Abstract
The effects of vitamin D on bone metabolism and calcium homeostasis have long been recognized. Emerging evidence has implicated vitamin D as a critical regulator of immunity, playing a role in both the innate and cell-mediated immune systems. Vitamin D deficiency has been found to be associated with several immune-mediated diseases, susceptibility to infection and cancer. Recently, there has been increasing interest in the possible link between vitamin D and asthma. Further elucidation of the role of vitamin D in lung development and immune system function may hold profound implications for the prevention and treatment of asthma.
Keywords: asthma, autoimmune disease, immune system regulation, T-regulatory cell, vitamin D, vitamin D deficiency
The role of vitamin D in the regulation of calcium and bone metabolism is well established. Recently, newer physiologic functions for vitamin D have been identified. Epidemiologic and genetic studies as well as research using animal models suggest vitamin D plays a vital and complex role in immune system function and regulation. Vitamin D insufficiency has been linked with susceptibility to infection, particularly respiratory infections [1–5], as well as to the development of a variety of cancers [6–11] and autoimmune diseases [12–16].
Asthma is one of the most common chronic diseases worldwide and has been increasing in prevalence over the last few decades [17,18]. Its exact cause remains unknown and likely has its origins in complex interactions among multiple genetic and environmental factors. Common risk factors for both asthma and vitamin D deficiency, such as an urbanized, westernized lifestyle [18–20], race/darker skin pigmentation [21–23] and obesity [24–26], along with increasing evidence of the immuno modulatory effects of vitamin D, have led to a hypo thesized link between the rising asthma prevalence and low vitamin D. This article will summarize some of the emerging evidence on the complex role of vitamin D in the immune system relevant to asthma, and provide an overview of investigations thus far linking vitamin D and asthma.
Vitamin D physiology
Vitamin D is an essential nutrient that humans obtain primarily through exposure to sunlight, and secondarily through diet and dietary supplements. Solar ultraviolet (UV) B (UVB) radiation converts 7-dehydrocholesterol in the skin to previtamin D3 and subsequently to vitamin D3. The ability to form this prohormone is influenced by skin pigmentation, sun protection, latitude, age, amount of UV radiation exposure and coverage by clothing, any of which may significantly affect vitamin D levels. In the diet, vitamin D is found mostly in oily fish (e.g., salmon and mackerel), and in fortified grain and dairy products. Vitamin D from the skin and diet is hydroxylated in the liver to 25-hydroxyvitamin D and stored. Under tight regulation by the parathyroid hormone, 25-hydroxyvitamin D is hydroxylated by 1-αhydroxylase in the kidney to the biologically active form, 1,25-dihydroxyvitamin D (1,25[OH]2D3), to maintain calcium–phosphate homeostasis. More recently, hydroxylation to 1,25-dihydroxyvitamin D has also been noted to occur at extra-renal sites, including cells of the immune system [27]. The vitamin D receptor (VDR) is part of the steroid hormone nuclear receptor family and acts to regulate gene transcription.
A patient's vitamin D status is determined by measuring 25-hydroxyvitamin D, which is the major circulating form. It has a half-life of 2 weeks in the circulation, and levels correlate with secondary hyperparathyroidism, osteomalacia and rickets [28]. 1,25-dihydroxyvitamin D levels may be normal or even elevated in a vitamin D-deficient state, and are thus not reliable in determining a patient's vitamin D status [29]. Most experts define vitamin D deficiency as a 25-hydroxyvitamin D level (hereafter referred to as ‘vitamin D level’) below 20 ng/ml (50 nmol/l), although there is no consensus as to what level is ideal [30]. Many vitamin D experts would argue that levels of 30–40 ng/ml (75–100 nmol/l) [31,32] or even greater [33] are needed for nonskeletal outcomes, although further research is needed to confirm this.
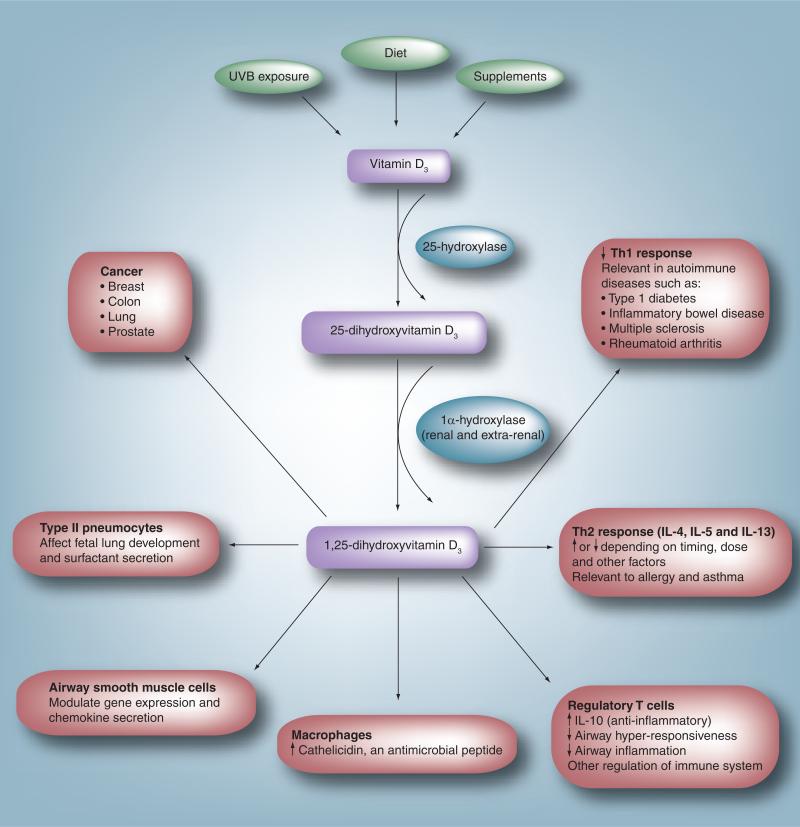
Low vitamin D levels have been documented in many different populations around the world, including those in latitudes where sun exposure would be presumed to be adequate such as Saudi Arabia [34,35], India [36], Israel [37], Costa Rica [38] and areas of southern and southeastern USA [39,40], among others [28]. At higher latitudes (i.e., >35°), very little vitamin D is produced in the skin during the late fall and winter months due to minimal UVB radiation exposure at this time of year [28,41]. In one study in Boston (MA, USA; latitude 42°), 42% of African–American and Hispanic children had vitamin D levels less than 20 ng/ml [42]. Another study in Boston showed 57% of in-patients in a general medical ward to be vitamin D deficient (levels ≤15ng/ml) [43]. Increased time spent indoors [44], wider use of sunscreen and skin coverage with clothing are all factors contributing to low vitamin D levels. Diseases traditionally associated with vitamin D deficiency include rickets and osteomalacia. Increasing data associate low vitamin D levels with other diseases such as autoimmune diseases [12–16], many cancers [6–10] and asthma (Figure 1).
