Abstract
Aims
Self-injurious behavior (SIB), which is deliberate infliction of self-injury without suicidal intent, is a significant human health problem. SIB is not unique to humans but is also manifested in a small percentage of captive macaques, typically as self-directed biting. Although the onset and maintenance of SIB have been linked to increased anxiety in both humans and nonhuman primates, no previous studies have directly tested the anxiety-SIB hypothesis. Here, we determined whether rhesus monkeys increase their self-directed biting following a challenge with the anxiogenic compound N-methyl-β-carboline-3-carboxamide (FG7142).
Main Methods
Ten rhesus monkeys (Macaca mulatta) with a veterinary record of self-wounding (SIB) as well as six age- and weight-matched non-wounding control monkeys were given intramuscular injections of 0.1, 0.3, or 1.0 mg/kg FG7142. Behavior was observed following drug administration with special attention to displacement behaviors (scratching, self-grooming, and yawning), locomotor stereotypy, and self-directed biting. Plasma cortisol and ACTH were also measured as physiological indices of stress.
Key Findings
Self-directed biting rates dose-dependently increased in a subset of SIB monkeys, but did not change in control animals. Furthermore, administration of FG7142 led to an increase in scratching, yawning, and locomotor stereotypy in all monkeys, but did not affect the frequency self-grooming. Additionally, there was a dose- dependent increase in plasma cortisol concentrations, but not ACTH, in all animals.
Significance
The present findings indicate that self-biting is anxiety-related in some but not all SIB monkeys, suggesting that this behavioral pathology is heterogeneous as has previously been suggested for SIB in humans.
Keywords: Self-injurious behavior (SIB), N-methyl-β-carboline-3-carboxamide (FG7142), Anxiety
Introduction
Self-injurious behavior (SIB), which is the deliberate infliction of self-injury without suicidal intent, is a significant human health problem. Common manifestations of SIB include cutting or burning of the skin, other forms of self-mutilation, and head-banging (Favazza 1998). One form of SIB, “impulsive” SIB, has been related to heightened anxiety or tension and may paradoxically serve to reduce negative emotions (Simeon and Favazza 2001). Impulsive SIB is often a symptom of anxiety-related and personality disorders (e.g., post-traumatic stress disorder (PTSD) and borderline personality disorder (BPD) respectively). Furthermore, patients with a co-occurrence of these disorders and SIB report a reduction in tension or anxiety immediately following an episode of self-injury (Coid 1993; Herpertz 1995; Weaver et al. 2004; Weierich and Nock 2008). Impulsive SIB appears to be functionally different than other forms of SIB, such as stereotypic or compulsive SIB (for an in-depth discussion of the classification of SIB in humans, see Simeon and Favazza 2001).
SIB also occurs spontaneously in macaque species and mainly takes the form of self-directed biting, which differs in form from most SIB in humans. Nonetheless, these animals may provide a valuable model to help us understand the causes of and possible treatments for this condition in humans. Self-inflicted wounding in individually housed monkeys (as a result of self-directed biting) has been reported in various macaque species (Macaca sp.), at a prevalence rate of about 5–15% across research facilities (Bayne et al. 1995; Bellanca and Crockett 2002; Lutz et al. 2003). Notably, these values fall within the range of prevalence rates reported in non-clinical human populations [4% (Briere and Gil 1998); 17% (Whitlock et al. 2006); and 35% (Gratz 2002)]. One major risk factor in the development of SIB in monkeys is social separation (i.e., permanent removal from a stable social group) and placement into individual housing at an early age, which is likely to be a highly stressful event (Lutz et al. 2003). Additional factors include prolonged exposure to individual housing following social separation and undergoing more minor veterinary procedures than non-SIB animals (Baker 2002; Bellanca and Crockett 2002; Lutz et al. 2003).
We have theorized that stressful events early in development in conjunction with repeated additional challenges later in life can lead to the development of SIB as a coping strategy to deal with stress and anxiety in both monkeys and humans (Tiefenbacher et al. 2005a). Support for this model comes from previous studies in our laboratory showing that monkeys that engage in SIB exhibit a persistent dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and a significant decrease in heart rate immediately after self-biting (suggesting a role for SIB in arousal reduction; Novak 2003; Tiefenbacher et al. 2004). Furthermore, we recently reported that biting rates in monkeys with SIB persistently increased as a consequence of an administratively mandated relocation of the animals to a new building (Davenport et al. 2007). Finally, self-biting rates were predicted by cerebrospinal fluid levels of the anxiogenic peptide corticotropin-releasing factor (Tiefenbacher et al. 2002), and treatment with the benzodiazepine (BDZ) diazepam significantly reduced self-directed biting and wounding frequency in 50% of the monkeys treated (Tiefenbacher et al. 2005b).
