Abstract
Caloric restriction (CR) without malnutrition delays aging and extends lifespan in diverse species; however, its effect on resistance to illness and mortality in primates is not clearly established. We report findings of a 20-year longitudinal adult-onset CR study in rhesus monkeys aimed at filling this critical gap in aging research. In a population of rhesus macaques maintained at the Wisconsin National Primate Research Center, moderate CR lowered the incidence of aging-related deaths. At the time point reported 50% of control fed animals survived compared with 80% survival of CR animals. Further, CR delayed the onset of age-associated pathologies. Specifically, CR reduced the incidence of diabetes, cancer, cardiovascular disease, and brain atrophy. These data demonstrate that CR slows aging in a primate species.
Evidence that mammalian longevity could be increased emerged in 1935 in a rodent study showing that caloric restriction (CR), without malnutrition, extended average and maximum lifespan and delayed the onset of age-associated pathologies(1). It was not until the 1990s that CR became widely viewed as a scientific model that could provide insights into retardation of the aging process(2) and thereby identify underlying mechanisms(3). The inverse relationship between calorie intake and increase in lifespan in mice suggests a role for regulators of energy metabolism in the mechanism of CR. Accordingly, CR-induced metabolic reprogramming may be a key event in the mechanism of lifespan extension(4). Studies in yeast, worms, flies and mice, point to a role for nutrient responsive signalling molecules including SIRT1, mTOR and PGC-1α in aging and CR(5). The relevance of these findings for human aging depends on conservation of the effects of CR on aging in primates.
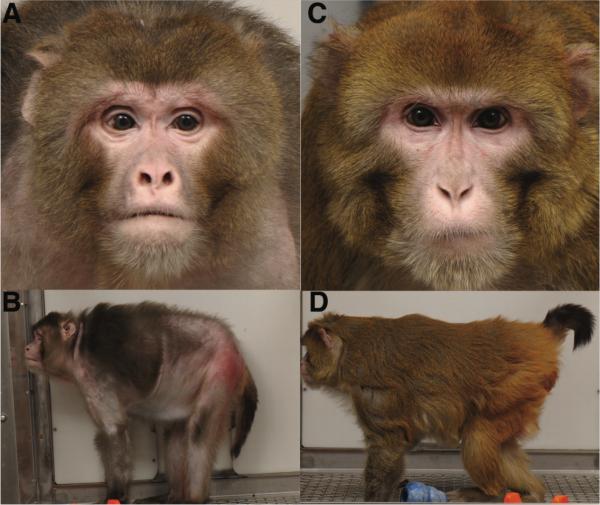
The marked anatomical, physiological and behavioral similarities between human and nonhuman primates make the latter uniquely suited for providing insights into the biology of human aging. Although animals on CR appeared subjectively younger than controls (Fig.1A–D), we sought to determine whether they were biologically younger than controls. Two critical indicators of aging retardation are delays in mortality and in the onset of age-associated disease. Incidence of disease increases with age and is a fundamental contributor to mortality(6). Thus, we examined age-associated conditions most prevalent in humans including diabetes, cancer, cardiovascular disease, and brain atrophy(7).
Fig. 1.
Animal appearance in old age. (A–B) Photographs of a typical control animal at 27.6 years of age (~age of average lifespan). (C–D) Photographs of an age-matched animal on CR.
