems) and 0.4μl of internal lane standard ILS 600 and denatured at 95°C for 3min. Amplified fragments were detected using the ABI PRISM 3100 Genetic Analyser (Applied Biosystems) and data were analysed with the GeneMapperID v3.2 software (Applied Biosystems). The statistical certainty of the profiles obtained from each reconstituted mouse was established by calculating the random match probability with correction for potential allele sharing with the mother (Weir, 2003). For the 19 week placenta recipient (Fig 2E, Table 1) displaying a complete profile, the probability of a match with a profile amongst a selection of unrelated and related individuals (i.e. not from the embryonic source) was estimated at 8.65×10−12. For the three 8 week placenta recipients showing a partial profile (Table 1), this probability was estimated at 6.87×10−11, 1.89×10−8 and 3.73×10−7 respectively. Estimations are based on the European population and may slightly vary if non-European populations are taken in account. The limit of sensitivity of STR profiling in which a complete profile could be obtained was 1 human cell in 1–2 × 103 mouse cells.
Supplementary Material
Acknowledgments
We thank the research-nurses, Joke van Rhee-Binkorst and Wilma Keller (Erasmus MC), the staff of the CASA clinics in Leiden and Rotterdam and other clinical personnel and most importantly the tissue donors for their pivotal contributions. We also thank Frederik Walberg, Christiane Kuhl, Tomomasa Yokomizo for experimental assistance and Kay Ballantyne for assistance in the statistical interpretation of STR profiles. Support was kindly provided by the Landsteiner Society for Blood Research (0614), NIH R37 (DK51077), Dutch BSIK Stem Cells in Development and Disease (03038), Dutch BSIK Tissue Engineering, NWO VIDI (917.76.345)(CR) and Marie Curie Fellowship (CT-2006-025333)(KB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, et al. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439–1448. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- Barcena A, Kapidzic M, Muench MO, Gormley M, Scott MA, Weier JF, Ferlatte C, Fisher SJ. The human placenta is a hematopoietic organ during the embryonic and fetal periods of development. Dev Biol. 2009;327:24–33. doi: 10.1016/j.ydbio.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Becker M, Nitsche A, Neumann C, Aumann J, Junghahn I, Fichtner I. Sensitive PCR method for the detection and real-time quantification of human cells in xenotransplantation systems. British journal of cancer. 2002;87:1328–1335. doi: 10.1038/sj.bjc.6600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman J, Bockhold K, Hogge DE, Eaves AC, Eaves CJ. Sustained proliferation, multi-lineage differentiation and maintenance of primitive human haemopoietic cells in NOD/SCID mice transplanted with human cord blood. Br J Haematol. 1997;98:1026–1036. doi: 10.1046/j.1365-2141.1997.3233140.x. [DOI] [PubMed] [Google Scholar]
- Challier JC, Galtier M, Cortez A, Bintein T, Rabreau M, Uzan S. Immunocytological evidence for hematopoiesis in the early human placenta. Placenta. 2005;26:282–288. doi: 10.1016/j.placenta.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang H, Gou X, Horikawa Y, Xing J, Chen Z. Upregulation of HAb18G/CD147 in activated human umbilical vein endothelial cells enhances the angiogenesis. Cancer letters. 2009;278:113–121. doi: 10.1016/j.canlet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Corbel C, Salaun J, Belo-Diabangouaya P, Dieterlen-Lievre F. Hematopoietic potential of the pre-fusion allantois. Dev Biol. 2007;301:478–488. doi: 10.1016/j.ydbio.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Coulombel L. Identification of hematopoietic stem/progenitor cells: strength and drawbacks of functional assays. Oncogene. 2004;23:7210–7222. doi: 10.1038/sj.onc.1207941. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand C, Robin C, Dzierzak E. Mesenchymal lineage potentials of aorta-gonad-mesonephros stromal clones. Haematologica. 2006;91:1172–1179. [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, Yoder MC. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Hann IM, Bodger MP, Hoffbrand AV. Development of pluripotent hematopoietic progenitor cells in the human fetus. Blood. 1983;62:118–123. [PubMed] [Google Scholar]
- Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543–553. doi: 10.1080/14653240410005366-1. [DOI] [PubMed] [Google Scholar]
- In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Song CH, Sung HJ, Yoo YD, Geum DH, Park SH, Yoo JH, Oh JH, Shin HJ, Kim SH, et al. Human placenta-derived feeders support prolonged undifferentiated propagation of a human embryonic stem cell line, SNUhES3: comparison with human bone marrow-derived feeders. Stem Cells Dev. 2007;16:421–428. doi: 10.1089/scd.2006.0098. [DOI] [PubMed] [Google Scholar]
- Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- Larochelle A, Vormoor J, Hanenberg H, Wang JC, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao XL, Kato I, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang P, Mao N. Isolation and Identification of a Multilineage Potential Mesenchymal Cell from Human Placenta. Placenta. 2005 doi: 10.1016/j.placenta.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Hayashi K, Suzuki T, Ichihara S, Yamada T, Kano Y, Yamabe T, Ito Y. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22:433–440. doi: 10.1634/stemcells.22-4-433. [DOI] [PubMed] [Google Scholar]
- Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- Pflumio F, Izac B, Katz A, Shultz LD, Vainchenker W, Coulombel L. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–3740. [PubMed] [Google Scholar]
- Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek DV. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664–673. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Mannucci A, Kimpton CP, Gill P. A rapid and quantitative DNA sex test: fluorescence-based PCR analysis of X-Y homologous gene amelogenin. BioTechniques. 1993;15:636–638. 640–631. [PubMed] [Google Scholar]
- Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lievre F, Peault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87:67–72. [PubMed] [Google Scholar]
- Tavian M, Hallais MF, Peault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development. 1999;126:793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- Tavian M, Robin C, Coulombel L, Peault B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- Tse W, Bunting KD, Laughlin MJ. New insights into cord blood stem cell transplantation. Curr Opin Hematol. 2008;15:279–284. doi: 10.1097/MOH.0b013e328304ae2c. [DOI] [PubMed] [Google Scholar]
- Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y, Fujimura Y, Tsuji T, Ikehara S, Sonoda Y. SCID-repopulating cell activity of human cord blood-derived CD34− cells assured by intra-bone marrow injection. Blood. 2003;101:2924–2931. doi: 10.1182/blood-2002-09-2782. [DOI] [PubMed] [Google Scholar]
- Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- Weir BS. Forensics. In: Balding DJM, Cannings C, editors. Handbook of Statistical Genetics, B. Hoboken, NJ: Wiley and Sons; 2003. pp. 830–850. [Google Scholar]
- Wulf GG, Viereck V, Hemmerlein B, Haase D, Vehmeyer K, Pukrop T, Glass B, Emons G, Trumper L. Mesengenic progenitor cells derived from human placenta. Tissue Eng. 2004;10:1136–1147. doi: 10.1089/ten.2004.10.1136. [DOI] [PubMed] [Google Scholar]
- Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Oberlin E, Tavian M, Peault B. Blood-forming endothelium in human ontogeny: lessons from in utero development and embryonic stem cell culture. Trends Cardiovasc Med. 2006;16:95–101. doi: 10.1016/j.tcm.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mitsuru A, Igura K, Takahashi K, Ichinose S, Yamaguchi S, Takahashi TA. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006;340:944–952. doi: 10.1016/j.bbrc.2005.12.091. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, Tang P, Mao N. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657–664. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.