Abstract
We determined reactivity of human blood to a vaccine based on the recombinant N-terminus of candidal Als3p (rAls3p-N) in preparation for future clinical trials. Healthy donor plasma had high immunoglobulin G titers (median, 1:51,200) and lower immunoglobulin A (median, 1:3,200) and immunoglobulin E (median, 1:128) titers to rAls3p-N by enzyme-linked immunosorbent assay. rAls3p-N stimulated interferon γ (IFN-γ) and interleukin (IL)–17, but not IL-4, from donor lymphocytes by enzyme-linked immunosorbent spot assay and IL-12 p70, IFN-γ, IL-17, and IL-10 by cytometric bead array. Donors reacted to diverse immunodominant epitopes. Thus, facile humoral and cellular assays can monitor immune responses to the rAls3p-N vaccine in planned clinical trials.
We are developing a vaccine, based on the recombinant N terminus of the Als3p surface adhesin from Candida albicans (rAls3p-N), which protects mice against otherwise-lethal disseminated candidiasis [1] and against disseminated Staphylococcus aureus infection [2]. T lymphocyte–derived interferon γ (IFN-γ) and interleukin (IL)–17 are required for vaccine-mediated protection in mice, whereas IL-4 is dispensable for protection [1, 3]. We also identified antibody titer cut-off values in vaccinated and control mice that correlated with survival versus mortality after infection [1, 4]. Hence, production of IFN-γ and IL-17 in response to vaccination, as well as anti-rAls3p-N antibody titers, are potential candidate assays for monitoring vaccine efficacy in future clinical trials.
As the rAls3p-N vaccine undergoes proper manufacturing development for a planned phase I clinical trial, it is necessary to establish relevant immunological assays to track vaccine immunogenicity. Such assays would become the basis for establishment of a surrogate marker of protection in future clinical testing in patients who are at risk for infection [5]. Because humans are colonized with Candida species (from which the rAls3p-N vaccine protein is derived), we hypothesized that healthy humans would have pre-existing immunity to the rAls3p-N protein. We developed humoral and cell-mediated immune assays to determine the magnitude and variability of immunity to rAls3p-N in healthy humans and sought to define the rAls3p-N epitope(s) responsible for these immune responses.
Materials and methods
Blood was drawn from healthy volunteers into Vacutainer CPT tubes (Becton Dickinson) for 1-step separation of plasma from peripheral blood mononuclear cells (PBMCs), in accordance with the manufacturer’s instructions. The human subjects component of the study was approved by the local institutional review board, and informed consent was obtained from all donors.
We developed human enzyme-linked immunosorbent assays (ELISAs) to detect immunoglobulin (Ig) G1, IgG2, IgG3, IgG4, total IgG, and IgA and IgE anti-rAls3p-N antibodies based on our previously described murine ELISAs [1]. In brief, rAls3p-N (amino acids 17 to 432 of Als1p) was produced in Saccharomyces cerevisiae and purified by Ni-NTA matrix affinity purification. Ninety-six–well plates were coated with rAls3p-N, blocked, and exposed to serial dilutions of human plasma. Negative control wells receive no primary antibody (ie, no plasma). Goat anti-human secondary antibody conjugated with horse-radish peroxidase was added, followed by washing and incubation with O-phenylenediamine to generate a colorometric reaction. The reaction was terminated after 20 min with sulfuric acid. The ELISA titer was taken as the reciprocal of the last serum dilution with a positive optical density (OD) reading (ie, >OD of negative control samples + [standard deviation × 2]).
PBMCs were plated at 2 × 105 cells per well in 100 μL of complete media on IFN-γ, IL-17, or IL-4 human enzyme-linked immunosorbent spot (ELISpot) kits (eBiosciences). Cells were stimulated with rAls3p-N (12.5 μg/mL) plus anti-CD28 antibody (BD Pharmingen), heat-killed (60°C for 1 h) C. albicans germlings (pre-germinated for 1 h at 37°C in Roswell Park Memorial Institute 1640 medium prior to killing) at a 2:1 ratio of organisms to PBMCs, media plus anti-CD28 antibody, or media alone. CD28 antibody was added to provide a second signal for T cell activation in the absence of professional antigen presenting cells in PBMCs. In some experiments, PBMCs were exposed to 20-mer peptides overlapping by 10 amino acids, spanning the length of the rAls3p-N protein (1 peptide per well at 50 μg/mL; peptides from Sigma). After 48 h of incubation, the plates were processed per the manufacturer’s instructions and read using a Biosys Bioreader 5000. Spot frequency in stimulated wells was corrected by subtracting background signal from wells with cells plus media alone and was normalized per 105 cells.