Figure 1. Extraskeletal effects of vitamin D.
In humans, vitamin D is obtained through ultraviolet B exposure, diet and supplement intake. It is converted to 25-hydroxyvitamin D3 by the liver. Circulating 25-hydroxyvitamin D3 is converted to the active form, 1,25-dihydroxyvitamin D3, in a variety of sites including the kidney and cells of the immune system. Experimental evidence suggests an effect of 1,25-dihydroxyvitamin D3 on multiple different processes and cell types. In the immune system it leads to a decrease in the Th1 response, thought to be the mechanism involved in the association between low vitamin D levels and a variety of autoimmune diseases. It modulates the Th2 response affecting cytokines such as IL-4, IL-5 and IL-13. This is one possible link between vitamin D and allergy/asthma. It has been shown to upregulate T-regulatory cells, leading to an increase in the synthesis of the anti-inflammatory cytokine IL-10. In macrophages, vitamin D upregulates synthesis of the antimicrobial peptide cathelicidin, which may enhance the ability to fight infections. In airway smooth muscle cells, it has been shown to modulate chemokine release. Vitamin D may play a role in fetal lung development and in the differentiation of type II pneumocytes and surfactant secretion. Vitamin D has also been associated with a lower incidence of and mortality from a variety of cancers.
Vitamin D & the immune system
Increasing evidence implicates a complex role for vitamin D in the regulation of immune responses. Multiple immune cell types express VDRs, including activated T and B cells, macrophages [45] and dendritic cells [46]. Expression of 1α-hydroxylase, the enzyme that catalyzes the synthesis of active 1,25-dihydroxyvitamin D from 25-hydroxyvitamin D, is expressed by cells at extra-renal sites, including epithelial cells, keratinocytes, activated macrophages and dendritic cells [27]. This not only highlights the capacity for extra-renal synthesis of the active form of vitamin D, but also the capacity to modulate innate [47] and adaptive [48] immune function at these sites.
Vitamin D inhibits the function of T lymphocytes both directly and via effects on antigen-presenting cells (APCs). It has potent antiproliferative effects on CD4+ T cells [49]. Essentially all published studies using both human and murine models report inhibition of Th1-associated cytokine production [46,49–52]. More recently, vitamin D has been reported to inhibit IL-17 production [53,54]. IL-17 is an inflammatory cytokine important for defense against extracellular bacteria, but is also involved in autoimmune and allergic diseases, including asthma [55].
The effects of vitamin D on Th2 responses are more complex. Most reviews simply state that vitamin D promotes Th2 responses; however, a closer look at the literature reveals reports of both the inhibition and enhancement of Th2 responses. Th2 cells play a central role in the pathogenesis of asthma, producing cytokines such as IL-4, IL-5 and IL-13, and inducing the production of IgE by B cells, as well as the growth and differentiation of relevant effector cells, namely mast cells and eosinophils [56]. In experimental models, Th2 cells from animals primed with antigen can induce experimental asthma, including airway hyper-responsiveness and elevated eosinophils in naive animals [57]. In culture, vitamin D has been reported to enhance IL-4 and IL-13 synthesis by murine T cells in one study [58], but to inhibit IL-4 when added from the onset of differentiation in a second study [59]. Notably, both studies used naive T cells.
There is a general consensus that vitamin D alleviates autoimmune disease in animal models [46], which is associated with the inhibition of IFN-γ production. Variable effects on Th2 cytokine production are reported in these models [60,61]. In models of allergic airway disease, the data are also conflicting. In one study of murine asthma, the administration of 1,25-dihydroxyvitamin D was found to inhibit airway inflammation, decrease levels of IL-4 in bronchoalveolar lavage fluid and decrease T-cell migration, inhibiting the inflammatory response [62]. In another study, mice irradiated with a single erythemal dose of UVB light prior to sensitization with antigen, probably increasing vitamin D levels, had less airway hyper-responsiveness and inflammation than mice who were not [63]. However, in a different study, vitamin D enhanced Th2 responses (IL-4 and IL-13) when given early during immunization, but inhibited both IL-5 and airway eosinophilia when administered later [64]. Additional evidence comes from VDR knock-out mice, which do not develop experimental asthma [65]. Although these mice do have the ability to prime and activate immune cells and have elevated levels of circulating mediators associated with experimental asthma (e.g., IL-5, IL-13 and IgE), it appears that VDR expression is necessary for lung development and inflammation to occur [57]. In this same experiment, vitamin D-deficient mice did not have the same phenotype as the VDR knockout mice [57]. Such results point to the complexity of the role of vitamin D in the inflammatory responses involved in the pathogenesis of asthma, as well as the importance of the timing and dose of exposure.
