Summary
Cytosolic internalization is a requirement for the toxicity of secretory ribonucleases. Here, we investigate the mechanism of internalization of Onconase® (ONC), a toxic protein, and ribonuclease A (RNase A), a nontoxic homolog. Microscopy studies indicate that both ribonucleases readily bind to the cell surface and are internalized via acidic vesicles. Blocking dynamin-dependent endocytosis prevents transferrin internalization, but does not hinder RNase A internalization. ONC and G88R RNase A, which is a toxic variant, demonstrate enhanced cytotoxicity in the absence of clathrin- and dynamin-mediated endocytosis. The cytosolic entry of ribonucleases does not require an acidic environment or transport to the ER, and likely occurs from endosomes. Thus, common proteins—secretory ribonuclease—enter the cytosol by a pathway that is distinct from that of other known toxins.
Keywords: dynamin, endocytosis, endosomes, ribonuclease, toxin
Introduction
The internalization of proteins by cells often occurs via a specific energy-dependent pathway—endocytosis. Endocytosis can occur via either clathrin-coated or non-clathrin-coated endosomes (Marsh, 2001). Historically, clathrin-mediated endocytosis has been synonymous with receptor-mediated endocytosis. Now, however, a growing number of proteins are known to be internalized by a receptor-mediated mechanism that is independent of clathrin (Nichols and Lippincott-Schwartz, 2001).
The characterization of alternative endocytic pathways has been due, in large part, to the creation of dominant-negative mutants that disrupt clathrin-mediated endocytosis. One such mutant involves dynamin, which is a GTPase required for the release of clathrin-coated pits from the cell surface (Schmid et al., 1998; McNiven et al., 2000). A variant of dynamin in which Lys44 is replaced with an alanine residue (K44A Dyn) is defective in GTP binding and hydrolysis (van der Bliek et al., 1993). Previous studies have shown that the overproduction of K44A Dyn in HeLa cells blocks clathrin-mediated endocytosis and inhibits the internalization of both transferrin and epidermal growth factor (van der Bliek et al., 1993; Damke et al., 1994). Dynamin is also required for the internalization of caveolae (Henley et al., 1998), as well as for vesicle budding from the trans-Golgi network (Jones et al., 1998). A poorly defined clathrin-independent endocytic pathway is upregulated when dynamin-dependent endocytosis is blocked (Damke et al., 1995).
Ribonuclease (RNase) A and its homologs are secretory proteins that manifest diverse biological activities following internalization (D’Alessio and Riordan, 1997; Raines, 1998). For example, bovine seminal ribonuclease demonstrates antitumor, antiviral, and immunosuppressive activity (D'Alessio et al., 1997; Matousek, 2001). Onconase® (ONC) demonstrates both antitumor and antiviral activity (Youle and D'Alessio, 1997; Leland and Raines, 2001), and is in Phase III clinical trials for the treatment of malignant mesothelioma (Mikulski et al., 2002). RNase A itself, however, does not have marked antitumor, antiviral, or immunosuppressive activity.
The mechanism of ribonuclease-mediated cell death consists of two major steps: (1) cytosolic internalization and (2) RNA cleavage. Several studies have focused on the contribution of the intracellular ribonucleolytic activity to cytotoxicity (Leland and Raines, 2001). In comparison, little is known about the internalization pathway of ribonucleases. Ribonucleases must reach the cytosol to degrade cellular RNA. Ribonucleases that are microinjected into the cytosol are more toxic than when added to cells externally (Saxena et al., 1991), suggesting that internalization limits toxicity. The potency of ONC can be enhanced by adding drugs that alter cellular routing (Wu et al., 1995). Likewise, conjugating ribonucleases to delivery molecules can enhance the specificity and potency of their cytotoxicity (Suzuki et al., 1999; Newton et al., 2001; De Lorenzo et al., 2002).
The pathway by which ribonucleases reach the cytosol is not known. The internalization of ONC could rely on an endocytic pathway, as its cytotoxicity is blocked by inhibitors of this energy-dependent pathway (Wu et al., 1995). Still, the endocytosis of ONC has not been observed directly. In addition, the type of vesicles that mediate ONC internalization have not been identified. Even less is known about the internalization of RNase A. Indeed, other workers have reported that RNase A is not even bound by cells (Leamon and Low, 1993).
The toxicity of a molecule can be a powerful tool for assessing the efficiency and delineating the pathway of its intracellular routing. As RNase A is not toxic to cultured cells, this approach cannot be used to probe its internalization. Variants of RNase A can, however, be toxic to cells. For example, replacing the glycine residue at position 88 with arginine (G88R) decreases the affinity of RNase A for the cytosolic ribonuclease inhibitor protein (RI) and makes RNase A cytotoxic, even when added to cells externally (Leland et al., 1998).
Here, we investigate the internalization of ONC and RNase A with tools from cell biology, pharmacology, and somatic cell genetics. In addition, we take advantage of the toxicity of G88R RNase A to probe the mechanism of its cytosolic entry. Our data reveal a pathway by which secretory ribonucleases are internalized, as well as the first evidence for differences in trafficking between ONC and RNase A, two homologous proteins.
Materials and methods
Production of ribonucleases and variants
The pBXR and pONC plasmids direct expression of wild-type RNase A and wild-type ONC in Escherichia coli (delCardayré et al., 1995; Leland et al., 1998). A plasmid that directs the expression of D16C ONC was created by using oligonucleotide-mediated site-directed mutagenesis (Kunkel et al., 1987).
Wild-type RNase A, G88R RNase A, K7A/G88R RNase A, and wild-type ONC were purified using methods described previously (delCardayré et al., 1995; Leland et al., 1998). A19C RNase A and D16C ONC were purified as described for the wild-type proteins, but with the following modifications (Kothandaraman et al., 1998). Refolding solutions were saturated with Ar(g) to remove O2(g). Immediately after anion-exchange chromatography, the sulfhydryl group of native Cys19 (A19C RNase A) or Cys16 (D16C ONC) was protected from oxidation by reaction with 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) at pH 9 (Messmore et al., 1995). DTNB-protected protein was isolated from unprotected protein with anion-exchange chromatography. Protein concentrations were determined by UV spectroscopy using ε = 0.72 ml mg−1 cm−1 at 277.5 nm for RNase A (Sela et al., 1957) and its variants and ε = 0.87 ml mg−1 cm−1 at 280 nm for ONC (Leland et al., 1998). All ribonucleases were dialyzed exhaustively versus phosphate-buffered saline (PBS), which contained (in 1 L) KCl (0.20 g), KH2PO4 (0.20 g), NaCl (8.0 g), and Na2HPO4 (2.16 g).
Fluorescent labeling of ribonucleases
DTNB-protected A19C RNase A and D16C ONC were deprotected immediatedly prior to fluorescent labeling. Protected protein was incubated with a 3-fold molar excess of dithiothreitol or tris(2-carboxyethyl) phosphine (TCEP) and a 40-fold molar excess of label for 25 minutes at 22°C. The fluorescent probes 5-iodoacetamidofluorescein (5-IAF), N-(4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene-2-yl)iodoacetamide (BODIPY® 507/545 IA), and 2′,7′-difluorofluorescein-iodoacetamide (Oregon Green™ 488 IA) were from Molecular Probes (Eugene, OR). Excess, unreacted probe was removed by gel filtration chromatography with a NICK column (Pharmacia Biotech; Piscataway, NJ). Protein concentration was determined with eq 1:
| (1) |
where Aprot is the actual A280 of the protein, Ameas is the measured absorbance at 280 nm, Amaxdye is the absorbance of the coupled dye, and cf is the A280/Amax ratio of the free dye. The cf for fluorescein is 0.2. The degree of protein labeled (DOL) was determined with eq 2:
| (2) |
where ε is the molar extinction coefficient of the dye at wavelength of maximum absorption.
Labeled protein was purified away from unlabeled protein by using reverse-phase HPLC. The HPLC peak containing labeled protein was dialyzed exhaustively versus PBS for biological assays.
Assay of thermal stability
Conformational stability assays were performed as described (Eberhardt et al., 1996), with the following modifications. The conformational stability of RNase A, ONC, and each labeled variant (in PBS) was determined by monitoring the change in absorbance at 287 nm as the temperature was increased from 25 to 75°C in 1-°C increments. The absorbance at 287 nm was recorded after a 7-minute equilibration at each temperature. The value of Tm is the temperature at the midpoint of the thermal denaturation. Data were analyzed with the program THERMAL (Varian Analytical Instruments; Walnut Creek, CA).
Assay of catalytic activity
Ribonucleolytic activity was measured by using 6-FAM–dArU(dA)2–6-TAMRA, a fluorogenic substrate (Kelemen et al., 1999). Assays were performed at 23°C in 2.00 ml of 0.10 M MES–NaOH buffer (pH 6.0) containing NaCl (0.10 M), substrate (50 nM), and enzyme (1.0–5.0 pM). Data were obtained and values of kcat/KM were calculated as described previously (Kelemen et al., 1999).