Production of IFN-γ, IL-4, IL-8, IL-10, IL-12 p70, and IL-17 were measured from ELISpot supernatants by Cytometric Bead Array Flex Sets (Becton Dickinson) and flow cytometry per the manufacturer’s recommendation. These cytokines were chosen because they reflect type 1 (IFN-γ and IL-12 p70), type 2 (IL-4, IL-10), and Th17 (IL-17, which induces IL-8 expression by other cells) responses.
The nonparametric Mann Whitney U test was used for unpaired comparisons, and the Wilcoxon signed rank test was used for paired comparisons. Correlations were determined by the Spearman rank test. P < .05 was considered to be statistically significant.
Results
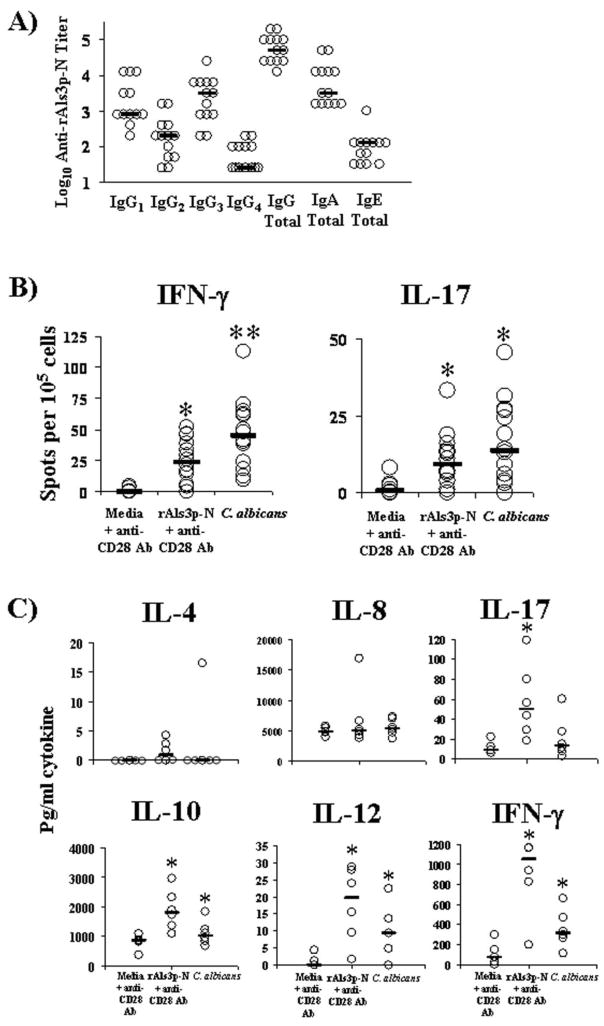
To determine whether healthy humans have baseline anti-rAls3p-N antibody titers, plasma samples from 13 healthy donors were screened for total IgG and IgG1, IgG2, IgG3, and IgG4 subtype titers. We found that all donors had high anti-rAls3p-N IgG serum titers (median value [inter-quartile range], 51,200 [102,400–25,600]) (Figure 1A). All donors also had detectable Ig subtype titers against rAls3p-N. Titers were higher for both IgG1 and IgG3 subtypes than they were for IgG2 and IgG4 subtypes (P < .001 for IgG1 or IgG3 titers vs. both IgG2 and IgG4 titers).
Figure 1.