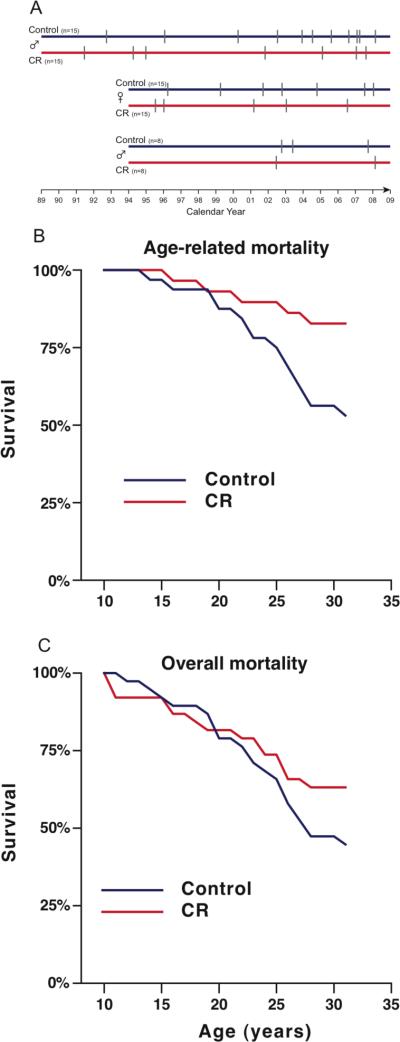
Our study was initiated in 1989 at the Wisconsin National Primate Research Center (WNPRC)(8)(Fig. 2A). Rhesus macaques (Macaca mulatta) have an average lifespan of ~27 years in captivity and a maximal lifespan of ~40 years. All animals were adults (7 to 14 years old) when introduced into the study. Initially the study included 30 males and the cohort was expanded in 1994 to include an additional 30 females and 16 males(9). Increased numbers improved statistical power and inclusion of females allowed us to monitor gender differences in the effect of CR. The animals were evenly matched and randomized to control or CR diets taking into consideration baseline food intake, body weight and age. Individualized food allotments were calculated based on daily food intake data that were collected for each animal over a 3 to 6 month period. Once animals were assigned to either control or CR groups, each CR animal's individually determined baseline intake was reduced by 10% per month over a 3-month period to reach the desired 30% restriction.
Fig. 2.
Longitudinal study design and mortality curves. (A) Study design. Initial group of 30 males and groups of 30 females and 16 males added in 1994. Hash marks represent deaths. (B) Age-related mortality. Animals that died from non-age-related causes are excluded. (C) All-cause mortality. These curves depict data for animals which died from any cause.
Any animal that died underwent a complete necropsy by a board-certified pathologist (who was blinded to the animal's diet group), and the cause of death was determined. On this basis, we distinguished deaths that were due to age-related causes from those due to acute conditions likely unrelated to aging. Of the original 76 animals, 37% (14/38) of the control animals died of age-related causes compared to only 13% (5/38) of the CR group. Survival analysis (Cox regression) considering only age-related deaths revealed a statistically significant effect of CR in increasing survival (p=0.03; Fig. 2B) with a hazard ratio (HR) of 3.0 indicating that at any point in time the control animals had 3 times the rate of death from an age-related cause when compared to animals under CR. Seven control and 9 animals subjected to CR died of non-age-related causes, which included complications of anesthesia, gastric bloat, endometriosis, and injury. The effect of CR on overall mortality is in the predicted direction, but is currently not statistically significant (p=0.16; Fig. 2C).
Age-associated diseases in rhesus monkeys have been well documented for animals at the WNPRC and are similar to those observed in humans(10). The most prevalent of such diseases are diabetes, cancer and cardiovascular disease. To determine the health and aging phenotype of each individual animal we assessed food intake, body weight, body composition, serum chemistry, glucose regulation, energy expenditure, activity measurement, endocrine profiles, electrocardiogram, blood pressure, brain MRI, and radiography. These measurements, and a minimum of twice daily observation of the animals, allowed disease conditions to be identified early and treated appropriately.
The effects of CR on body composition and metabolic function were robust. Body weight was reduced in animals on CR compared to that of control animals, primarily due to a decrease in total body fat mass(11). The age-associated decline in muscle mass (sarcopenia) was also attenuated in animals exposed to CR(12). Dual-energy x-ray absorptiometry analysis of lean muscle mass throughout the study revealed onset of sarcopenia at 15.5 years with statistically significant maintenance of lean muscle mass in the animals on CR compared to that of controls that has been sustained in animals at old age.