In studies with human cells, vitamin D inhibits IFN-γ synthesis [52,66–68]. At least two reports describe the capacity of vitamin D to enhance [66,69] and to inhibit Th2 responses in culture; of the latter, one was in blood from adults [68] and the second in cord blood [70]. The reason for these differences is unclear, but responsiveness to vitamin D has been suggested to differ according to the stage of differentiation and activation status of CD4+ T cells [49]. Differential dose-dependent effects may also exist [66,68]. Importantly, studies looking at Th2 cytokines in peripheral blood post vitamin D supplementation [71] or calcitriol ingestion [68] show no evidence of Th2 enhancement, although the individuals were not challenged with antigen at the same time.
Several laboratories have described the capacity of vitamin D to promote regulatory T cells (Tregs) [72,73]. Tregs play an important role in the control of the immune response and inhibit Th2 responses, as well as airway inflammation and airway hyper-responsiveness [74,75], which may be the key to the potential role of vitamin D in asthma. Vitamin D acts on dendritic cells, which play a central role in the activation of T-cell-mediated immune responses to induce a tolerogenic phenotype associated with decreased expression of MHC class II and costimulatory ligands, decreased secretion of the immunostimulatory cytokine IL-12 [50] and increased IL-10, an anti-inflammatory cytokine with potent inhibitory effects on Th1 and Th2 responses [68]. Vitamin D also acts directly on T cells to promote an IL-10-secreting Treg phenotype either alone [68] or in concert with glucocorticoids [76]. In experimental models of allergic airway disease, IL-10 consistently shows anti-inflammatory properties [75,77,78] and in a number of studies also decreased airway hyper-responsiveness [79–84], although a few studies have reported the capacity of IL-10 to increase airway hyper-responsiveness [85,86], possibly via effects on airway smooth muscle [87]. The reasons for this discrepancy in effects on airway hyper-responsiveness (either increased or decreased) have not yet been clarified. In animal models, both oral [73] and topical [88] administration of vitamin D has been demonstrated to enhance Tregs. In an animal model of allergic experimental encephalomyelitis, IL-10 signaling was essential for 1,25-dihydroxyvitamin D-mediated inhibition of the disease [89]. Recently, 1,25-dihydroxyvitamin D was shown to potentiate the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma, which was dependent on IL-10 and TGF-β [90]. Vitamin D also enhances IL-10 synthesis by dendritic cells [46] and B cells [91], while inhibiting antibody production, including IgE.
Human studies demonstrate that vitamin D supplementation is associated with increased serum levels of TGF-β1 [71]. TGF-β is a complex cytokine with a role in the peripheral induction of Foxp3+ Treg immunosuppression, but also in wound healing and repair [92]. In addition, two studies demonstrate that vitamin D increases IL-10 synthesis. In one, calcitriol ingestion increased IL-10 gene expression by T cells assessed directly ex vivo pre- and postingestion [68], while a double-blind, randomized, placebo-controlled trial demonstrated that vitamin D supplementation in patients with congestive heart failure improved cytokine profiles by enhancing IL-10 and improving tumor necrosis factor:IL-10 ratios [93]. A recent study shows a nonlinear relationship between vitamin D levels and IgE, with both low (<25 nmol/l) and high (>135 nmol/l) vitamin D levels associated with elevated IgE [94]. Of note, only a small percentage of the total sampled population (58 subjects or ~0.8%) were in the group with the highest vitamin D level.
Vitamin D in fetal lung & immune system development
In the fetus, vitamin D appears to play a role in immune system and lung development. In early gestation, 1,25-dihydroxyvitamin D is synthesized by human decidual cells [95], and may exert autocrine or paracrine effects on the fetus’ developing immune system. In addition, in human cord blood cells, vitamin D has been shown to decrease IFN-γ as well as IL-4 and IL-13 [70]. Although these effects seem contradictory to some described previously (i.e., that vitamin D increases IL-4), this re-emphasizes that the timing of vitamin D exposure in ‘naive’ versus mature T cells may determine the type of response and cytokine profile elicited.
Vitamin D receptors have been found in fetal type II alveolar pneumocytes of rats, and may play a role in lung development, pneumocyte differentiation and surfactant secretion [96–99]. Recent evidence suggests that type II alveolar pneumocytes may play a role in the induction of Tregs and self-tolerance [100]. Lung mechanics studied in rats showed decreased compliance in those born to mothers deprived of vitamin D [101]. The relationship between vitamin D and surfactant production appears to be more complex in studies of human fetal lung tissue [102,103].
Vitamin D in asthma & human disease
Multiple recent studies have noted the effects of vitamin D on the development of immune-related disorders and certain cancers. Epidemiologic studies have shown an association between low vitamin D levels and increased risk of, and/or higher mortality from, colon, breast and lung cancer [6–10]. Low vitamin D levels or intake have also been found to be associated with other immune-mediated diseases including Type I diabetes [13], multiple sclerosis [15,16], rheumatoid arthritis [14] and inflammatory bowel disease [12]. These are primarily Th1-mediated diseases.