Cell culture
K-562, JAR, and HeLa cells were obtained from the American Type Culture Collection (Manassas, VA). K-562 and JAR cells were grown in RPMI medium 1640. HeLa cells were grown in DMEM medium. All culture medium contained FBS (10% v/v), penicillin (100 units/ml), and streptomycin (100 µg/ml). Cell culture medium and supplements were from Life Technologies (Gaithersburg, MD). Cells were cultured at 37°C in a humidified incubator containing CO2 (g; 5% v/v). All studies were performed using asynchronous log-phase cultures.
Immunohistochemistry
Wild-type RNase A, G88R RNase A, or ONC was added to a culture of K-562 cells at 4°C. After 20 minutes, cells were washed twice with PBS and fixed for 30 minutes at 4°C in PBS containing paraformaldehyde (2% w/v) and Triton X-100 (0.1% w/v). After being fixed, the cells were rinsed three times in PBS and incubated for 1 hour at 37°C with primary antibody. Rabbit IgG raised against RNase A was from Biodesign International (Kennebunk, ME), and was used at a concentration of 1 µg/ml in PBS containing Tween-20 (0.1% v/v) (PBST). Chicken antiserum raised against ONC was the generous gift from Alfacell Corporation (Bloomfield, NJ), and was used at a 1:100 dilution in PBST. After incubation with primary antibodies, cells were washed three times in PBST and incubated with the appropriate secondary antibodies (1:500 in PBST) conjugated to fluorescein or rhodamine (Molecular Probes; Eugene, OR). After a 1-hour incubation with secondary antibodies, cells were washed three times with PBST, and stained with propidium iodide (1 µg/ml in PBS) for 5 minutes. Then, cells were washed twice with PBST and mounted onto glass microscope slides using Vectashield (Vector Laboratories; Burlingame, CA). Fluorescence staining of cells was visualized on a Zeiss Axiovert 100 TV microscope (Zeiss; Germany). Images were analyzed with the programs BioRad MRC 1024 Laser Scanning Confocal Imaging System (Hercules, CA) and Adobe Photoshop (San Jose, CA).
Assays of internalization
The internalization of RNase A was visualized directly in living cells. K-562 cells in PBS (1–2 × 106 cells/ml) were incubated with either BODIPY-labeled A19C RNase A (BODIPY–RNase A) or fluorescein-labeled A19C RNase A (fluorescein–RNase A) (1 µM) at 4°C for 20 minutes. Cells were washed three times with ice-cold PBS to removed unbound protein. Then, cells were incubated for 5 minutes at 37°C in a humidified incubator containing CO2 (g; 5% v/v). After incubation, living cells were washed three times in PBS and placed on a microscope slide; fluorescence was monitored immediately.
To quantitate the pH-sensitivity of fluorescein–RNase A, K-562 cells were incubated at 4°C for 20 minutes with fluorescein–RNase A or OG–RNase A (10 µM). Cells were washed with ice-cold PBS, and the fluorescence of each cell was measured after a 0–6-minute incubation at 37°C with a FACScan flow cytometer and a 530DF30 bandpass filter (Becton Dickenson, San Jose, CA).
To probe the co-internalization of ONC and RNase A, JAR or HeLa cells were grown on coverslips in the wells of a 6-well plate for 24 hours before the assay. Oregon Green-labeled D16C ONC (OG–ONC) and BODIPY–RNase A (both at a concentration of 1 µM and labeled to ~10%) were incubated with cells at 4°C for 20 minutes. Cells were washed three times with ice-cold PBS to remove unbound protein, and then incubated for 5 minutes at 37°C in a humidified incubator containing CO2 (g; 5% v/v). Cells were washed three times with ice-cold PBS, and fixed as described for immunohistochemistry. After fixing, cells were mounted on microscope slides, and visualized immediately.
To study the dose-dependence of RNase A and ONC, both proteins were labeled with Oregon Green, and used at a labeling efficiency of 30%. HeLa cells were incubated with increasing concentrations of either OG–RNase A or OG–ONC (0.01, 0.1, 1, and 10 µM). Samples were pulsed, fixed, and visualized as described for the ribonuclease co-internalization studies. To quantitate the fluorescence in each cell, pulsed cells were detached with trypsin and analyzed by flow cytometry.
To investigate the internalization of RNase A with endocytic markers, cells were pulsed with BODIPY–RNase A (1 µM), OG–RNase A (1 µM), FM™ 1–43 (1 µg/ml; Molecular Probes; Eugene, OR), BODIPY-FL-transferrin (50 µg/ml; Molecular Probes; Eugene, OR), or tetramethylrhodamine-labeled transferrin (TAMRA–transferrin; 50 µg/ml; Molecular Probes; Eugene, OR). Endocytosis assays were performed as described (Lamaze et al., 2001).
Assays of cytotoxicity
The effect of ONC, RNase A, and G88R RNase A on cell proliferation was determined by measuring [methyl-3H]thymidine uptake into DNA. Briefly, cells (95 µl of a solution of 5 × 104 cells/ml) were incubated with PBS containing a known concentration of a ribonuclease (5 µl) in a 96-well plate for 20 hours at 37°C in a humidified incubator containing CO2 (g; 5% v/v). Data on cell proliferation were obtained and analyzed as described previously (Haigis et al., 2002).
For metabolic inhibition studies, cells were incubated in the presence of NaN3 (10 mM) and 2-deoxyglucose (50 mM) before the addition of a ribonuclease. For pharmacological studies, cells were incubated in the presence of BFA (5.0 µg/ml), NH4Cl (20 mM), or monensin (10 µM) before the addition of a ribonuclease. Data were analyzed as the percent of [methyl-3H]thymidine incorporated compared to cells incubated instead with PBS.
To investigate the role of dynamin in ribonuclease toxicity, the toxicity of ONC and G88R RNase A was measured in a cell line overproducing K44A Dyn (Damke et al., 1994). Stably transformed tTA-HeLa cells with tightly regulated expression of dynamin and K44A Dyn was a generous gift from S.L. Schmid (Scripps Research Institute; LaJolla, CA). Briefly, transformed tTA-HeLa cells were cultured in DMEM containing FBS (10% v/v), penicillin (100 units/ml), streptomycin (100 µg/ml), G418 (400 µg/ml), and tetracycline (2 µg/ml) as described previously (Damke et al., 1994). To induce the overexpression of HA-tagged dynamin (or K44A Dyn), subconfluent cultures (<50%) were washed several times in PBS, detached with trypsin, and plated onto 10-cm culture dishes in the absence of tetracycline. After 48 hours, the overproduction of dynamin was monitored by immunoblot analysis using antibodies to the HA epitope. Then, toxicity assays were performed as described above using both uninduced and induced cells.
Results
Design of ribonuclease variants
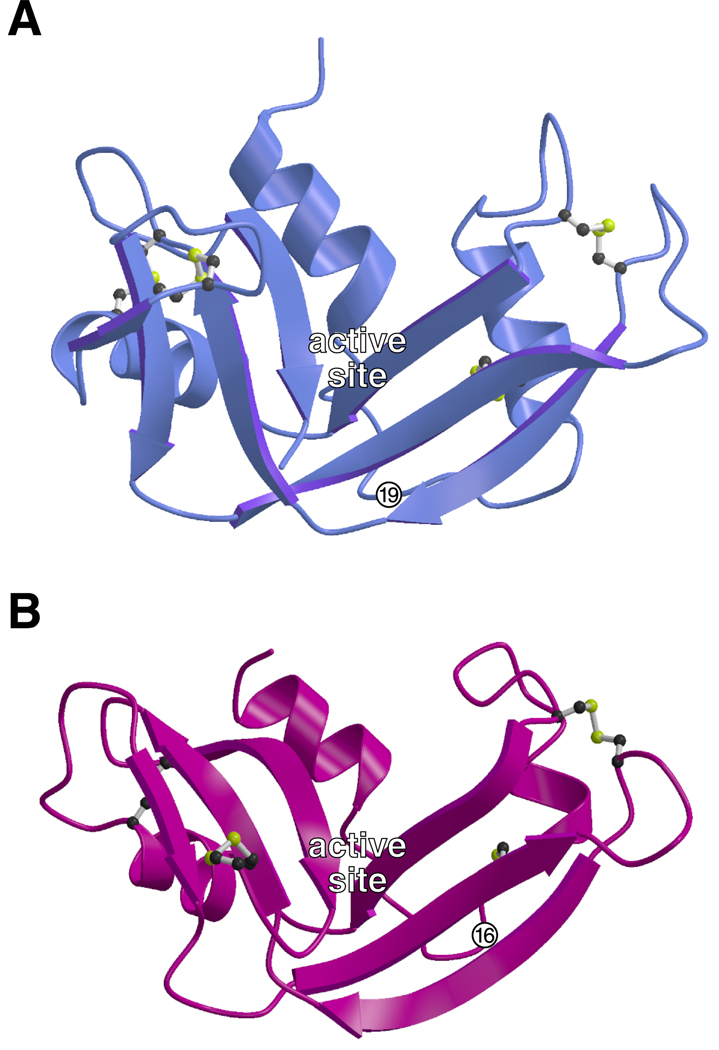
To visualize the internalization of RNase A and ONC in living cells, we linked a fluorophore to each protein. Ala19 (RNase A) and Asp16 (ONC) are located in a solvent-exposed surface loop far from each enzymic active site (Fig. 1), and not conserved in the RNase A superfamily (Beintema et al., 1988). As neither RNase A nor ONC contain a reduced cysteine residue, replacing these alanine residues with a cysteine enabled site-specific, nondisruptive labeling with a thiol-reactive fluorescent probe.