Healthy human donors have preexisting immunity to the rAls3p-N vaccine protein. A, Serum samples from 13 donors were tested in immunoglobulin (Ig) G, IgG1, IgG2, IgG3, and IgG4 enzyme-linked immunosorbent assays to quantify rAls3p-N titers. Bars denote median titers. B, Peripheral blood mononuclear cells (PBMCs) from healthy human donors produce interferon (IFN)–γ and interleukin (IL)–17 in response to the rAls3p-N protein and to pre-germinated, heat-killed Candida albicans. Results from 14 donors are shown in both graphs. Spots from each well were normalized per 105 PBMCs. Bars denote median values. *P <.05 versus media; **P <.05 versus both rAls3p-N and media. C, Supernatants from 6 donor PBMCs were assayed for cytokines by Cytometric Bead Array. Bars denote median values. *P <.05 versus media control.
IgA and IgE titers were also detectable in all donors, although they were lower than IgG titers (Figure 1A). Median (inter-quartile range) titers were 3200 (1600–12,800) and 128 (32–128) for IgA and IgE, respectively. IgA titers correlated with IgG titers in individual donors (ρ = 0.7; P<.001); IgE titers did not correlate with IgG titers (ρ = 0.4; P = .1).
IFN-γ and IL-17 have been shown to be critical to vaccine efficacy in preclinical studies; in contrast, IL-4 was dispensable for protection [1, 3]. To determine the strength of cell-mediated immunity to the vaccine protein, we evaluated IFN-γ, IL-17, and IL-4 production by PBMCs in response to rAls3p-N, as well as to pregerminated, heat-killed C. albicans (which expresses Als3p on its cell surface [6]). Thirteen of 14 donors demonstrated an IFN-γ response to the vaccine protein; all donors demonstrated an IFN-γ response to heat-killed C. albicans (Figure 1B). Overall IFN-γ production was significantly greater in response to the vaccine protein and to C. albicans than it was to media alone (P < .01 for both comparisons). IFN-γ production was also greater in response to C. albicans than it was to rAls3p-N (P = .001). PBMCs from most donors did not produce detectable levels of IL-4 (data not shown).
Eleven of fourteen donors demonstrated an IL-17 response to the vaccine protein; 12 donors demonstrated an IL-17 response to C. albicans (Figure 1B). IL-17 production was significantly greater in response to the vaccine protein and C. albicans than it was to media alone (P < .05 for both comparisons). IL-17 production in response to C. albicans and vaccine protein were similar. In paired PBMC samples, IL-17 production in response to both C. albicans and vaccine protein was less than IFN-γ production (P < .01 for both comparisons).
We also sought to determine whether there was a relationship between the strength of the humoral and the PBMC-mediated response to the vaccine protein in individual donors. In individual donors, serum anti-rAls3p-N titers did not correlate with PBMC production of either IFN-γ or IL-17 in response to rAls3p-N in ELISpot assays.
To determine the general profile of cytokines produced in PBMCs in the ELISpot assays, supernatants from the ELISpot wells were harvested prior to processing the plates. Cytokine levels in the supernatants were measured by Cytometric Bead Array Flex Sets. rAls3p-N induced expression of IL-12 p70 and IFN-γ, indicative of a type 1 immune response, as well as IL-17 (Figure 1C). rAls3p-N also induced expression of IL-10, but it did not induce expression of IL-4 or of IL-8.
Because the rAls3p-N–induced IFN-γ response was stronger than the IL-17 response in PBMCs from healthy donors, we used the IFN-γ ELISpot assay to identify immundominant epitopes from rAls3p-N. ELISpot plates were coated with 20-mer peptides overlapping by 10 amino acids each, spanning the length of the rAls3p-N molecule (1 peptide per well). After 48 h incubation with PBMCs from individual donors, the number of spots in each well was quantified. Peptides inducing responses at the 95th percentile of magnitude (ie, ≥2 standard deviations above the mean response of all peptides) were considered to be immunodominant (Figure 2). The predominant IFN-γ inducing epitopes for individual donors spanned the entire length of the protein. Epitopes to which >1 donor was reactive were identified at multiple peptide positions. However, the highest number of reactive donors for any individual immunodominant peptide was only 4 (peptide 5 and 29). Furthermore, individual donors reacted to widely separated peptides.
Figure 2.