Although our previous findings support a link between SIB and anxiety in rhesus monkeys, these studies provide correlational data only. In order to more fully understand the relationship of SIB and anxiety, it is important to determine whether increased anxiety can serve as a trigger for SIB, a question that has not previously been addressed either in the primate or human literature. Accordingly, the present study was designed to test the anxiety-SIB hypothesis by measuring behavioral and physiological responses to an anxiety-provoking pharmacological challenge. Monkeys with a veterinary record of self-wounding (SIB) along with age-matched non-wounding controls were given injections of N-methyl-β-carboline-3-carboxamide (FG7142), a partial inverse agonist at the BDZ receptor. FG7142 and other drugs in the β-carboline family have been shown to induce anxiety-related behaviors in humans (Dorow et al. 1983) and rhesus monkeys (Crawley et al. 1985; Takamatsu et al. 2003; Kalin et al. 1992), and to stimulate HPA axis activity (Takamatsu et al. 2003; for review of the pharmacology of FG7142, see Evans and Lowry 2007). We predicted that administration of FG7142 would result in dose-dependent increases in plasma adrenocorticotropic hormone (ACTH) and cortisol concentrations as well as increased frequencies of anxiety-related behaviors in all animals. Most importantly, we further predicted that the frequency of self-directed biting would increase in SIB monkeys following treatment with the drug.
Materials and Methods
Animals
Sixteen individually-housed adult male rhesus monkeys (Macaca mulatta) housed at the New England Primate Research Center were tested. Ten of the monkeys had a previous veterinary record of self-inflicted wounding (i.e., wounding requiring suturing or analgesics) and thus constituted the SIB group. It is important to note that, although monkeys classified as SIB had a previous record of self-wounding, the incidence of wounding in these animals is very low despite a relatively high frequency of biting. Therefore, the risk of wounding during the study was considered to be minimal. Six non-wounding monkeys served as controls. One control and seven SIB monkeys were mother-reared, whereas four control and three SIB monkeys were nursery peer-reared. Rearing history was not available for one control monkey. Control animals entered individual housing at an average age of 34.5 ± 8.9 months and SIB monkeys were placed in individual housing at 18.0 ± 5.6 months. There were no significant age or weight differences between the two groups (Control: Mage=17.5 ± 2.6 years, Mweight=14.7 ± 0.9 kg; SIB: Mage=19.7 ± 1.0 years, Mweight=16.4 ± 0.6 kg; M ± SEM). Monkeys were fed formulated monkey chow twice daily and had access to water ad libitum. Their diet was supplemented daily with fresh fruit. The animals were maintained on a 13-h light:dark cycle with light onset at 0600 h; however, they were also exposed to natural light through windows in each of the colony rooms. All environments were enriched with toys and perches. Animal housing, diet, and environmental enrichment were all conducted in accordance with the Standing Committee on Animals of Harvard Medical School, the Animal Welfare Act (United States Department of Agriculture 1991), and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996). All research protocols were approved by the IACUC of Harvard Medical School.
Drug Administration
The monkeys received intramuscular (IM) injections of 0.1 mg/kg, 0.3 mg/kg, or 1.0 mg/kg doses of N-methyl-β-carboline-3-carboxamide (FG7142; Tocris Cookson Inc., Ellisville, Missouri, USA) dissolved in a mixture of 1:1 propylene glycol and sterile water. These doses were based on previous findings that IM administration of similar doses of FG7142 elicited dose-dependent behavioral and physiological changes in adult male rhesus monkeys (Schino et al. 1996; Takamatsu et al. 2003). Injection volumes were based on animal weight and ranged from 0.06 to 0.2 ml/kg. Administration of vehicle alone served as a control procedure. All animals received one treatment with each dose, given in a randomized order across groups. No more than two monkeys were tested per day to facilitate data collection and ensure close monitoring for potential adverse reactions. Injections were given in each monkey’s home cage and occurred at approximately 0815 h, with an interval of 1 min between dosing of the two subjects. The monkeys were briefly restrained (~5–10 s) during injections using a standard “squeeze-back” mechanism that was part of each cage. All monkeys were familiar with this procedure. We did not anticipate that FG7142 would elicit either severe biting or any type of wounding in the SIB animals; nonetheless, a dose of the BDZ diazepam was on hand throughout the study to reverse the effects of FG7142 in case such reversal was required.