Fig. 1.
Three-dimensional structure of ribonuclease A and Onconase®. Ribbon diagrams of (A) RNase A and (B) ONC were created using the atomic coordinates derived by x-ray diffraction analysis (Wlodawer, 1985; Mosimann et al., 1994) and the program MOLSCRIPT (Kraulis, 1991). The location of the active site clefts and residues 19 (RNase A) and 16 (ONC), which were replaced with cysteine for labeling experiments, are indicated.
Conformational stability and ribonucleolytic activity
Data from experiments with incubations at 37°C can be interpreted properly only with information that the ribonuclease is folded at that temperature. Fluorescein-labeled A19C RNase A (fluorescein–RNase A) and DTNB-labeled D16C ONC (DTNB–ONC) had Tm values of 61 and 88°C, respectively (Table I). These values are similar to those reported for the wild-type ribonucleases (Leland et al., 2000).
Table I.
Biophysical and biochemical properties of ribonucleases
| Ribonuclease | Tm (°C)a | kcat/KM (M−1s−1)b |
|---|---|---|
| RNase A | 63 | (4.3 ± 0.3) × 107 |
| fluorescein–RNase A | 61 | (0.54 ± 0.02) × 107 |
| ONC | 90 | (3.5 ± 0.1) × 102 |
| DTNB–ONC | 88 | (1.7 ± 0.2) × 102 |
Values of Tm (± 1 °C) were determined in PBS by UV spectroscopy.
Values of kcat/KM (± SE) are for the catalysis of 6-FAM–dArU(dA)2–6-TAMRA cleavage at (23 ± 2) °C in 0.10 M MES–NaOH buffer (pH 6.0) containing NaCl (0.10 M).
The cytotoxicity of ribonucleases relies on their ribonucleolytic activity. Alkylation of ONC results in the loss of catalytic as well as cytotoxic activity (Wu et al., 1993). RNase A variants with altered active-site residues have lowered enzymatic activity and cytotoxic activity (Bretscher et al., 2000). We measured the ribonucleolytic activity of labeled ONC and RNase A variants using 6-FAM–dArU(dA)2–6-TAMRA as the substrate (Table I). The values of kcat/KM for RNase A and ONC were found to be 4.3 × 107 M−1s−1 and 3.5 × 102 M−1s−1, which are in good agreement with kcat/KM values reported previously (Kelemen et al., 1999; Leland et al., 2000). The values of kcat/KM for fluorescein–RNase A and DTNB–ONC were found to be 0.54 × 107 M−1s−1 and 1.7 × 102 M−1s−1 respectively. Thus, thermal stability and ribonucleolytic activity are retained in the modified variants.
Binding and internalization of ribonucleases
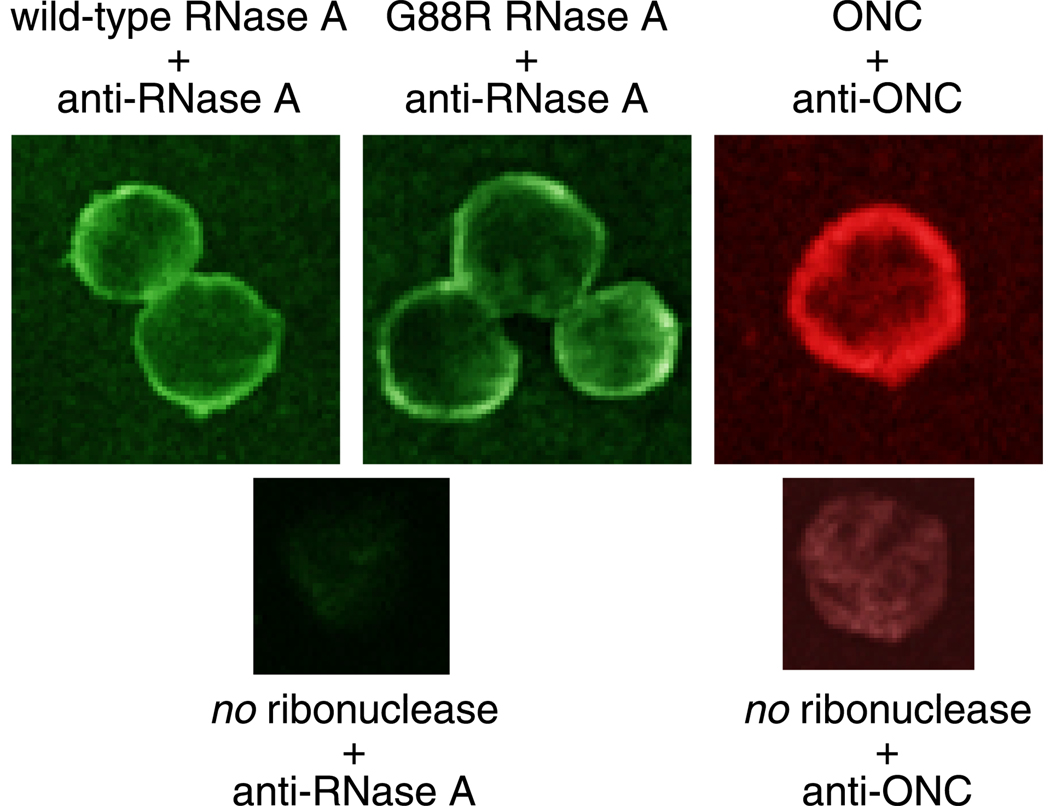
First, we investigated whether ribonucleases interacted with the cell surface. Previous studies have reported a high-affinity interaction (Kd = 62 nM) between ONC and the surface of 9L cells (Wu et al., 1993). An interaction between RNase A and the cell surface has not been reported previously. We investigated the binding of RNase A, G88R RNase A, and ONC to the plasma membrane of human leukemia (K-562) cells. All three proteins are bound to the cell surface after a 30-minute incubation at 4°C (Fig. 2). The signal intensity does not increase significantly after incubating for 2 hours at 4°C (data not shown). Both RNase A and ONC accumulate at the cell surface at 4°C and do not cross the plasma membrane, even after 2 hours. These results suggest that the internalization of ribonucleases does not occur at 4°C, and thus likely relies on an endocytic process. A preincubation of metabolic inhibitors protects K-562 cells from the toxicity of onconase and K7A/G88R RNase A (Fig. 3A), which is the most toxic known variant of RNase A (Haigis et al., 2002). In addition, metabolic inhibitors block the internalization of RNase A (Fig. 3B).
Fig. 2.
Binding of unlabeled RNase A, G88R RNase A, and ONC to the surface of K-562 cells. Cells were incubated with a ribonuclease (1 µM) for 30 minutes at 4°C. Cells were then washed, fixed, and processed for indirect immunofluorescence with antibodies generated against either RNase A or ONC. The appropriate FITC or TRITC-conjugated secondary antibody was used to visualize RNase A (green), G88R RNase A (green), and ONC (red) binding. Negative control samples were incubated in PBS in the absence of protein and processed with primary and secondary antibodies as described above.
Fig. 3.
Effect of metabolic inhibitors on ribonuclease-mediated cytotoxicity. (A) Cells were incubated with NaN3 (10 mM) and 2-deoxyglucose (50 mM) or PBS control for 1 hour at 37°C. ONC (10 mM) or K7A/G88R RNase A (25 mM) was then added, and cells were incubated for an additional 6 hours. DNA synthesis was measured as described in Materials and Methods. (B) Cells were incubated with NaN3 (10 mM) and 2-deoxyglucose (50 mM). BODIPY–RNase A (1 µM) was then added, and cells were incubated for an additional 30 minutes at 4°C. Cells were pulsed at 37°C for 30 minutes, and the fluorescence of BODIPY–RNase A was visualized directly.