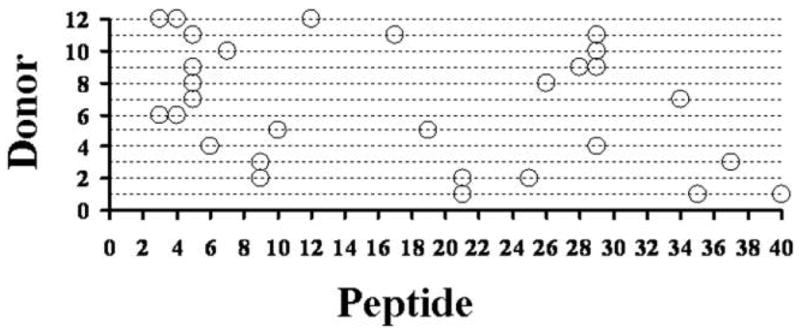
Immunodominant epitopes span the entire rAls3p-N sequence. Peripheral blood mononuclear cells (PBMCs) from 12 individual donors were cultured with individual 20-mer peptides, overlapping by 10 amino acids each, spanning the length of the rAls3p-N molecule. Each individual donor (Y axis) is plotted against the sequential 20-mer peptide numbers (X axis) which induced the highest number of interferon γ (IFN-γ) spots in the enzyme-linked immunosorbent spot assay for that donor’s PBMCs. Peptides inducing responses ≥95th percentile of responses (ie, ≥2 standard deviations above the mean response to all peptides) are plotted for each donor.
Discussion
It is necessary to identify reliable immunoassays to be measured during future clinical trials of the rAls3p-N vaccine, to identify immune surrogates or correlates of protection [5]. Our data indicate that antibody titers by ELISA, PBMC production of IFN-γ and IL-17 by ELISpot, and PBMC production of IL-12 p70, IFN-γ, IL-17, and IL-10 by Cytometric Bead Array are potential candidates for immunoassays to be explored in future clinical trials.
Because they are colonized with Candida species, we hypothesized that most humans would have preexisting immunity to a vaccine protein based on the Als3p adhesin. Indeed, we found high IgG antibody titers and cellular production of both IFN-γ and IL-17 in response to rAls3p-N in healthy donors, confirming that humans have preexisting memory lymphocytes specific for the protein. The magnitude of cytokine responses detected were similar to those found to be produced by immune lymphocytes from vaccinated mice that were protected against infection [3, 7].
The IFN-γ response to whole C. albicans was greater than the response to rAls3p-N alone, suggesting that Th1 cells in healthy donors recognize other antigens aside from rAls3p-N, as well. However, the IL-17 response to whole C. albicans was similar to the response to the rAls3p-N protein, suggesting that Als3p may be a predominant antigen recognized by Th17 cells in healthy donors. The identification of numerous immunodominant epitopes in the rAls3p-N protein, which span the entire sequence of the protein both between and within individual donors, indicates that fragments of the vaccine protein are less likely to be broadly reactive to patient populations than is the whole vaccine protein.
In contrast to mice, which are neither colonized with nor pre-immune to Candida species and mount a naive T cell response to the first dose of the rAls3p-N protein [1], our data indicate that humans should mount a memory response to the first dose of vaccine in future clinical trials. We have shown that the rAls3p-N vaccine induces protection against disseminated candidiasis in mice as soon as 1 week after a single dose of vaccine [1]. Therefore, acutely at-risk humans are likely to derive an even more rapid survival advantage after vaccination. Because the mean (± standard deviation) time to onset of disseminated candidiasis is 22 ± 1 days of hospitalization [8], it should be feasible to vaccinate acutely at-risk patients to prophylax them from infection, based on early recognition of well established risk factors [9].