To date, studies that have investigated the link between vitamin D and asthma have been few and have had conflicting results. Initial evidence of a link was found in genetic association studies. The VDR maps to chromosome 12, a region of the genome previously found to be linked with asthma [104]. Two different family-based studies in North American subjects reported an association between VDR polymorphisms and asthma [104,105]; however, two German studies involving fewer subjects did not show this link [106,107]. Subsequent work by the same German group showed transmission disequilibrium in genes involved in vitamin D metabolism other than the VDR gene in children with asthma [108].
Two epidemiologic studies, one in a cohort in the Boston area and another in Aberdeen (Scotland, UK; latitude 57°), have demonstrated an association between pregnant women with lower vitamin D intake and a higher risk of wheeze in their children [109,110]. Each study involved over 1000 mothers and both showed that in mothers who had the highest vitamin D intake, there was a greater than 50% reduction in the risk of recurrent wheeze in their children. These studies assessed intake using food frequency questionnaires; vitamin D levels were not measured. A large cross-sectional study looking at over 14,000 subjects showed a strong correlation between higher serum vitamin D levels and better lung function in the Third National Health and Nutrition Examination Survey (NHANES III) data set [111].
Studies by other groups have shown conflicting data. One study from Finland showed an increased risk for asthma and atopy in adults who were supplemented with vitamin D during childhood [112]. However, this study did not assess vitamin D intake in mothers, or levels in either the subjects or their mothers. Another study from the UK showed an increased risk of eczema at 9 months of age, and asthma at 9 years of age in the children of women with higher vitamin D levels. This study had a large loss to follow-up, with only approximately 30% of the initial cohort retained at the time of analysis for asthma [113]. There continues to be a debate regarding the true relationship of vitamin D with asthma, with some authors believing that the increase in asthma prevalence is due to the fortification of foods with vitamin D [114]. In addition to the development of asthma, others have examined the relationship between vitamin D and asthma severity or response to treatment in those with existing asthma. After demonstrating that the administration of vitamin D in cell culture increases glucocorticoid-induced Treg secretion of the inhibitory cytokine IL-10, Xystrakis et al. administered vitamin D to steroid-resistant asthmatics and demonstrated a similar response in vivo, which may have treatment implications in this group [115]. Banerjee et al. showed that, in addition to immune system effects in asthmatics, vitamin D may also modulate chemokines secreted by airway smooth muscle cells, an additional pathway by which airway reactivity may be modulated by vitamin D [116]. Similarly, another group looked at the VDR in bronchial smooth muscle cells and showed vitamin D to be a regulator of the expression of over 400 genes, some of which have been implicated in the pathogenesis of asthma [117]. This pathway may be involved in the airway remodeling seen in asthmatics. Our group analyzed a cohort of asthmatic children in Costa Rica and demonstrated that lower vitamin D levels correlated with markers of severity of asthma, including hospitalizations, the use of anti-inflammatory medications and airway hyper-responsiveness [38].
Infection, asthma & vitamin D
Infants who wheeze with viral respiratory infections are more likely to develop asthma. It is unclear whether the infection and/or specific viral agents may be causal. Some studies have shown a link between specific viruses and the subsequent development of asthma or allergy such as respiratory syncytial virus [118,119] or, more recently, rhinovirus [120]. A detailed discussion of the controversy surrounding the connection between early-life respiratory viral infections and wheeze or asthma is beyond the scope of this article but is covered elsewhere in detail [121]. Vitamin D may have an indirect link with the development of asthma through its association with susceptibility to infections [122].
It has been previously observed that children with rickets have an increased risk of respiratory infections [5,123–125]. Vitamin D increases the production of cathelicidin in macrophages [1], which enhances antimicrobial activity, especially in Mycobacterium tuberculosis, but also in other infections [126,127]. Airway epithelial cells have been found to express high levels of 1α-hydroxylase, converting 25-hydroxyvitamin D to its active form, leading to the increased production of both cathelicidin and the Toll-like receptor coreceptor CD14, important in the recognition of Gram-positive and -negative bacteria [128]. Vitamin D also enhances the differentiation and recruitment of macrophages, which may lead to an increased ability to fight infection [50]. Not all in vitro and animal models show a beneficial effect of vitamin D on the clearance of infections [129].
Low vitamin D levels have been found to be associated with respiratory infections in infants and adults [3–5]; higher levels may be associated with fewer influenza or other viral respiratory infections [130]. Ecological arguments have been made that seasonal vitamin D deficiency (worse in winter months) and influenza epidemics follow similar patterns [131–133]. Recent analysis of over 18,000 subjects in the NHANES III data set showed a strong independent association between low vitamin D levels and recent upper respiratory tract infections after adjusting for multiple demographic and clinical characteristics. This association was even stronger in individuals with asthma or chronic obstructive pulmonary disease [2]. However, a randomized, controlled trial of vitamin D supplementation in adults showed no difference in the incidence or severity of upper respiratory tract infections [134]. Mean 25-hydroxyvitamin D levels in the treatment arm were only 35 ng/ml (88.5 nmol/l), and it is unclear what level is adequate for optimal immune function. In addition, it may take up to 3 months to achieve steady-state levels [135], which was the duration of the study. In general, intervention studies are lacking and have been disappointing thus far [136,137]; more studies are needed to elucidate the clinical relevance of findings from in vitro studies of immune function. A recent review on vitamin D, asthma and infections covers this subject in greater detail [138].