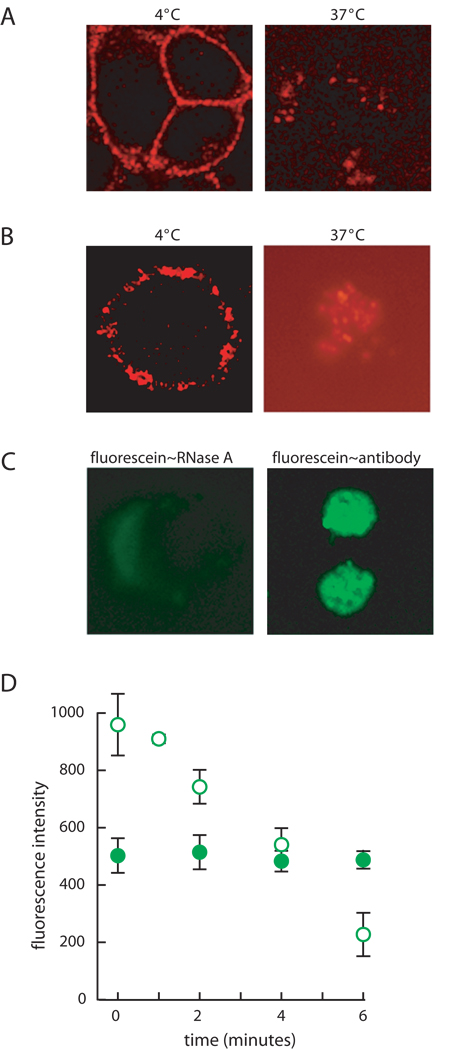
Next, we examined the initial events of internalization. A19C RNase A was labeled with fluorescein (fluorescein–RNase A), which has pH-sensitive fluorescence, or BODIPY (BODIPY–RNase A), which is a pH-insensitive probe. The pH-dependent properties of fluorescein and BODIPY are retained when linked to a specific site on RNase A (data not shown). We incubated JAR cells at either 4°C or 37°C. The fluorescence of the former cells is located uniformly on the plasma membrane (as in Fig. 2 and 3B), and that of the latter cells is located internally (Fig. 4A and 4B). Next, we pulsed K-562 cells at 37°C with either fluorescein–RNase A or BODIPY–RNase A, and then analyzed their fluorescence by microscopy. Cells pulsed with BODIPY–RNase A have vivid internal fluorescence (Fig. 4A and 4B). In contrast, cells incubated with fluorescein–RNase A lack strong internal fluorescence (Fig. 4C). To verify that unlabeled RNase A is likewise internalized by vesicles, we pulsed K-562 cells with unlabeled RNase A and then analyzed them by immunohistochemistry. RNase A appears in vesicles similar in morphology to those seen in living cells (Fig. 4C). The addition of the fluorescent label does not hinder or alter the mode of ribonuclease internalization.
Fig. 4.
Binding and internalization of RNase A. (A) JAR cells were incubated with BODIPY–RNase A (1 µM) for 30 minutes at 4°C, and their fluorescence was visualized directly or after a 5-min incubation at 37°C. (B) K-562 cells were treated as in Panel A. (C) K-562 cells were incubated with unlabeled RNase A as described for panel A. After a 5-min incubation at 37°C, cells were visualized directly or fixed, and internalized RNase A was detected by using an appropriate primary and secondary antibody. (D) K-562 cells were incubated with fluorescein–RNase A (open; 1 µM) or OG–RNase A (filled; 1 µM) for 20 minutes at 4°C. Fluorescence intensity was measured after a 0–6-minute incubation at 37°C with a FACScan flow cytometer. Each data point represents the mean (±SE) of the fluorescence from 10,000 cells in two separate experiments.
To monitor the pH surrounding an internalized ribonuclease, K-562 cells were pulsed with fluorescein–RNase A or RNase A labeled with Oregon Green™ (OG–RNase A), which has a lower pKa (= 4.7) than the pKa (= 6.4) fluorescein and hence retains much more of its fluorescence at low pH. Fluorescence was measured by using flow cytometry after timed incubations at 37°C. The fluorescence of fluorescein–RNase A decreases with longer incubations at 37°C, providing evidence that ribonucleases are internalized through acidic endosomes (Fig. 4D). In contrast, the fluorescence of OG–RNase A does not decrease significantly, indicating that it is in an environment with a pH > 5 (Fig. 4D). All 37°C pulses were for ≤6 minutes, so as to probe only the initial phase of ribonuclease internalization. Cells incubated with unconjugated flourescein, BODIPY, or Oregon Green (10 µM) are not fluorescent (data not shown).
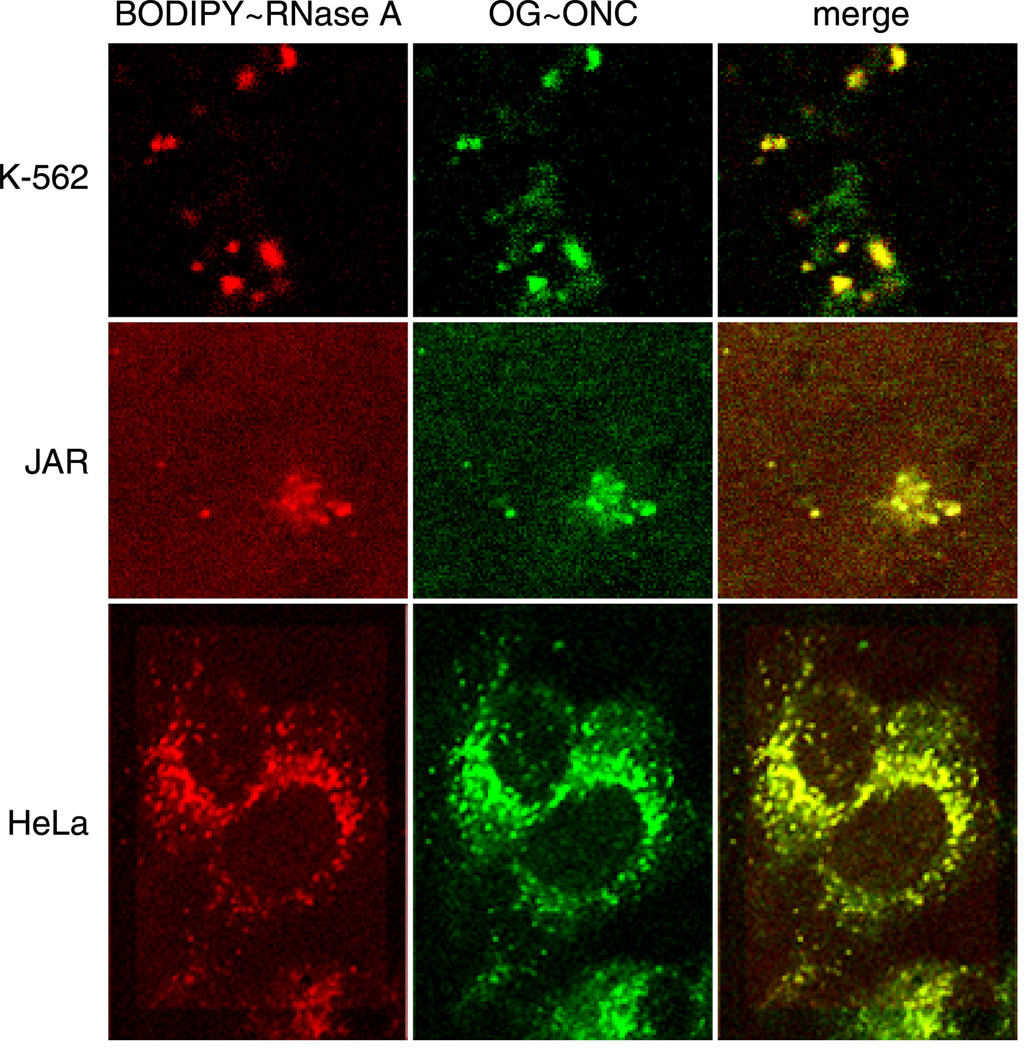
We then investigated whether ONC and RNase A are co-internalized. We labeled D16C ONC at residue 16 with Oregon Green™ (OG–ONC) to allow for co-localization studies with BODIPY–RNase A. K-562 cells internalize OG–ONC and BODIPY–RNase A using the same compartments (Fig. 5). OG–ONC and BODIPY–RNase A are also internalized in the same compartments in cervical epithelioid carcinoma (HeLa) and choriocarcinoma (JAR) cells. Identical results were seen in living cells that were viewed without fixation after incubation with ribonucleases (data not shown). The colocalization was not due to fluorescence bleed-through, as determined by cells pulsed with either OG–ONC or BODIPY–RNase A alone (data not shown).
Fig. 5.
Co-internalization of BODIPY–RNase A and OG–ONC in K-562, JAR, and HeLa cells. Cells were incubated with OG–ONC (green) or BODIPY–RNase A (red; 1 µM, 10% labeled) for 20 minutes at 4°C. After a 5-minute incubation at 37°C, cells were washed three times with ice-cold PBS and fixed; and their fluorescence was visualized directly.