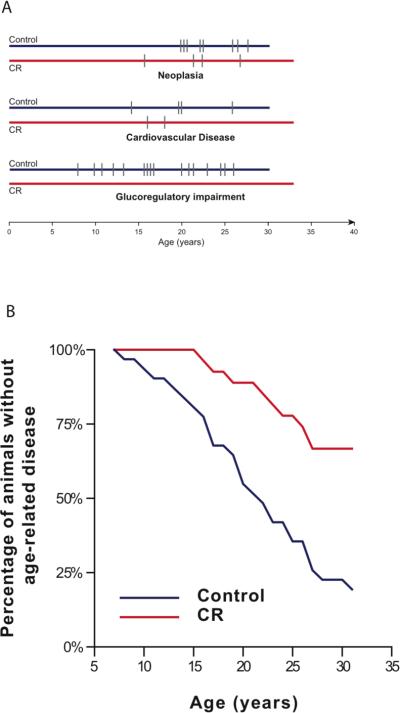
Improvements in metabolic function conferred by CR, specifically insulin sensitivity, have been consistent and striking(9, 13). We found that improved glucose homeostasis was maintained and that diabetes was prevented by CR. Of the initial 38 control animals, 5 progressed to diabetes and an additional 11 were classed as pre-diabetic. In contrast, all animals on CR (even those with compromised metabolic function at baseline) show no impairment of glucose homeostasis (Fig. 3A). These data are consistent with CR providing long-term health benefits in protection against diabetes.
Fig. 3.
Effect of CR on age-associated disease. (A) Incidence of three major age-related conditions. Hash marks represent age of diagnosis. Individual animals with multiple discrete diagnoses are represented multiple times. (B) Data represent first occurrence of any age-related disease in each individual animal.
The incidence of cancer increases with age in rhesus monkeys and intestinal adenocarcinoma is the most commonly diagnosed cancer in these animals(14). The methods used to detect and determine the type of cancer are described(7). The incidence of neoplasia was reduced by 50% in the animals undergoing CR compared to that in controls (Fig. 3A). The most common form of neoplasia was gastrointestinal adenocarcinoma which was identified in 7 of the 8 cases in the control animals, and 2 of the 4 cases in the animals on CR.
As it is in humans, cardiovascular disease is a prevalent age-associated disorder in rhesus monkeys. The methods used to diagnose cardiovascular disease are described(7). The most common diagnosis in living monkeys was leak of the mitral valve. The most frequently observed lesions at necropsy were valvular endocardiosis, cardiomyopathy, and myocardial fibrosis. The incidence of cardiovascular disease was reduced by 50% in the animals subject to CR compared to that in controls (Fig. 3A).
To assess the overall incidence of age-associated disease we recorded the age at which animals experienced their first age-associated diagnosis. The diseases mentioned above and other clinical conditions including diverticulosis and clinically relevant arthritis were monitored. The effect of CR in reducing disease onset was statistically significant (p =0.008; HR of 2.9). Age-related diseases were detected in control animals at ~3 times the rate they were detected in animals on CR(Fig. 3B). Animals on CR, thus, appear to be biologically younger than the normally fed animals.
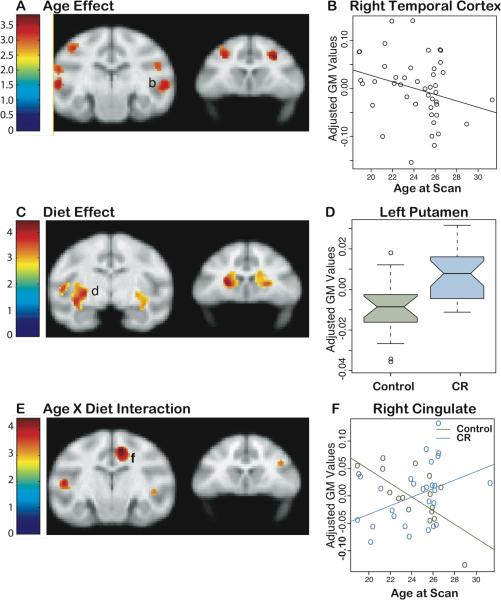
Brain atrophy is a characteristic of human aging that is not accurately reproduced in smaller mammals(15). We therefore determined the regional effects of age, diet, and age x diet interactions on gray matter (GM) volume(16). There were several cortical regions (bilateral frontal and temporal cortex) where decreases in volume with age were observed independent of diet(Fig.4A–C) (17). However, animals subjected to CR had statistically significant preservation of GM volume in subcortical regions (Fig.4D–F) including the caudate and putamen and the left insula. Examination of group differences in the slope of age-related GM atrophy (age x diet group interaction) reveal regions where CR significantly modified the aging effect (Fig.4G–I) in the midcingulate cortex, lateral temporal cortex bilaterally, and right dorsolateral frontal lobe, indicating relative preservation of volume with age in the CR group. Thus, CR reduced age-associated brain atrophy in key regions that subserve motor function and aspects of executive function.