Behavioral Data Collection
After a 15-min post-injection period, animals were videotaped for the next 60 min (between 0830 and 0930 h). Data were obtained from the videotapes using a modified frequency scoring system in which the presence/absence of 35 categories of behavior was noted in 5-min blocks (comprised of twenty 15-s intervals) over 60 min (twelve 5-min observations). Particular attention was paid to the frequency of self-directed biting, locomotor stereotypy (i.e., pace), and anxiety-associated displacement behaviors such as scratching, self-grooming, and yawning (Maestripieri et al. 1992; Schino et al. 1996). With respect to scoring, “event” behaviors such as self-directed biting were recorded each time they occurred, regardless of duration. For “state” behaviors such as self-grooming, the behavior was recorded as continuously occurring until the monkey either changed behaviors or paused for longer than 3 s. In the case of repetitive stereotypies such as pacing, the behavior was scored if the animal displayed a fixed, repeated, pattern (at least four consecutive cycles of the behavior) with no more than a 3-s pause during the behavior. A behavior could occur multiples times in a 15-s interval and also could continue into adjacent intervals; however the presence of any behavior was only counted once per individual time interval. Data are reported as the mean modified frequency score of each behavior (i.e., the mean number of 15-s intervals in which a behavior occurred per 5-min block of time). The experimenter who recorded the behavioral data was blind to the drug treatments and met an inter-observer reliability criterion of at least 90% agreement.
Hormone Assays
Monkeys were sedated with ketamine (10.0 mg/kg IM) immediately after the end of videotaping (0930 h), after which 20 ml of femoral blood was drawn into a 10-ml EDTA-containing Vacutainer tube and a 10-ml heparin-containing Vacutainer tube for subsequent analysis of ACTH and cortisol respectively (time from ketamine administration to blood drawing = 11.0 ± 0.31 min; M ± SEM). Blood samples were centrifuged at 2600 rpm for 15 min, after which plasma was aliquotted into 2.0-ml cryovials and stored at −80°C until assayed in duplicate using a commercially available enzyme immunoassay kit for ACTH (Alpco Diagnostics, Salem, NH) and a radioimmunoassay kit for cortisol (Siemens Medical Solutions Diagnostics, Los Angeles, CA). The intra-assay coefficients of variance for the ACTH and cortisol assays were 14.7 % and 3.6 % respectively.
Statistical Analysis
Behavioral data were analyzed using mixed-design analyses of variance (ANOVAs) with drug dose and group (SIB vs. control) as within- and between-subjects factors respectively. If Mauchly’s tests of sphericity yielded significance, Huynh-Feldt corrections are reported. Unpaired and paired-samples t-test contrasts were conducted to explore interactions of the independent variables, even if there was no significance in the initial ANOVA (see Wilcox, 1987, for a justification of this approach). Cortisol and ACTH values were analyzed using mixed-design ANOVAs as described above. Pearson product-moment correlations were performed to determine whether drug dose was positively correlated to either plasma cortisol or ACTH concentrations.
After an initial set of analyses, we observed a high degree of variability among the SIB animals at different doses of FG7142 for several behavioral responses, including self-biting (i.e., Mauchly’s tests of sphericity were significant). Based on our previous finding that only half of SIB monkeys tested responded positively to treatment with the anxiolytic BDZ diazepam (Tiefenbacher et al. 2005b), we theorized that this high level of variability was caused by differential responding to FG7142. Therefore, we conducted a visual inspection of the self-biting data for each animal at the various doses of FG7142. Subsequently, we observed that, as in the diazepam study, half of the SIB group appeared to respond in a pronounced manner to drug administration, whereas the remaining SIB monkeys did not respond at all. To test this possibility statistically, we performed an additional set of analyses in which SIB monkeys were assigned to two subgroups designated responders and non-responders. Thus, control monkeys were first compared to all SIB animals and then to the individual subgroups of SIB.