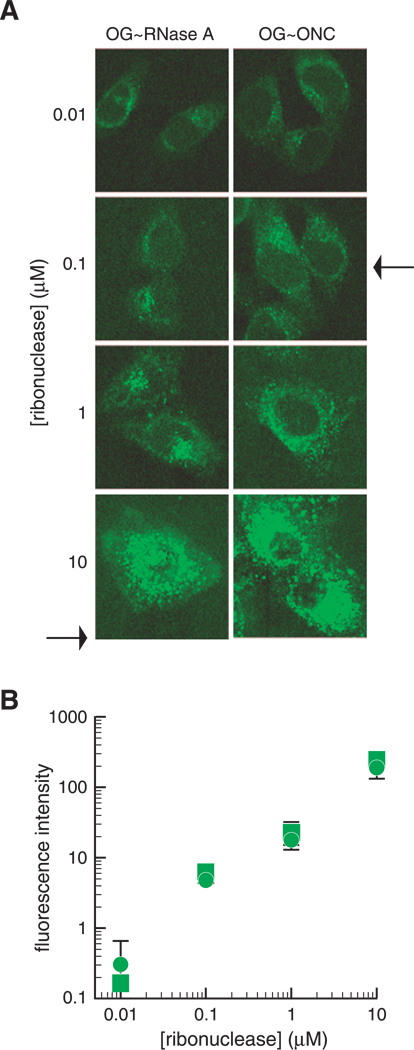
To compare the dose dependence of ONC and RNase A internalization, we pulsed HeLa cells with increasing concentrations of either OG–ONC or OG–RNase A and analyzed the cells by microscopy or flow cytometry. The amount of OG–ONC and OG–RNase A internalized by cells is identical and increases with increasing protein concentration (Fig. 6A,B). In addition, the 10 µM samples show a clear increase in the number of ribonuclease-filled punctate vesicles (Fig. 6A). The data indicate that ribonuclease internalization is not saturable (Fig. 6B); in addition, the fluorescence intensity of OG–RNase A (1 µM) is not diminished by the addition of a 100-fold excess of either unlabeled RNase A or ONC (data not shown).
Fig. 6.
Dose-dependent internalization of OG–RNase A and OG–ONC. (A) HeLa cells were grown on coverslips in the wells of a 6-well plate, incubated with 30% labeled OG–RNase A or OG–ONC (0.01, 0.1, 1, and 10 µM), washed, and fixed as described for Fig. 5. The arrows correspond to the IC50 values for G88R RNase A and ONC (Table II). (B) HeLa cells were pulsed with OG–RNase A (circles) or OG–ONC (squares) as described for panel A, except that cells were treated with trypsin before fixation. Fluorescence was quantitated using a FACScan flow cytometer. Each data point represents the mean (±SE) of the fluorescence from 10,000 cells in two separate experiments.
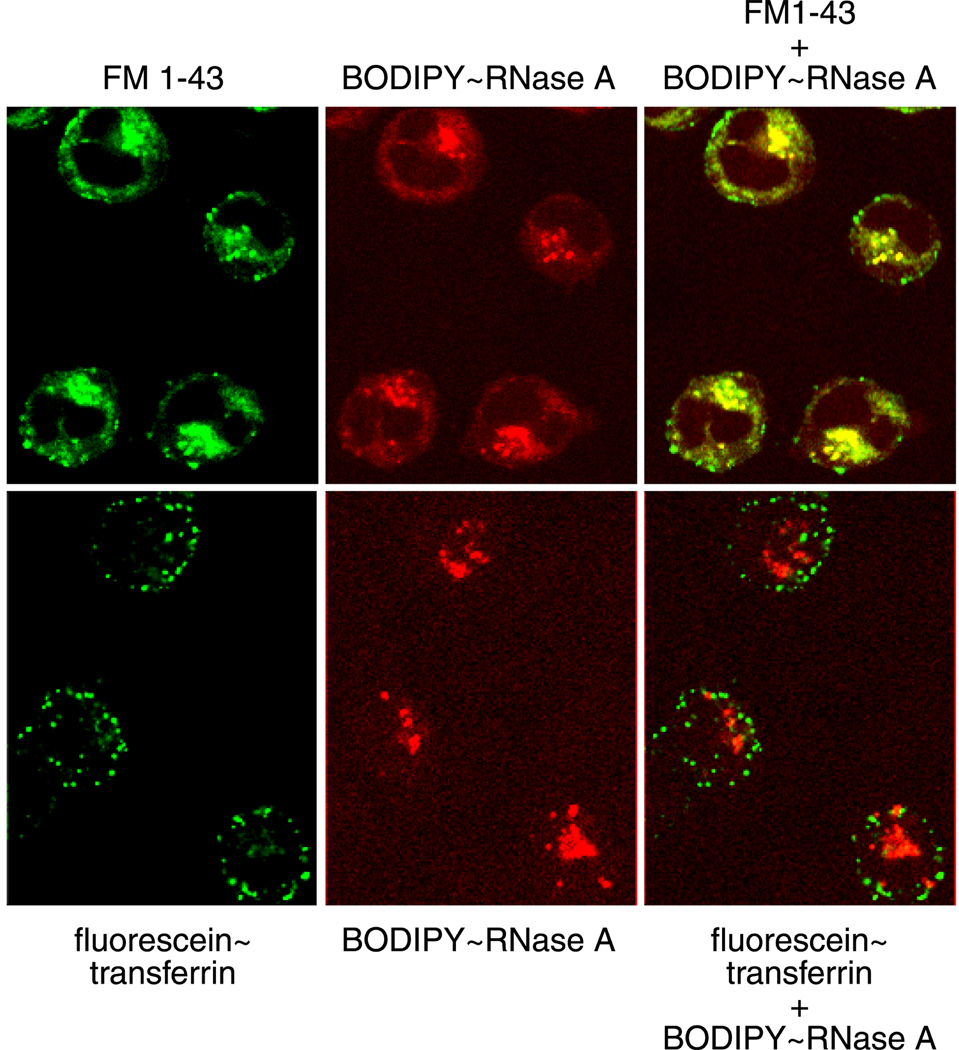
We analyzed the fluid-phase endocytosis of K-562 cells by using FM 1–43™, which is a non-specific probe for membrane internalization. We find that the K-562 cells undergo constant endocytosis (Fig. 7). In addition, BODIPY–RNase A colocalizes with FM 1–43 (Fig. 7). An enhancement in endocytosis is not detectable upon the addition of RNase A (data not shown).
Fig. 7.
Endocytosis in K-562 cells. K-562 cells were co-incubated with BODIPY–RNase A (1 µM) and either FM1–43 (1 µg/ml) or BODIPY-FL-labeled transferrin (50 µg/ml) for 20 minutes at 4°C. Cells were pulsed at 22°C for 15 minutes, washed, and fixed; and the fluorescence of BODIPY–RNase A (red), FM 1–43 (green), and BODIPY-FL-labeled transferrin (green) was visualized directly.
We analyzed the clathrin-mediated endocytosis of K-562 cells by using BODIPY FL-labeled transferrin. We find that transferrin, a marker for clathrin-mediated endosomes and recycling endosomes, is readily internalized by K-562 cells, but does not colocalize with BODIPY–RNase A (Fig. 7). In addition, RNase A and transferrin do not colocalize in cells pulsed for 2, 5, or 15 minutes at 37°C, which eliminates a scenario of co-internalization and then post-endocytic sorting (data not shown). Thus, BODIPY–RNase A is internalized by a pathway distinct from that expected for clathrin-mediated endocytosis.
Dynamin-independent toxicity and internalization of ribonucleases
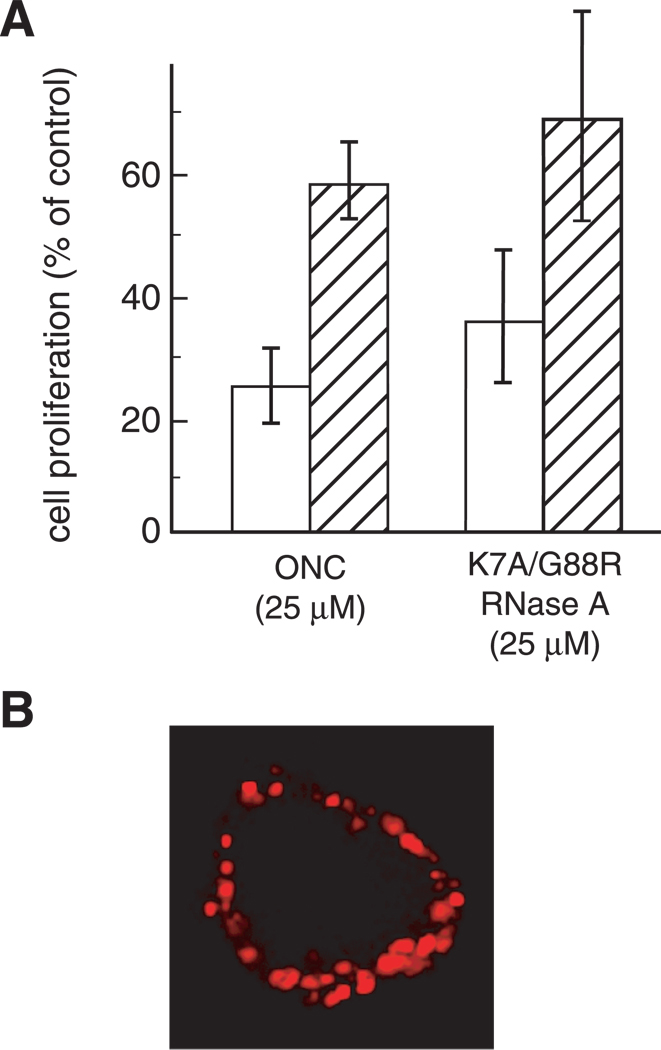
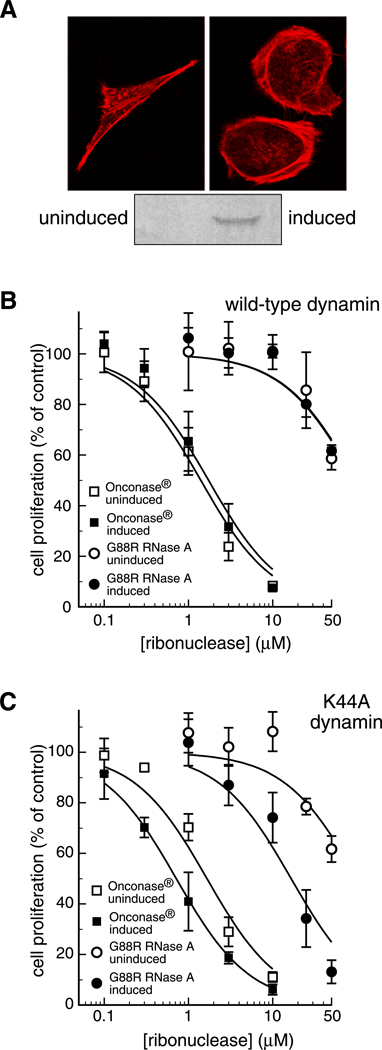
We investigated the toxicity of ONC and G88R RNase A by using tTA HeLa cell lines that overexpress the gene encoding wild-type dynamin or its K44A variant (Damke et al., 1994). Removal of tetracycline results in the overproduction of K44A Dyn, as shown by immunoblotting (Fig. 8A), as well as morphological changes in these cells that are similar to those described previously (Damke et al., 1994). Specifically, cells overproducing K44A Dyn are flatter, and have rounder edges and distinct actin formation (Fig. 8A).