Expert commentary & five-year view
The role of vitamin D in the pathogenesis of immune-mediated diseases such as asthma is only beginning to be understood. Several ongoing or planned human clinical trials are aimed at clarifying this link in the next 5 years. These include both prevention trials, such as one by our group of vitamin D supplementation in pregnant women for the prevention of allergy and asthma in their children [201], as well as treatment in patients with known severe or steroid-resistant asthma [202]. In addition, studies of vitamin D supplementation in other disease states including TB, multiple sclerosis, lupus and cancer, along with ancillary laboratory investigations, for example of Treg cell function, will aid in our understanding of the role of vitamin D in the modulation of the immune system and its effects on disease. The elucidation of the precise roles of vitamin D in the immune system and in the pathogenesis of multiple diseases has the potential to have profound effects on our ability to prevent and treat these disorders.
Key issues.
Recent evidence points to vitamin D as an essential immune system regulator.
Vitamin D deficiency and insufficiency are widespread, regardless of latitude.
Low vitamin D levels have been linked with many immune-mediated diseases and cancers.
Basic science and animal models demonstrate the multiplicative effects of vitamin D on cells of the immune system and cytokine profiles.
Genetic and epidemiologic studies have shown an association between asthma and vitamin D.
The rising prevalence of asthma may be linked to vitamin D deficiency.
Further investigation is needed to fully understand the role of vitamin D in the development of allergy and asthma.
Financial & competing interests disclosure
Scott Weiss has received funding from the NIH/NHLBI grant (U01 HL091528) ‘Randomized Trial: Maternal Vitamin D Supplementation to Prevent Childhood Asthma’ and the NIH grant (R21 HL089842) ‘Vitamin D in Obstructive Lung Diseases’. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Nancy E Lange, Channing Laboratory, Division of Pulmonary and Critical Care Medicine, Brigham & Women's Hospital, 181 Longwood Avenue, Boston, MA 02115, USA Tel.: +1 617 525 0874 Fax: +1 617 525 0958 nlange@partners.org.
Augusto Litonjua, Channing Laboratory, Division of Pulmonary and Critical Care Medicine, Brigham & Women's Hospital, 181 Longwood Avenue, Boston, MA 02115, USA Tel.: +1 617 525 0874 Fax: +1 617 525 0958 reaal@channing.harvard.edu.
Catherine M Hawrylowicz, King's College London, Department of Asthma Allergy and Respiratory Science, Guy's Hospital, London, SE1 9RT, UK Tel.: +44 020 7188 0598 Fax: +44 020 7403 8640 catherine.hawrylowicz@kcl.ac.uk.
Scott Weiss, Channing Laboratory, Division of Pulmonary and Critical Care Medicine, Brigham & Women's Hospital, 181 Longwood Avenue, Boston, MA 02115 USA Tel.: +1 617 525 0874 Fax: +1 617 525 0958 scott.weiss@channing.harvard.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 2•.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [Recent study looking at Third National Health and Nutrition Examination Survey data showing an association between low 25-hydroxyvitamin D levels and recent upper respiratory tract infection. This association was stronger in patients with lung disease such as asthma or coronary obstructive pulmonary disease] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur. J. Clin. Nutr. 2007;63(4):473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 4.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/l with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 5.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur. J. Clin. Nutr. 2004;58(4):563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 6.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am. J. Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W, Heist RS, Liu G, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. J. Clin. Oncol. 2007;25(5):479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 9.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am. J. Prev. Med. 2007;32(3):210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Crew KD, Gammon MD, Steck SE, et al. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev. Res. (Phila. Pa) 2009;2(6):598–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26(4A):2687–2699. [PubMed] [Google Scholar]
- 12.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog. Biophys. Mol. Biol. 2006;92(1):60–64. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 14.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50(1):72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 15.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 16.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 17.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma – United States, 1980–1999. MMWR Surveill. Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 18.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 19.Manicourt DH, Devogelaer JP. Urban tropospheric ozone increases the prevalence of vitamin D deficiency among Belgian postmenopausal women with outdoor activities during summer. J. Clin. Endocrinol. Metab. 2008;93(10):3893–3899. doi: 10.1210/jc.2007-2663. [DOI] [PubMed] [Google Scholar]
- 20.Aligne CA, Auinger P, Byrd RS, Weitzman M. Risk factors for pediatric asthma. Contributions of poverty, race, and urban residence. Am. J. Respir. Crit. Care Med. 2000;162(3 Pt 1):873–877. doi: 10.1164/ajrccm.162.3.9908085. [DOI] [PubMed] [Google Scholar]
- 21.Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J. Clin. Endocrinol. Metab. 2008;93(1):40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weitzman M, Byrd RS, Auinger P. Black and white middle class children who have private health insurance in the United States. Pediatrics. 1999;104(1 Pt 2):151–157. [PubMed] [Google Scholar]
- 23.Carter-Pokras OD, Gergen PJ. Reported asthma among Puerto Rican, Mexican–American, and Cuban children, 1982 through 1984. Am. J. Public Health. 1993;83(4):580–582. doi: 10.2105/ajph.83.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007;175(7):661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J. Allergy Clin. Immunol. 2005;115(5):925–927. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 26.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 27.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holick MF. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest. 2006;116(8):2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 30•.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [Excellent, comprehensive review of vitamin D physiology. Describes both well-established and recently discovered effects of vitamin D deficiency on a variety of diseases] [DOI] [PubMed] [Google Scholar]