Results
Behavioral Responses
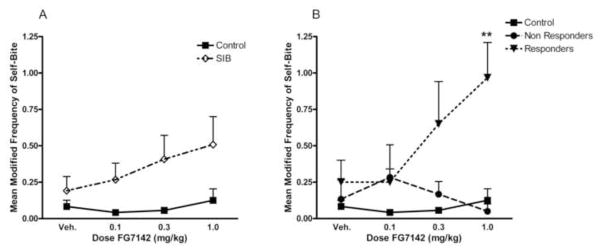
None of the monkeys wounded themselves either during the study or during post-study monitoring, and none required diazepam treatment. However, as expected, overall rates of self-biting across the study were higher in the SIB monkeys than in the controls (F(1,4) = 4.78, p<0.05). An ANOVA examining biting behavior across all drug doses revealed no overall effect of FG7142 on biting frequency in either SIB or control animals (FH-F(2.48,34.73) = 0.87, ns); however, there was a non-significant pattern such that the mean biting frequency increased from baseline to each dose of FG7142 in the SIB group (Figure 1a). A second ANOVA comparing control animals to the two subgroups of SIB monkeys (responders and non-responders) revealed a significant interaction between dose and group status (FH-F(5.13,33.32) = 2.79, p<0.05). This interaction was further characterized in two ways. First, post-hoc trend analyses revealed a significant dose-related linear trend in biting for the responder group (F(1,4) = 9.56, p<0.05) but not for the non-responder (F(1,4) = 0.33, ns) or control groups (F(1,5) = 0.37, ns). Second, post-hoc t-tests showed that the responder monkeys bit themselves significantly more than either non-responders (t(8) = −3.70, p<0.025) or control monkeys (t(9) = −3.57, p<0.025) at the highest dose of FG7142 (Figure 1b).
Figure 1.
Dose-dependent effects of FG7142 on self-biting behavior for (A) both SIB and control monkeys and (B) monkeys by subgroup. In this figure as well as in Figures 2–4, the data are presented as the mean (± SEM) modified frequency score (the mean number of 15-s intervals in which the behavior occurred per 5-min time block). Asterisks indicate a difference between the responders and both the non-responders and control monkeys (**p<0.025).
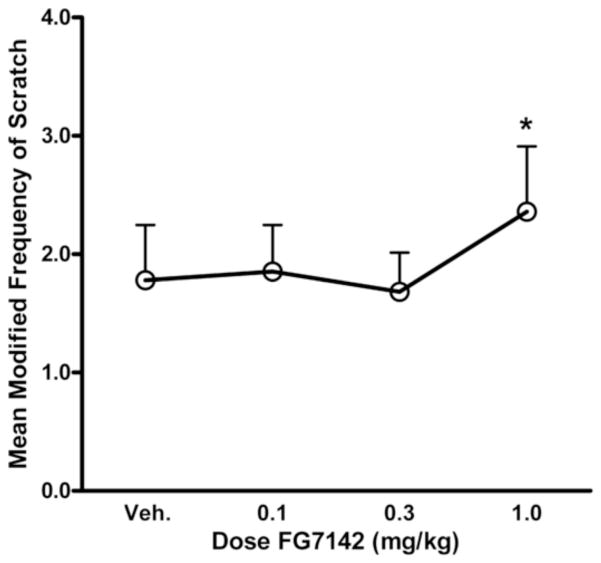
With respect to other behavioral responses to the drug challenge, there was a significant main effect of drug dose for scratch that reflected an overall increase in the frequency of this behavior across both SIB and control monkeys (F(3,42) = 3.81, p<0.025). Post-hoc testing revealed a significant increase in scratching between the 0.3 mg/kg and 1.0 mg/kg doses (t(15) = −2.22, p<0.05; Figure 2). There were no group differences in scratch either when considering all SIB monkeys (F(1,14) = 0.01, ns) or the individual subgroups (F(2,13) = 1.04, ns). Likewise, there were no differential effects of drug dose on scratching behavior of the different subgroups of monkeys (F(6,39) = 0.73, ns).
Figure 2.
Dose-dependent effects of FG7142 on scratching for all monkeys. Asterisks indicate a difference between mean scratching frequency at the 0.3 mg/kg and 1.0 mg/kg doses of FG7142 (*p<0.05).