Fig. 8.
Effect of dynamin on ribonuclease-mediated cytotoxicity. (A) K44A Dyn cells grown for 2 days in the presence (induced) or absence (uninduced) of tetracycline were fixed and stained with TRITC-conjugated phalloidin to detect actin filaments (red). The production of wild-type dynamin and its K44A variant was monitored by immunoblotting using anti-HA antibodies. (B) The toxicity of G88R RNase A and ONC was measured for cells overproducing wild-type dynamin or K44A dynamin. Cells were grown in the absence of tetracycline for 2 days to induce overproduction. Then, increasing concentrations of G88R RNase A and ONC were added. After a 48-hour incubation at 37°C, DNA synthesis was measured as described in Materials and Methods. As a control, the effects of G88R RNase A and ONC were measured on cells not overproducing either form of dynamin. Each point represents the percent of [methyl-3H]thymidine incorporated compared to a PBS control, and is the mean (± SE) of at least three separate experiments with triplicate samples.
We find that HeLa cells are not equally susceptible to ONC- and G88R RNase A-mediated toxicity (Fig. 8B,C). ONC and G88R RNase A kill HeLa cells with IC50 values of 1 and >50 µM, respectively (Table II). The cytotoxicity of ONC and G88R RNase A is unaffected by the overproduction of wild-type dynamin (Fig. 8B; Table II).
Table II.
IC50 values of cytotoxic ribonucleases
| IC50 (µM) | ||
|---|---|---|
| HeLa Cells | ONC | G88R RNase A |
| wild-type | 1.5 ± 0.1 | >50a |
| wild-type dynamin, uninduced | 1.4 ± 0.1 | >50a |
| wild-type dynamin, induced | 1.8 ± 0.2 | >50a |
| K44A dynamin, uninduced | 1.6 ± 0.1 | >50a |
| K44A dynamin, induced | 0.72 ± 0.05 | 17 ± 3 |
A protein concentration of 50 µM resulted in <50% cell death.
We next measured the toxicity of ONC and G88R RNase A for cells that overproduce K44A Dyn (Fig. 8C). The toxicity of ONC and G88R RNase A for cells not overproducing K44A Dyn does not differ significantly from that for cells containing wild-type dynamin (Table II). Surprisingly, we find that the overproduction of K44A Dyn makes cells more susceptible to ribonuclease-mediated toxicity. The IC50 value for ONC decreases from 1.6 to 0.7 µM in uninduced versus induced cells, respectively (Table II). We observed an even more dramatic enhancement to the cytotoxicity of G88R RNase A, as its IC50 value decreases from >50 to 17 µM in uninduced versus induced cells, respectively.
The increase in cytotoxicity upon K44A Dyn overproduction suggests that ONC and G88R RNase A are internalized via a dynamin-independent mechanism. To test this hypothesis, we pulsed cells overproducing K44A Dyn with OG–RNase A and TAMRA–transferrin. OG–RNase A is internalized in cells overproducing K44A Dyn (Fig. 9). The majority of transferring remains at the surface of these cells (Fig. 9). Control cells not overproducing the dynamin variant internalize both OG–RNase A and labeled transferrin by vesicles that do not overlap (Fig. 9). These data show that ribonucleases can be internalized in the absence of clathrin- and dynamin-mediated endocytosis.
Fig. 9.
Dynamin-independent internalization of RNase A. (A) Induced and uninduced K44A Dyn cells were co-incubated with OG–RNase A (1 µM) or TAMRA–transferrin (50 µg/ml) for 20 minutes at 4°C. After a 15-minute incubation at 22°C, cells were washed and fixed; and the fluorescence of OG–RNase A (green) and TAMRA–transferrin (red) was visualized directly. (B) Fraction of total uninduced (white) or induced (hatched) cells that contained internalized OG–RNase A or TAMRA–transferrin. Data are the mean (± SE) of two separate experiments with n = 65 and 105 cells for uninduced and induced samples, respectively.
Routing of toxic ribonucleases to the cytosol
The pharmacological agents NH4Cl, monensin, and brefeldin A (BFA) have distinct and established effects on cellular compartments (Pelham, 1991; Sciaky et al., 1997; Dinter and Berger, 1998). We used these drugs to examine the intracellular pathway(s) by which ribonucleases reach the cytosol. In all experiments, K-562 cells were incubated with a drug for 2 hours prior to the addition of a ribonuclease.
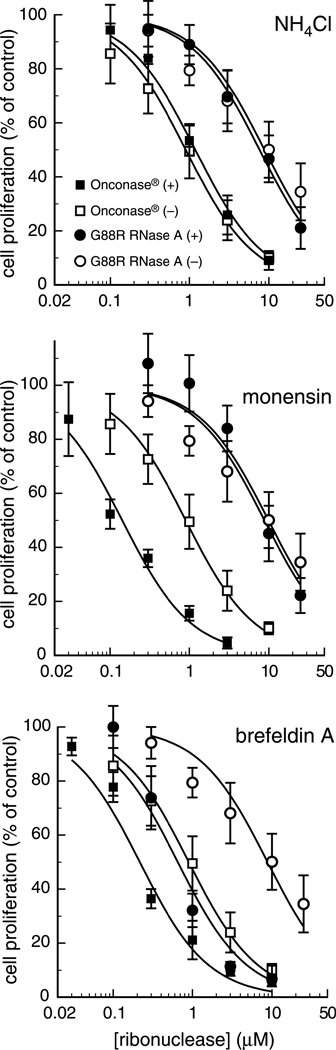
NH4Cl is a weak base that increases the endosomal pH. We find that NH4Cl (20 mM) has no effect on the cytotoxicity of either ONC or G88R RNase A, indicating that routing to the cytosol is not perturbed in the presence of deacidified vesicles (Fig. 10; Table III). An NH4Cl concentration of 30 mM also has no effect on ribonuclease cytotoxicity (data not shown).
Fig. 10.
Effect of drugs on ribonuclease-mediated cytotoxicity. The effect of G88R RNase A and ONC inhibition of cellular DNA synthesis was measured in the presence of NH4Cl (20 mM), monensin (10 µM), and brefeldin A (10 µM). K-562 cells were pre-incubated with each drug for 2 hours at 37°C, and then with G88R RNase A (circles) and ONC (squares). Open symbols represent control samples without drug treatment; filled symbols represent samples pre-incubated with drug. Each point represents the percent of [methyl-3H]thymidine incorporated compared to a PBS control (with or without drug), and is the mean (± SE) of at least three separate experiments with triplicate samples.
Table III.
Effect of drugs on the IC50 values of cytotoxic ribonucleases for K-562 cellsa
| IC50 (µM) | ||
|---|---|---|
| Drug | ONC | G88R RNase A |
| (none) | 0.89 ± 0.07 | 7.7 ± 0.9 |
| NH4Cl | 1.2 ± 0.1 | 8.0 ± 0.8 |
| Monensin | 0.15 ± 0.02 | 8.0 ± 1.4 |
| Brefeldin A | 0.23 ± 0.03 | 0.66 ± 0.07 |
Cells were preincubated with drug for 2 hours prior to the addition of ribonucleases.