- 31.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 32.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am. J. Clin. Nutr. 2007;85(3):649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 33.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: an important tool to define adequate nutritional vitamin D status. J. Steroid Biochem. Mol. Biol. 2007;103(3–5):631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharyya AK. Nutritional rickets in the tropics. World Rev. Nutr. Diet. 1992;67:140–197. doi: 10.1159/000419463. [DOI] [PubMed] [Google Scholar]
- 35.Taha SA, Dost SM, Sedrani SH. 25-hydroxyvitamin D and total calcium: extraordinarily low plasma concentrations in Saudi mothers and their neonates. Pediatr. Res. 1984;18(8):739–741. doi: 10.1203/00006450-198408000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Marwaha RK, Tandon N, Reddy DR, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am. J. Clin. Nutr. 2005;82(2):477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 37.Nehama H, Wientroub S, Eisenberg Z, Birger A, Milbauer B, Weisman Y. Seasonal variation in paired maternal-newborn serum 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D concentrations in Israel. Isr. J. Med. Sci. 1987;23(4):274–277. [PubMed] [Google Scholar]
- 38••.Brehm JM, Celedon JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am. J. Respir. Crit. Care Med. 2009;179(9):765–71. doi: 10.1164/rccm.200808-1361OC. [Recent study in a large cohort of children with asthma in Costa Rica showing an association between low vitamin D levels (<30 ng/ml) and asthma severity in terms of hospitalizations, medication use and airway responsiveness] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayward I, Stein MT, Gibson MI. Nutritional rickets in San Diego. Am. J. Dis. Child. 1987;141(10):1060–1062. doi: 10.1001/archpedi.1987.04460100038018. [DOI] [PubMed] [Google Scholar]
- 40.Stein EM, Laing EM, Hall DB, et al. Serum 25-hydroxyvitamin D concentrations in girls aged 4–8 y living in the southeastern United States. Am. J. Clin. Nutr. 2006;83(1):75–81. doi: 10.1093/ajcn/83.1.75. [DOI] [PubMed] [Google Scholar]
- 41.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 42.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch. Pediatr. Adolesc. Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 43.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N. Engl. J. Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 44.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2008;168(6):577–586. doi: 10.1093/aje/kwn163. discussion 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 46.Adorini L, Penna G, Giarratana N, et al. Dendritic cells as key targets for immunomodulation by vitamin D receptor ligands. J. Steroid Biochem. Mol. Biol. 2004;89–90(1–5):437–441. doi: 10.1016/j.jsbmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 48.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007;8(3):285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 49.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell Biochem. 2003;89(5):922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 50.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu. Rev. Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 51.Iho S, Kura F, Sugiyama H, Takahashi T, Hoshino T. The role of monocytes in the suppression of PHA-induced proliferation and IL 2 production of human mononuclear cells by 1,25-dihydroxy vitamin D3. Immunol. Lett. 1985;11(5–6):331–336. doi: 10.1016/0165-2478(85)90116-6. [DOI] [PubMed] [Google Scholar]
- 52.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 α,25-dihydroxyvitamin D3 inhibits γ-interferon synthesis by normal human peripheral blood lymphocytes. Proc. Natl Acad. Sci. USA. 1987;84(10):3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th)1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 54.Tang J, Zhou R, Luger D, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J. Immunol. 2009;182(8):4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louten J, Boniface K, de Waal Malefyt R. Development and function of Th17 cells in health and disease. J. Allergy Clin. Immunol. 2009;123(5):1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Holgate ST. Pathogenesis of asthma. Clin. Exp. Allergy. 2008;38(6):872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 57.Wittke A, Chang A, Froicu M, et al. Vitamin D receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Arch. Biochem. Biophys. 2007;460(2):306–313. doi: 10.1016/j.abb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 59.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J. Immunol. 2002;168(3):1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 60.Mattner F, Smiroldo S, Galbiati F, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur. J. Immunol. 2000;30(2):498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 61.Overbergh L, Decallonne B, Waer M, et al. 1α,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543). Diabetes. 2000;49(8):1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 62.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004;34(4):1068–1076. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 63.McGlade JP, Gorman S, Zosky GR, et al. Suppression of the asthmatic phenotype by ultraviolet B-induced, antigen-specific regulatory cells. Clin. Exp. Allergy. 2007;37(9):1267–1276. doi: 10.1111/j.1365-2222.2007.02750.x. [DOI] [PubMed] [Google Scholar]
- 64.Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of Th1/Th2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 2003;112(3):585–592. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 65.Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J. Immunol. 2004;173(5):3432–3436. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 66.Jirapongsananuruk O, Melamed I, Leung DY. Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on Th1, but not Th2, responses. J. Allergy Clin. Immunol. 2000;106(5):981–985. doi: 10.1067/mai.2000.110101. [DOI] [PubMed] [Google Scholar]