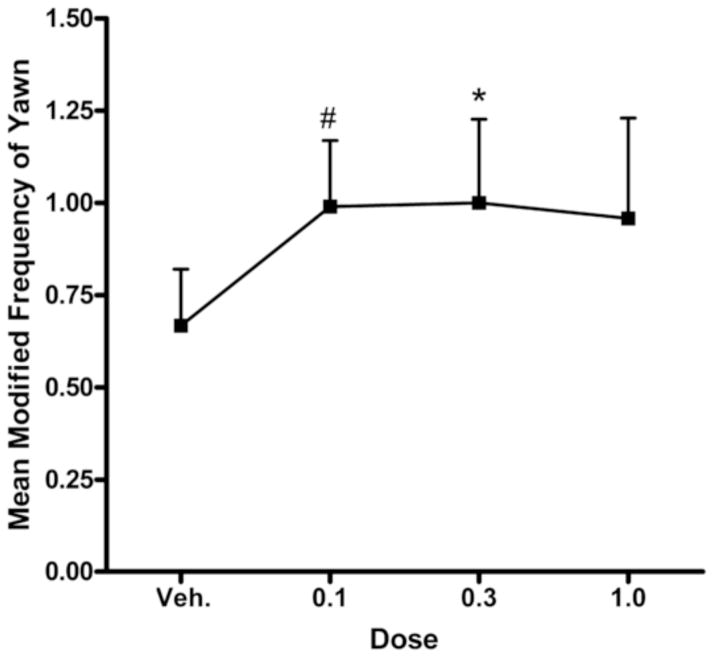
Repeated measures ANOVA revealed no significant dose-dependent increase in the frequency of yawning (F(3,42) = 1.21, ns) or drug dose by group interaction (F(6,39) = 0.08, ns). Neither were there group differences in yawning either when considering all SIB monkeys (F(1,14) = 0.06, ns) or the individual subgroups (F(2,13) = 0.03, ns). However, given the possibility that nonsignficant F tests can lead to type II errors if further group (planned) comparisons are not conducted (Wilcox, 1987), we performed paired-samples t-tests comparing each individual dose of the drug to vehicle. These analyses revealed either a marginal or a significant increase in yawning as compared to the vehicle control at the 0.1 mg/kg (t(15) = −2.01, p<0.07) and 0.3 mg/kg doses (t(15) = −2.35, p<0.05; Figure 3). There were no effects of FG7142 on self-grooming (data not shown).
Figure 3.
Effects of FG7142 on yawning for all monkeys. Asterisks indicate a difference between mean yawning at each dose and the vehicle control (#p<0.07; *p<0.05).
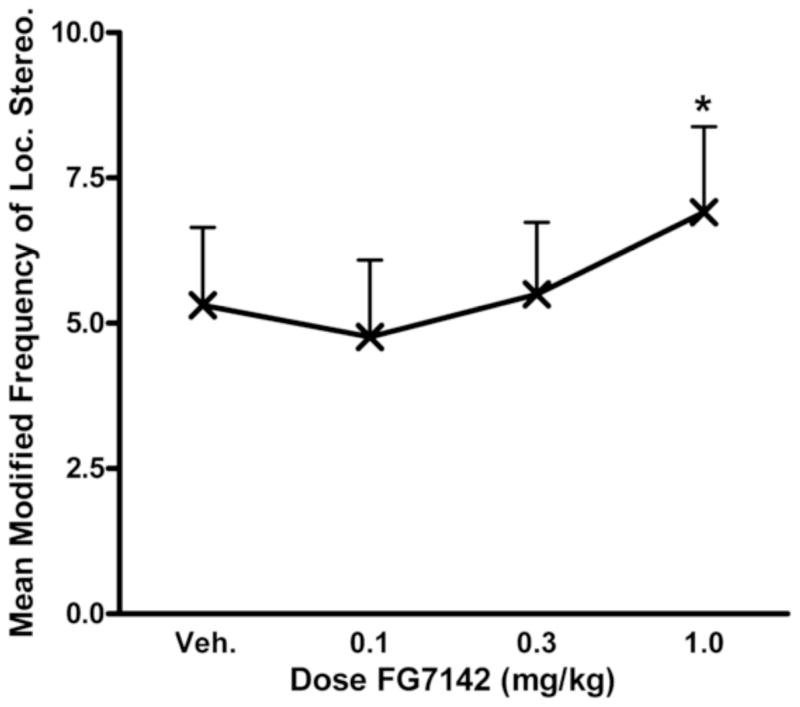
There was also a main effect of dose on the frequency of locomotor stereotypy manifested as pacing behavior (F(3,42) = 4.63, p<0.01). Post-hoc testing revealed a significant increase in stereotypy from the 0.3 mg/kg to the 1.0 mg/kg dose (t(15) = −3.17, p<0.01; Figure 4). There were no differences in stereotypy between SIB and control monkeys at either the whole-group (F(1,14) = 0.02, ns) or subgroup (F(2,13) = 0.11, ns) levels, and there was no interaction between drug dose and subgroup (F(6,39) = 0.88, ns).