Monensin is a carboxylate ionophore that also leads to the deacidification of endosomes and can disrupt Golgi trafficking. We find that monensin (10 µM) potentiates the cytotoxicity of ONC by 10-fold, but has little effect on the cytotoxicity of G88R RNase A (Fig. 10). These data reveal that G88R RNase A is internalized via a monensin-insensitive pathway, and verify that the internalization of ONC or G88R RNase A is not dependent on a low pH environment.
To investigate whether downstream events in the retrograde pathway are important for cytotoxicity, we analyzed the toxicity of ONC and G88R RNase A in the presence and absence of BFA. BFA disassembles the Golgi stack and disrupts retrograde transport from the Golgi to the ER (Pelham, 1991), and has been a powerful tool for studying the internalization pathway of other protein toxins (Hudson and Grillo, 1991). We find that BFA potentiates the cytotoxicity of both ONC and G88R RNase A by 10-fold (Fig. 10; Table III). Hence, retrograde transport from the Golgi to the ER is not an essential component of ribonuclease translocation to the cytosol.
Discussion
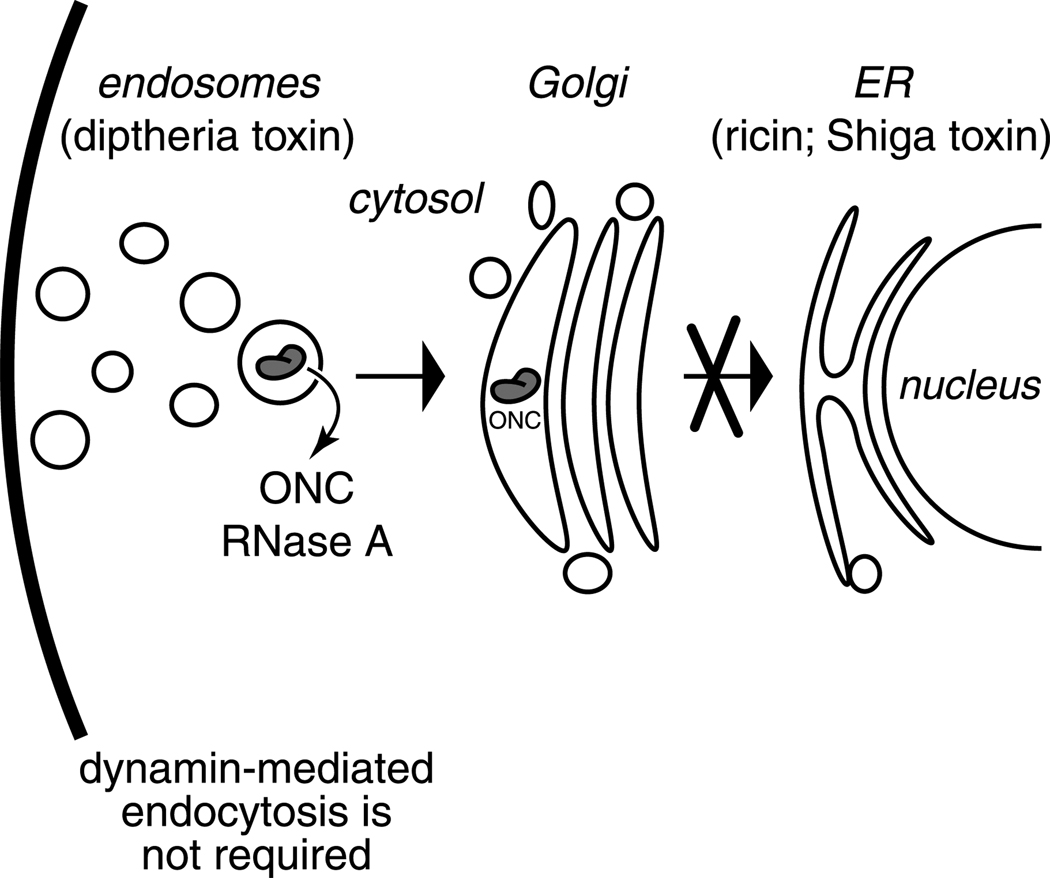
Secretory ribonucleases must enter the cytosol to reach their substrate. Here, we have used RNase A, ONC, and their variants to analyze the pathway(s) of ribonuclease internalization with a multifaceted approach. Our data are summarized in Fig. 11, which depicts a detailed model of ribonuclease internalization. ONC, RNase A, and G88R RNase A bind to the cell-surface and are not internalized at 4°C (Fig. 2; Fig. 4A and 4B) or at 37°C in the presence of metabolic inhibitors (Fig. 3B). At 37°C, internalization of ribonucleases occurs through acidic endosomes (Fig. 4A–C). Unlike transferrin, ribonucleases do not require dynamin for internalization (Fig. 9A,B). Nor is the acidic environment of endosomes necessary for translocation to the cytosol (Fig. 10). Once internalized, ribonucleases translocate to the cytosol from a pre-ER compartment.
Fig. 11.
Model for the cytotoxic internalization of ribonucleases. RNase A and ONC bind to the cell surface and are internalized by dynamin-independent endocytosis. Like diptheria toxin, both ribonucleases likely reach the cytosol by translocation from an endosome. Unlike diphtheria toxin, the low pH of acidic endosomes is not a prerequisite for the translocation of RNase A and ONC to the cytosol. The entry of ONC to the Golgi is inefficient for cytotoxicity. Unlike ricin and Shiga toxin, both ribonucleases reach the cytosol by translocation from a pre-ER compartment.
We were surprised to discover that secretory ribonucleases are more toxic to cells in the absence of dynamin-mediated endocytosis (Fig. 8; Table II). These data can be explained by invoking known mechanisms of dynamin action. The dynamin-dependent endocytic pathways, such as clathrin- and caveolae-mediated endocytosis, could represent an inefficient route for the cytosolic entry of ribonucleases. By bypassing a dead-end pathway, ribonucleases could enter the cytosol more efficiently, leading to enhanced toxicity. Thus, a block in dynamin function could alter the translocation of ribonucleases, as well as their initial uptake. Finally, some clathrin- and dynamin-independent pathways, such as pinocytosis, are up-regulated in cells that lack functional dynamin (Damke et al., 1995). Ribonucleases are indeed endocytosed by K44A Dyn cells that do not internalize transferrin, which is a marker for clathrin-mediated endocytosis (Fig. 9). Hence, inactivating dynamin-dependent pathways could increase the influx and cytotoxicity of ribonucleases (Fig. 8).
We were also surprised to learn that monensin potentiates the cytotoxicity of ONC by 10-fold, but does not alter significantly the cytotoxicity of G88R RNase A (Fig. 10). Monensin deacidifies vesicles by exchanging protons for other cations, but it can also disrupt cellular functions, such as trafficking through the Golgi apparatus. Our results provide evidence for a possible difference between ONC and G88R RNase A trafficking through the Golgi. For example, the Golgi could be an inefficient site for ONC translocation to the cytosol; monensin could prevent such inefficient routing of ONC to the Golgi, resulting in enhanced cytotoxicity.
Perturbing intracellular trafficking by using drugs may have indirect consequences on cellular pathways and must be interpreted with caution. For example, BFA has diverse effects on endocytosis and has been shown to interfere with the sorting of the transferrin receptor (Wang et al., 2001). Such a block could lead to the decrease of a protein or lipid receptor at the cell surface, which would result in a decrease of toxicity. The toxicity of G88R RNase A and ONC is enhanced when cells are treated with BFA (Fig. 10). These data provide evidence that ribonuclease cytotoxicity is not diminished by this indirect affect. Cells treated with BFA or monensin internalize the same amount of ribonuclease as do control cells in flow cytometry assays (data not shown).
Ribonucleases are internalized in a dose-dependent manner (Fig. 6), which is consistent with their dose-dependent cytotoxicity (Fig. 8B,C; Fig. 10). We propose that as the extracellular concentration of a ribonuclease increases, so does its cytosolic concentration. The arrows in Fig. 6 indicate the protein concentrations that correspond to the IC50 values of >50 and 1 µM for G88R RNase A and ONC, respectively (Table II). We were surprised to find that ONC and G88R RNase A have a similar dose-dependent influx into HeLa cells, despite having IC50 values that differ by more than 50-fold (Fig. 7; Table II). These results imply that the difference in the cytotoxicity of ONC and G88R RNase A is not due to differences in cell-surface binding and internalization. Still, ribonuclease-mediated toxicity also requires translocation to the cytosol. Once in the cytosol, uninhibited ribonucleolytic activity is necessary for toxicity. Thus, ribonucleases must evade binding and inhibition by cytosolic RI. ONC has low affinity for RI (Wu et al., 1993; Boix et al., 1996), while RNase A binds RI with fM affinity (Lee et al., 1989; Vicentini et al., 1990). Our data suggests that either (or both) of these hurdles is responsible for the difference in the toxicity of ONC and G88R RNase A for HeLa cells. Indeed, much work has demonstrated that evasion of RI is a requirement for the cytotoxicity of mammalian ribonucleases (Cafaro et al., 1995; Kim et al., 1995b; Leland et al., 1998; Bretscher et al., 2000; Haigis et al., 2002).