- 67.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J. Nutr. 1995;125(6 Suppl):1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 68.Urry Z, Xystrakis E, Richards DF, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1α,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Invest. 2009;119(2):387–398. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thien R, Baier K, Pietschmann P, Peterlik M, Willheim M. Interactions of 1 α,25-dihydroxyvitamin D3 with IL-12 and IL-4 on cytokine expression of human T lymphocytes. J. Allergy Clin. Immunol. 2005;116(3):683–689. doi: 10.1016/j.jaci.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 α,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr. Res. 2002;52(1):12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J. Neuroimmunol. 2003;134(1–2):128–132. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 72.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001;167(4):1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 73.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1α,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 74.Umetsu DT, Akbari O, Dekruyff RH. Regulatory T cells control the development of allergic disease and asthma. J. Allergy Clin. Immunol. 2003;112(3):480–487. quiz 488. [PubMed] [Google Scholar]
- 75.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 76.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)-and Th2-inducing cytokines. J. Exp. Med. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 78.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 1996;97(6):1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 79.Henry E, Desmet CJ, Garze V, et al. Dendritic cells genetically engineered to express IL-10 induce long-lasting antigen-specific tolerance in experimental asthma. J. Immunol. 2008;181(10):7230–7242. doi: 10.4049/jimmunol.181.10.7230. [DOI] [PubMed] [Google Scholar]
- 80.Nakagome K, Dohi M, Okunishi K, et al. In vivo IL-10 gene delivery suppresses airway eosinophilia and hyperreactivity by down-regulating APC functions and migration without impairing the antigen-specific systemic immune response in a mouse model of allergic airway inflammation. J. Immunol. 2005;174(11):6955–6966. doi: 10.4049/jimmunol.174.11.6955. [DOI] [PubMed] [Google Scholar]
- 81.Presser K, Schwinge D, Wegmann M, et al. Coexpression of TGF-β1 and IL-10 enables regulatory T cells to completely suppress airway hyperreactivity. J. Immunol. 2008;181(11):7751–7758. doi: 10.4049/jimmunol.181.11.7751. [DOI] [PubMed] [Google Scholar]
- 82.Koya T, Matsuda H, Takeda K, et al. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J. Allergy Clin. Immunol. 2007;119(5):1241–1250. doi: 10.1016/j.jaci.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 83.Akbari O, Freeman GJ, Meyer EH, et al. Antigen-specific regulatory T cells develop via the ICOS–ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8(9):1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 84.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202(11):1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Makela MJ, Kanehiro A, Dakhama A, et al. The failure of interleukin-10-deficient mice to develop airway hyperresponsiveness is overcome by respiratory syncytial virus infection in allergen-sensitized/challenged mice. Am. J. Respir. Crit. Care Med. 2002;165(6):824–831. doi: 10.1164/ajrccm.165.6.2105062. [DOI] [PubMed] [Google Scholar]
- 86.van Scott MR, Justice JP, Bradfield JF, Enright E, Sigounas A, Sur S. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278(4):L667–L674. doi: 10.1152/ajplung.2000.278.4.L667. [DOI] [PubMed] [Google Scholar]
- 87.Grunstein MM, Hakonarson H, Leiter J, et al. Autocrine signaling by IL-10 mediates altered responsiveness of atopic sensitized airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281(5):L1130–L1137. doi: 10.1152/ajplung.2001.281.5.L1130. [DOI] [PubMed] [Google Scholar]
- 88.Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J. Immunol. 2007;179(9):6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 89.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177(9):6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 90.Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1α,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-β. J. Immunol. 2008;180(8):5211–5221. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- 91.Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008;38(8):2210–2218. doi: 10.1002/eji.200838216. [DOI] [PubMed] [Google Scholar]
- 92.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 93.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 94.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE – a significant but nonlinear relationship. Allergy. 2009;64(4):613–620. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 95.Evans KN, Nguyen L, Chan J, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol. Reprod. 2006;75(6):816–822. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen M, Guillozo H, Garabedian M, Balsan S. Lung as a possible additional target organ for vitamin D during fetal life in the rat. Biol. Neonate. 1987;52(4):232–240. doi: 10.1159/000242714. [DOI] [PubMed] [Google Scholar]
- 97.Nguyen M, Trubert CL, Rizk-Rabin M, et al. 1,25-dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J. Steroid Biochem. Mol. Biol. 2004;89–90(1–5):93–97. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen TM, Guillozo H, Marin L, et al. 1,25-dihydroxyvitamin D3 receptors in rat lung during the perinatal period: regulation and immunohistochemical localization. Endocrinology. 1990;127(4):1755–1762. doi: 10.1210/endo-127-4-1755. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am. J. Physiol. 1996;271(3 Pt 1):L392–L399. doi: 10.1152/ajplung.1996.271.3.L392. [DOI] [PubMed] [Google Scholar]
- 100.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4+ T cells and induce Foxp3+ regulatory T cells. Am. J. Respir. Crit. Care Med. 2009;179(5):344–355. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- 101.Gaultier C, Harf A, Balmain N, Cuisinier-Gleizes P, Mathieu H. Lung mechanics in rachitic rats. Am. Rev. Respir. Dis. 1984;130(6):1108–1110. doi: 10.1164/arrd.1984.130.6.1108. [DOI] [PubMed] [Google Scholar]
- 102.Rehan VK, Torday JS, Peleg S, et al. 1A,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1α,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol. Genet. Metab. 2002;76(1):46–56. doi: 10.1016/s1096-7192(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 103.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1α,25-dihydroxyvitamin D3. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289(4):L617–L626. doi: 10.1152/ajplung.00129.2004. [DOI] [PubMed] [Google Scholar]
- 104•.Raby BA, Silverman EK, Lazarus R, Lange C, Kwiatkowski DJ, Weiss ST. Chromosome 12q harbors multiple genetic loci related to asthma and asthma-related phenotypes. Hum. Mol. Genet. 2003;12(16):1973–1979. doi: 10.1093/hmg/ddg208. [Along with [105], two separate genetic studies from different populations published simultaneously showing a significant association between polymorphisms in the vitamin D receptor and asthma] [DOI] [PubMed] [Google Scholar]