Figure 4.
Dose-dependent effects of FG7142 on locomotor stereotypy for all monkeys. Asterisks indicate a difference between mean stereotypy at the 0.3 mg/kg and 1.0 mg/kg doses of FG7142 (*p<0.05).
Hormonal Responses
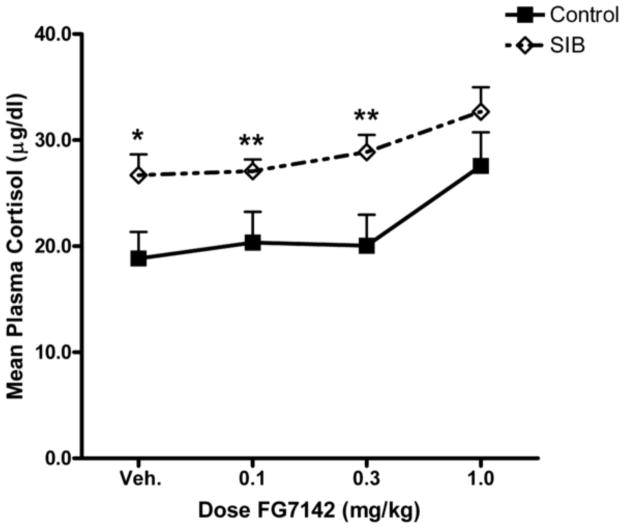
There was a significant FG7142 dose effect on plasma cortisol concentrations across both groups of monkeys (FH-F(2.58, 36.14) = 11.82, p<0.001), but no significant group by dose interaction (FH-F(2.58, 36.14) = 0.68, ns). However, there was a significant group difference in overall cortisol levels with the values for the SIB monkeys being significantly greater than those for the controls (F(1,14) = 6.77, p<0.025). Post-hoc testing at each dose indicated that this difference was statistically significant at the vehicle control (t(14) = −2.48, p<0.05), 0.1 mg/kg (t(14) = −2.56, p<0.025) and 0.3 mg/kg (t(14) = −2.88, p<0.025) doses (Figure 5). Examination of subgroups revealed the same overall dose-related increase in cortisol (FH-F(2.51, 32.59) = 11.42, p<0.001); however, there was only a nonsignificant trend towards a difference in cortisol concentrations between the subgroups (F(2,13) = 3.20, p<0.075). Overall, responder monkeys had significantly higher plasma cortisol concentrations than controls (F(1,9) = 5.69, p<0.05), but the non-responders did not differ significantly from either the responders (F(1,8) = 0.08, ns) or the controls (F(1,9) = 3.32, ns) (data not shown). Plasma cortisol concentrations were significantly correlated with dose when all animals were taken together (rtotal = 0.37, p<0.01) and when the SIB and control groups were analyzed separately (rSIB = 0.40, p<0.025; rcont.= 0.46, p<0.025).
Figure 5.
Dose-dependent effects of FG7142 on plasma cortisol for SIB and control monkeys. Data are presented as mean ± SEM. Asterisks indicate a difference between SIB and control animals (*p<0.05; **p<0.025).
ACTH concentrations did not change significantly as a function of FG7142 dose at either the whole-group (F(3,42 )= 1.94, ns) or subgroup level (F(3,39) = 2.53, ns). Neither did ACTH concentrations differ between SIB and control monkeys (F(1,14) = 0.02, ns) or between subgroups (F(2,13) = 0.06, ns). Likewise, there was no correlation between plasma ACTH concentrations and dose in either group of monkeys (rtotal = 0.16, ns; rcont.= 0.16, ns; rSIB.= 0.16, ns). ACTH was either significantly or marginally correlated with cortisol at vehicle and all three doses of FG7142 (rveh.= 0.60, p<0.01; r.01 = 0.47, p<0.05; r0.3 = 0.42, p<0.06; r1.0 = 0.62, p<0.01). Neither cortisol nor ACTH were significantly related to the time of drug treatments or the latency between ketamine administration and blood drawing (data not shown).