Fig. 4.
Effects of CR and age on loss of GM in the brain. (A) Statistical parametric map of the t-contrast (SPMt) displayed on coronal slices depicting regions where an age-related decrease in GM volume was observed. (B) plot of the age effect on GM volume at the labelled location in (A). (C) SPMt indicating regions where CR monkeys exhibited preserved volume relative to controls. (D) Notched box plots for each group at the location labelled in (C) indicating the mean (center line), the 95% confidence interval (notches) and the 5th, 25th, 75th and 95th percentiles (horizontal lines) representing the range of variability in the data. (E) SPMt depicting regions where the slope between GM volume and age differs as a function of group. (F) Scatter plot of the location labelled in (E). All comparisons included sex and total brain volume as covariates. The probability threshold for each t contrast was p<0.005 (uncorrected). The color bars represent the value of the t-statistic in panels A, C and E. The left side of the brain is on the left in the images.
Our data indicate that adult-onset, moderate CR delays the onset of age-associated pathologies and promotes survival in a primate species. In two related nonhuman primate studies, the benefits of CR on health and longevity were less overt, possibly due to differences in study design(18, 19). Given the obvious parallels between rhesus monkey and human, the beneficial effects of CR may also occur in humans. This prediction is supported by studies of people on long-term CR, who show fewer signs of cardiovascular aging(20). The effect of controlled, long-term CR on maximal lifespan in humans may never be known, but our extended study will eventually provide such data in rhesus monkeys.
Supplementary Material
Acknowledgments
We acknowledge the excellent technical assistance provided by S. Baum, J. Christensen, J. A. Adriansjach, C. E. Armstrong, D. G. McLaren, C. Dizack, and the Animal Care, Veterinary and Pathology Staff of the WNPRC. R.W is a cofounder and member of the board of LifeGen Technologies, a company focused on nutritional genomics including the impact of dietary interventions on the aging process. This work was supported by NIH grants P01 AG-11915 and P51 RR000167. This research was conducted in part at the WNPRC which received support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This research was supported in part by facilities and resources at the Wm. S. Middleton Memorial Veterans Hospital.
Footnotes
Adult-onset, moderate caloric restriction delays the onset of age-associated diseases and prolongs survival in rhesus monkeys.
References
- 1.McCay CM, Crowell MF, Maynard LA. Nutrition. 1935;5:155. [PubMed] [Google Scholar]
- 2.Weindruch RH, Walford RL. The retardation of aging and disease by dietary restriction. Charles C Thomas; Springfield, Illinois, USA: 1988. [Google Scholar]
- 3.Kennedy BK, Steffen KK, Kaeberlein M. Cell Mol Life Sci. 2007;64:1323. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RM, Weindruch R. Interdiscip Top Gerontol. 2007;35:18. doi: 10.1159/000096554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mair W, Dillin A. Annu Rev Biochem. 2008;77:727. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 6.Vita AJ, Terry RB, Hubert HB, Fries JF. N Engl J Med. 1998;338:1035. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 7.Detailed information is supplied as Supporting Online Material.
- 8.Kemnitz JW, et al. J Gerontol. 1993;48:B17. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey JJ, et al. Exp Gerontol. 2000;35:1131. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 10.Uno H. Age. 1997;20:1. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. Aging (Milano) 1998;10:83. doi: 10.1007/BF03339642. [DOI] [PubMed] [Google Scholar]
- 12.Colman RJ, Beasley TM, Allison DB, Weindruch R. J Gerontol A Biol Sci Med Sci. 2008;63:556. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gresl TA, et al. Am J Physiol Endocrinol Metab. 2001;281:E757. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez NA, et al. J Med Primatol. 2002;31:74. doi: 10.1034/j.1600-0684.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 15.Yankner BA, Lu T, Loerch P. Annu Rev Pathol. 2008;3:41. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 16.Good CD, et al. Neuroimage. 2001;14:21. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 17.Alexander GE, et al. J Neurosci. 2008;28:2710. doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. J Gerontol A Biol Sci Med Sci. 2003;58:212. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 19.Mattison JA, Lane MA, Roth GS, Ingram DK. Exp Gerontol. 2003;38:35. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 20.Holloszy JO, Fontana L. Exp Gerontol. 2007;42:709. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.