- 105•.Poon AH, Laprise C, Lemire M, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am. J. Respir. Crit. Care Med. 2004;170(9):967–973. doi: 10.1164/rccm.200403-412OC. [Along with [104], two separate genetic studies from different populations published simultaneously showing a significant association between polymorphisms in the vitamin D receptor and asthma] [DOI] [PubMed] [Google Scholar]
- 106.Vollmert C, Illig T, Altmuller J, et al. Single nucleotide polymorphism screening and association analysis – exclusion of integrin β 7 and vitamin D receptor (chromosome 12q) as candidate genes for asthma. Clin. Exp. Allergy. 2004;34(12):1841–1850. doi: 10.1111/j.1365-2222.2004.02047.x. [DOI] [PubMed] [Google Scholar]
- 107.Wjst M. Variants in the vitamin D receptor gene and asthma. BMC Genet. 2005;6(1):2. doi: 10.1186/1471-2156-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wjst M, Altmuller J, Faus-Kessler T, Braig C, Bahnweg M, Andre E. Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir. Res. 2006;7:60. doi: 10.1186/1465-9921-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109•.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am. J. Clin. Nutr. 2007;85(3):788–795. doi: 10.1093/ajcn/85.3.788. [Along with [110], two studies published simultaneously in two different cohorts, both showing an association between lower intake of vitamin D in pregnant women and higher risk of recurrent wheeze in their children] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110•.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am. J. Clin. Nutr. 2007;85(3):853–859. doi: 10.1093/ajcn/85.3.853. [Along with [110], two studies published simultaneously in two different cohorts, both showing an association between lower intake of vitamin D in pregnant women and higher risk of recurrent wheeze in their children] [DOI] [PubMed] [Google Scholar]
- 111.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 112.Hypponen E, Sovio U, Wjst M, et al. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann. NY Acad. Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 113.Gale CR, Robinson SM, Harvey NC, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy. 1999;54(7):757–759. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 115••.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Invest. 2006;116(1):146–155. doi: 10.1172/JCI21759. [Study both in vitro and ex vivo of the effect of vitamin D on IL-10 production by T-regulatory cells. The investigators show that in steroid-resistant asthmatics, the administration of vitamin D reverses the inability to secrete the inhibitory cytokine IL-10 in response to steroids, resulting in the inhibition of allergen-induced Th2 cytokine production] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Banerjee A, Damera G, Bhandare R, et al. Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells. Br. J. Pharmacol. 2008;155(1):84–92. doi: 10.1038/bjp.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117••.Bosse Y, Maghni K, Hudson TJ. 1α,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol. Genomics. 2007;29(2):161–168. doi: 10.1152/physiolgenomics.00134.2006. [Demonstrated the presence of the vitamin D receptor in bronchial smooth muscle cells, as well as the upregulation of many different genes that are likely involved in airway remodeling in these cells after stimulation with 1,25-hydroxyvitamin D] [DOI] [PubMed] [Google Scholar]
- 118.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000;161(5):1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 119.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 120.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carroll KN, Hartert TV. The impact of respiratory viral infection on wheezing illnesses and asthma exacerbations. Immunol. Allergy Clin. North Am. 2008;28(3):539–561. viii. doi: 10.1016/j.iac.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr. Res. 2009 doi: 10.1203/PDR.0b013e31819dba91. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.El-Radhi AS, Majeed M, Mansor N, Ibrahim M. High incidence of rickets in children with wheezy bronchitis in a developing country. J. R. Soc. Med. 1982;75(11):884–887. doi: 10.1177/014107688207501112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muhe L, Lulseged S, Mason KE, Simoes EA. Case–control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349(9068):1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 125.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J. Trop. Pediatr. 2004;50(6):364–368. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 126.Hiemstra PS. The role of epithelial β-defensins and cathelicidins in host defense of the lung. Exp. Lung Res. 2007;33(10):537–542. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- 127.Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin. Biol. Ther. 2007;7(9):1449–1461. doi: 10.1517/14712598.7.9.1449. [DOI] [PubMed] [Google Scholar]
- 128.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Helming L, Bose J, Ehrchen J, et al. 1α,25-dihydroxyvitamin D3 is a potent suppressor of interferon γ-mediated macrophage activation. Blood. 2005;106(13):4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 130.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol. Infect. 2007;135(7):1095–1096. doi: 10.1017/S0950268807008308. author reply 1097–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grant WB. Hypothesis – ultraviolet-B irradiance and vitamin D reduce the risk of viral infections and thus their sequelae, including autoimmune diseases and some cancers. Photochem. Photobiol. 2008;84(2):356–365. doi: 10.1111/j.1751-1097.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 132.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E. On the epidemiology of influenza. Virol. J. 2008;5:29. doi: 10.1186/1743-422X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li-Ng M, Aloia JF, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009;137(10):1396–1404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 135.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001;73(2):288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 136.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr. Pract. 2009;15(5):438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2009;179(9):843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 138.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr. Allergy Asthma Rep. 2009;9(1):81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
Websites
- 201.ClinicalTrials.gov. Randomized trial: Maternal Vitamin D Supplementation to Prevent Childhood Asthma (VDAART) http://clinicaltrials.gov/ct2/show/NCT00920621.
- 202.ClinicalTrials.gov. Vitamin D for the Treatment of Severe Asthma http://clinicaltrials.gov/ct2/show/NCT00712205.