Discussion
To our knowledge, this is the first report of increased SIB (self-directed biting) in monkeys following an experimentally-induced state of anxiety. Previous research has shown FG7142 to be an anxiogenic drug, and this notion was supported in the present study by an increase in displacement behaviors (scratching and yawning) in both groups of subjects. Other changes in behavior, such as the elevated frequency of locomotor stereotypy, could likewise indicate elevated levels of anxiety in the monkeys as a result of the drug challenge.
Plasma cortisol concentrations were significantly elevated in both SIB and control animals following FG7142 administration, which is consistent with previous work demonstrating a stimulatory effect of this compound on the HPA axis (Takamatsu et al. 2003). Yet, despite this evidence that FG7142 produced a physiological stress response in all monkeys, only SIB animals showed an increase in self-biting. These results indicate that self-biting behavior is a specific response of some SIB monkeys (i.e., animals with a prior history of self-wounding) to anxiety, not a generalized reaction, for example increased scratching or HPA activation that tends to be shown by all animals.
The present finding that only 50% of the SIB monkeys increased their self-directed biting following FG7142 administration suggests that (1) this behavioral pathology is heterogeneous in monkeys, and (2) that anxiety is a trigger for SIB only in one form of the disorder. Indeed, it is interesting to note that a previous study from our laboratory involving treatment of a largely separate group of monkeys with diazepam (only one monkey was involved in both studies) found that only half of the animals responded positively (i.e., with decreased SIB) to this compound (Tiefenbacher et al. 2005b). Together, these findings implicate the BDZ site on the γ-aminobutyric acidA (GABAA) receptor as playing an important role in the hypothesized anxiety-related subtype of SIB. Unfortunately, little is presently known about the potential role of GABA and the GABAA receptor in either the etiology or maintenance of SIB. Indeed, it is possible that the GABAergic system modulates SIB in a manner independent of changes in anxiety. However, given the known anxiolytic and anxiogenic effects of diazepam and FG7142 respectively, we propose that genetic polymorphisms of the GABAA receptor or a functional dysregulation of the GABA system may underlie anxiety-related forms of SIB in both monkeys and humans. Additional studies are needed to test this hypothesis.
In the present animal study, we were able to perform a novel test of the anxiety-SIB hypothesis by administering an anxiogenic compound to our subjects. Although we found no evidence for this kind of manipulation in the human SIB literature, other findings support a link between SIB and anxiety. First, one of the most commonly reported reasons for engaging in self-injury is to relieve negative affect in the form of heightened tension or anxiety (reviewed by Klonsky 2007). Second, several studies have found elevated symptoms of anxiety in non-clinical populations that exhibit SIB (Gollust et al. 2008; Klonsky and Olino 2008; Klonsky et al. 2003; Ross and Heath 2002). Finally, consistent with the monkey study cited earlier (Novak 2003), Haines and coworkers (1995) found that changes in heart rate and other psychophysiological measures support the notion that SIB reduces tension in people that engage in this behavior. These results provide strong evidence for a relationship between SIB and anxiety (sometimes labeled tension) in humans. On the other hand, reasons other than tension reduction are also offered by some individuals who engage in SIB (Klonsky 2007). Thus, it will be important in future research to elucidate the relative importance of anxiety in the various proposed categories of SIB in human populations and to determine how these categories differ in their underlying neurobiological mechanisms (including potential involvement of the GABA system).