Although Candida colonization clearly leads to skin test reactivity in humans, the preexisting immunity to the organism is not protective, because colonization is a risk factor for disseminated candidiasis, not a protective factor [9]. The purpose of vaccinating at-risk hospitalized patients is to rapidly boost immunity to the organism during the critical acute-risk time period. Specific evidence in support of the ability of vaccination to enormously decrease disease in populations already colonized with and pre-immune to the targeted pathogen derives from the experience with Hemophilus influenza and Streptococcus pneumoniae. The conjugated H. influenzae b vaccine has decreased invasive disease due to H. influenza by >95% in the United States [10]. Similarly, pneumococcal vaccines have significantly reduced the incidence of invasive infection [11]. This vaccine efficacy has occurred despite the fact that, akin to Candida species, both children and adults had significant oropharyngeal colonization rates (up to 60% in some studies) of H. influenza and S. pneumoniae in the prevaccine era [12, 13], but vaccination successfully increased antibody titers, increased the rate of eradication of colonization, and decreased subsequent invasive disease [14, 15]. Conversely, it is also possible that pre-existing immunity to Candida is nonprotective because the strength of preexisting immunity acutely decreases in highly at-risk patients. Thus, it is conceivable that boosting of the preexisting immune response may be blunted by acute immunocompromising conditions in at-risk patients. Future studies that determine the qualitative and quantitative immune responses to Candida species in acutely at-risk patients, as opposed to healthy donors, will be critical to determine whether preexisting immune deficiency can be boosted or circumvented by vaccination in future clinical trials.
In summary, we have confirmed that healthy humans have lymphocytes that have been previously primed to generate both a humoral and cell-mediated immune response specific to the rAls3p-N vaccine protein. Hence, vaccination will likely induce a memory response in acutely at-risk patients, rapidly boosting their immunity during the critical at-risk period. These data support the continued development of the rAls3p-N vaccine.
Acknowledgments
Financial support: Public Health Service (grants R01 AI072052 to B.S. and AI063382 to J.E.E.
Footnotes
Potential conflicts of interest: B.S., A.S.I, Y.F., and J.E.E own equity in NovaDigm Therapeutics, which is developing vaccine technologies. NovaDigm Therapeutics provided no financial support for these studies.
References
- 1.Spellberg B, Ibrahim AS, Lin L, et al. An antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J Infect Dis. 2008;197:957–971. doi: 10.1086/529204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spellberg B, Ibrahim AS, Yeaman M, et al. The anti-fungal rAls3p-N vaccine protects mice against the bacterium Staphylococcus aureus. Infect Immun. 2008;76:4574–4580. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin L, Ibrahim AS, Xu X, et al. Th1–Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathogens. doi: 10.1371/journal.ppat.1000703. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L, Ibrahim AS, Baquir B, Avanesian V, Fu Y, Spellberg B. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS Immunol Med Microbiol. 2009;55(3):293–295. doi: 10.1111/j.1574-695X.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998;33:451–459. doi: 10.1007/s002940050359. [DOI] [PubMed] [Google Scholar]
- 7.Lin L, Ibrahim AS, Avanesian V, et al. Vaccine immunogenicity and efficacy vary considerably by diluent used for aluminum hydroxide adjuvant. Clin Vaccine Immunol. 2008;15:582–584. doi: 10.1128/CVI.00427-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 9.Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 10.Peter CG. Responses of children immunized with capsular polysaccharide of Hemophilus influenzae type b, by David H. Smith, MD, et al, Pediatrics, 1973; 52:637–644 and Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100 000 vaccinees 3 months to 5 years of age in Finland, by Heikki Peltola, MD et al, Pediatrics, 1977; 60:730–737. Pediatrics. 1998;102:252–254. [PubMed] [Google Scholar]
- 11.Lynch JP, 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. 2009;30:189–209. doi: 10.1055/s-0029-1202938. [DOI] [PubMed] [Google Scholar]
- 12.Gray BM, Dillon HC., Jr Epidemiological studies of Streptococcus pneumoniae in infants: antibody to types 3, 6, 14, and 23 in the first two years of life. J Infect Dis. 1988;158:948–955. doi: 10.1093/infdis/158.5.948. [DOI] [PubMed] [Google Scholar]
- 13.Lifshitz S, Dagan R, Shani-Sekler M, et al. Age-dependent preference in human antibody responses to Streptococcus pneumoniae polypeptide antigens. Clin Exp Immunol. 2002;127:344–353. doi: 10.1046/j.1365-2249.2002.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskola J, Kayhty H, Takala AK, et al. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990;323:1381–1387. doi: 10.1056/NEJM199011153232004. [DOI] [PubMed] [Google Scholar]
- 15.Musher DM, Groover JE, Rowland JM, et al. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin Infect Dis. 1993;17:66–73. doi: 10.1093/clinids/17.1.66. [DOI] [PubMed] [Google Scholar]