Conclusions
In conclusion, adult male rhesus monkeys with a history of SIB as well as control animals were administered several different doses of the anxiogenic BDZ partial inverse agonist FG7142. Drug treatment led to dose-dependent increases in displacement behaviors, locomotor stereotypy, and plasma cortisol concentrations in both subject groups. More importantly, there was a dose-dependent increase in self-biting behavior in half of the SIB monkeys but none of the controls. These results suggest that SIB in rhesus monkeys is a heterogenous disorder, and that anxiety is an important trigger for self-biting in some but not all animals with the disorder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker K. Rearing and housing history of rhesus macaques (Macaca mulatta) displaying self-injurious and noninjurious abnormal behaviors. American Journal of Primatology. 2002;57:82. [Google Scholar]
- Bayne K, Haines M, Dexter S, Woodman D, Evans C. Nonhuman primate wounding prevalence: a retrospective analysis. Lab Animal. 1995;24:40–44. [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. American Journal of Primatology. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Ninan PT, Pickar D, Chrousos GP, Linnoila M, Skolnick P, Paul SM. Neuropharmacological antagonism of the beta-carboline induced ‘anxiety’ response in rhesus monkeys. Journal of Neuroscience. 1985;5:477–485. doi: 10.1523/JNEUROSCI.05-02-00477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. A rhesus monkey model of self-injury: Effects of relocation stress on behavior and neuroendocrine function. Biological Psychiatry. 2008;63:990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorow R, Horowski R, Paschelke G, Amin M, Braestrup C. Severe anxiety induced by FG 7142, a β-carboline ligand for benzodiazepine receptors. Lancet. 1983;322:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Evans AK, Lowry CA. Pharmacology of the β-carboline FG7142, a partial inverse agonist at the benzodiazepine allosteric site of the GABAA receptor: Neurochemical, neurophysiological, and behavioral effects. CNS Drug Reviews. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favazza AR. The coming of age of self-mutilation. Journal of Nervous and Mental Disease. 1998;186:259–268. doi: 10.1097/00005053-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Gollust SE, Eisenberg D, Golberstein E. Prevalence and correlates of self-injury among university students. Journal of American College Health. 2008;56:491–498. doi: 10.3200/JACH.56.5.491-498. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. American Journal of Orthopsychiatry. 2002;72:128–140. doi: 10.1037//0002-9432.72.1.128. [DOI] [PubMed] [Google Scholar]
- Haines J, Williams CL, Brain KL, Wilson GV. The psychophysiology of self-mutilation. Journal of Abnormal Psychology. 1995;104:471–489. doi: 10.1037//0021-843x.104.3.471. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Turner JG. Effects of β-carboline on fear-related behavioral and neurohormonal responses in infant rhesus monkeys. Biological Psychiatry. 1992;31:1008–1019. doi: 10.1016/0006-3223(92)90094-g. [DOI] [PubMed] [Google Scholar]
- Klonsky ED. The functions of deliberate self-injury: A review of the evidence. Clinical Psychology Review. 2007;27:226–239. doi: 10.1016/j.cpr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Klonsky ED, Olino TM. Identifying clinically distinct subgroups of self-injurers among young adults: A latent class analysis. Journal of Consulting and Clinical Psychology. 2008;76:22–27. doi: 10.1037/0022-006X.76.1.22. [DOI] [PubMed] [Google Scholar]
- Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: Prevalence and psychological correlates. American Journal of Psychiatry. 2003;160:1501–1508. doi: 10.1176/appi.ajp.160.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak MA. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: displacement activities as an indicator of emotions in primates. Animal Behavior. 1992;44:967–979. [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: new insights on etiology, physiology, and treatment. American Journal of Primatology. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Ross S, Heath N. A study of the frequency of self-mutilation in a community sample of adolescents. Journal of Youth and Adolescence. 2002;31:67–77. [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Simeon D, Favazza AR. Self-injurious behaviors: Phenomenology and assessment. In: Simeon D, Hollander E, editors. Self-injurious Behaviors: Assessment and Treatment. American Psychiatric Publishing; Washington D.C: 2001. pp. 1–28. [Google Scholar]
- Takamatsu H, Noda A, Kurumaji A, Murakami Y, Tatsumi M, Ichise R, Nishimura S. A PET study following treatment with a pharmacological stressor, FG7142, in conscious rhesus monkeys. Brain Research. 2003;980:275–280. doi: 10.1016/s0006-8993(03)02987-1. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher S, Fahey MA, Rowlett JK, Meyer JS, Pouliot AL, Jones BM, Novak MA. The efficacy of diazepam treatment for the management of acute wounding episodes in captive rhesus macaques. Comparative Medicine. 2005b;55:387–392. [PubMed] [Google Scholar]
- Tiefenbacher S, Gabry KE, Novak MA, Pouliot AL, Gold PW, Meyer JS. Central levels of CRF and NPY in male rhesus monkeys with self-injurious behavior. Thirty-second Annual Meeting of the Society for Neuroscience; Orlando, Florida. November 2–7, 2002. [Google Scholar]
- Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. The physiology and neurochemistry of self-injurious behavior: A nohuman primate model. Frontiers in Bioscience. 2005a;10:1–11. doi: 10.2741/1500. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Marinus LM, Chase WK, Miller JA, Meyer JS. Altered hypothalamic-pituitary-adrenocortical function in rhesus monkeys (Macaca mulatta) with self-injurious behavior. Psychoneuroendocrinology. 2004;29:501–515. doi: 10.1016/s0306-4530(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939–1948. doi: 10.1542/peds.2005-2543. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. New Statistical Procedures for the Life Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1987. [Google Scholar]