Is there a cell-surface receptor for secretory ribonucleases? We hypothesize that ribonucleases bind to the cell-surface without interacting with a specific protein receptor on the cell surface. We find that ribonucleases bind to the cell surface in a non-saturable manner (Fig. 6B). This non-specific binding is not perturbed by treatment of the cells with proteases (K. A. Dickson and R. T. Raines, unpublished results). In addition, homolog-scanning mutagenesis results suggest that there is not a single protein receptor for bovine seminal ribonuclease (Kim et al., 1995a). Finally, the random cationization of RNase A increases both its cellular uptake and cytotoxicity (Futami et al., 2001; Futami et al., 2002).
A distinct internalization pathway for a protein toxin
Ribonucleases are internalized by a pathway that is distinct from that of toxins characterized previously (Fig. 11). Protein toxins characterized to date, that act within the cytosol, cross the plasma membrane from two subcellular locations: endosomal or post-endosomal compartments (Lord and Roberts, 1998). The best characterized toxin that translocates from an endosomal compartment is diphtheria toxin (Collier, 2001) (Fig. 11). Ricin is among the best characterized toxins that translocates from a post-endosomal compartment (Olsnes and Kozlov, 2001) (Fig. 11). Diphtheria- and ricin-like toxins have evolved special mechanisms for entering and killing mammalian cells. Such toxins are comprised of distinct domains—one to provide catalytic activity and another to facilitate cytosolic entry. During toxin action, these domains physically dissociate. In a striking contrast, secretory ribonucleases have a single globular domain that is responsible for cell-surface binding, internalization, and catalytic activity (Fig. 1).
The internalization pathways of ribonucleases and diphtheria toxin differ by their modes of uptake and translocation. The internalization of diphtheria toxin is contingent on clathrin-dependent endocytosis (Simpson et al., 1998). Blocking this pathway by overexpressing a dominant negative variant of dynamin protects cells from diphtheria toxin (Simpson et al., 1998). In contrast, ribonucleases are more toxic in the absence of dynamin-dependent endocytosis (Fig. 8). The translocation of diphtheria toxin is highly dependent on the low pH of the endosome, as this acidic environment induces a conformational change in the B domain that forms a pore through the membrane and allows the A domain to cross (Draper and Simon, 1980; Sandvig and Olsnes, 1980; Sandvig and Olsnes, 1981; Sandvig and Olsnes, 1982). Agents such as NH4Cl and monensin, which neutralize acidic environments within the cell, block cytosolic entry and thereby protect cells from diphtheria toxin. These drugs do not protect cells from the toxicity of ribonucleases (Fig. 10).
The internalization pathway of ribonucleases has similarities and differences with that of ricin (Sandvig and van Deurs, 2000; Olsnes and Kozlov, 2001). Like some RNase A homologs (Irie et al., 1998), ricin is a lectin, and binds carbohydrates at the cell surface. ONC, RNase A, and ricin can be internalized and cytotoxic in the absence of dynamin-mediated endocytosis (Llorente et al., 1998) (Fig. 9). In addition, a low pH is not required for the cytotoxicity of ONC, G88R RNase A, or ricin. Unlike ribonucleases, ricin translocates to the cytosol from the ER after retrograde transport from the Golgi network. Cells with a BFA-disrupted Golgi stack are protected from ricin (Yoshida et al., 1991), but not from ribonucleases (Fig. 10). These data are consistent with the finding that BFA does not block the toxicity of ONC for 9L cells (Wu et al., 1995). Olsnes and coworkers, demonstrated that transport of the ricin A chain from the Golgi to the ER was a prerequisite for translocation (Rapak et al., 1997). Moreover, introduction of a KDEL tail to the ricin A chain dramatically enhances its cytotoxicity (Wales et al., 1993; Tagge et al., 1996), but the presence of a KDEL tail on G88R RNase A has no effect on its cytotoxicity (P.A. Leland and R.T. Raines, unpublished results). Hence, an increased ribonuclease concentration in the ER does not enhance its cytotoxicity. Combined, these results indicate that ONC and G88R RNase A do not translocate to the cytosol from the ER.
The study of toxin internalization has facilitated important discoveries in the field of intracellular transport (Lord and Roberts, 1998). Studies of Shiga toxin showed for the first time that the secretory pathway is completely reversible (Sandvig et al., 1992). In addition, Shiga toxin and ricin are models for dissecting retrograde transport and as well as the molecular details of endocytosis (Mallard et al., 1998; Sandvig and van Deurs, 2000). Studies using cholera toxin binding and entry have clarified pathways of lipid trafficking (Orlandi and Fishman, 1998; Radhakrishnan et al., 2000). Information about protein translocation from the ER to the cytosol can also be gained using Shiga toxin and ricin. Likewise, secretory ribonucleases can be a tool for studying clathrin- and dynamin-independent endocytosis, as well as for investigating how cationic proteins enter cells.
RNase A and its homologs are secreted proteins. Yet, the cytosol of every cell contains RI (Hofsteenge, 1997; Shapiro, 2001), indicating an imperative to protect cells from internalized ribonucleases. The prevalence of RI suggests that ribonuclease internalization is a widespread and important occurrence. Our work has revealed aspects of a distinct pathway by which these common proteins—secretory ribonucleases—can enter the cytosol (Fig. 11).
Acknowledgments
This research was support by Grant CA73808 (NIH). We are grateful to S.L. Schmid, K.M. Haigis, and R.L. Abel for helpful suggestions and critical reading of the manuscript, and A.K. Menon, S.Y. Bednarek, A.D. Attie, and M.L. Nibert for contributive discussions. We thank J.F. Wilkins and K.A. Dickson for help in the preparation of D16C ONC. We thank J. Kimble for use of her Zeiss Axiovert 100 TV microscope.
References
- Beintema JJ, Schüller C, Irie M, Carsana A. Molecular evolution of the ribonuclease superfamily. Prog. Biophys. Molec. Biol. 1988;51:165–192. doi: 10.1016/0079-6107(88)90001-6. [DOI] [PubMed] [Google Scholar]
- Boix E, Wu Y, Vasandani VM, Saxena SK, Ardelt W, Ladner J, Youle RJ. Role of the N terminus in RNase A homologues: differences in catalytic activity, ribonuclease inhibitor interaction and cytotoxicity. J. Mol. Biol. 1996;257:992–1007. doi: 10.1006/jmbi.1996.0218. [DOI] [PubMed] [Google Scholar]
- Bretscher LE, Abel RL, Raines RT. A ribonuclease A variant with low catalytic activity but high cytotoxicity. J. Biol. Chem. 2000;275:9893–9896. doi: 10.1074/jbc.275.14.9893. [DOI] [PubMed] [Google Scholar]
- Cafaro B, De Lorenzo C, Piccoli R, Bracale A, Mastronicola MR, Di Donato A, D’Alessio G. The antitumor action of seminal ribonuclease and its quaternary conformations. FEBS Lett. 1995;359:31–34. doi: 10.1016/0014-5793(94)01450-f. [DOI] [PubMed] [Google Scholar]
- Collier RJ. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- D'Alessio G, Di Donato A, Mazzarella L, Piccoli R. Seminal ribonuclease: the importance of diversity. In: D'Alessio G, Riordan JF, editors. Ribonucleases: Structures and Functions. New York: Academic Press; 1997. pp. 383–423. [Google Scholar]
- D’Alessio G, Riordan JF. Ribonucleases: Structures and Functions. New York: Academic Press; 1997. [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J. Cell. Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell. Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo C, Nigro A, Piccoli R, D’Alessio G. A new RNase-based immunoconjugate selectively cytotoxic for ErbB2-overexpressing cells. FEBS Lett. 2002;516:208–212. doi: 10.1016/s0014-5793(02)02527-9. [DOI] [PubMed] [Google Scholar]
- delCardayré SB, Ribó M, Yokel EM, Quirk DJ, Rutter WJ, Raines RT. Engineering ribonuclease A: production, purification, and characterization of wild-type enzyme and mutants at Gln11. Protein Engng. 1995;8:261–273. doi: 10.1093/protein/8.3.261. [DOI] [PubMed] [Google Scholar]
- Dinter A, Berger EG. Golgi-disturbing agents. Histochem. Cell Biol. 1998;109:571–590. doi: 10.1007/s004180050256. [DOI] [PubMed] [Google Scholar]
- Draper RK, Simon MI. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J. Cell Biol. 1980;87:849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt ES, Wittmayer PK, Templer BM, Raines RT. Contribution of a tyrosine side chain to ribonuclease A catalysis and stability. Protein Sci. 1996;5:1697–1703. doi: 10.1002/pro.5560050823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami J, Maeda T, Kitazoe M, Nukui E, Tada H, Seno M, Kosaka M, Yamada H. Preparation of potent cytotoxic ribonucleases by cationization: enhanced cellular uptake and decreased interaction with ribonuclease inhibitor by chemical modification of carboxyl groups. Biochemistry. 2001;40:7518–7524. doi: 10.1021/bi010248g. [DOI] [PubMed] [Google Scholar]
- Futami J, Nukui E, Maeda T, Kosaka M, Tada H, Seno M, Yamada H. Optimum modification for the highest cytotoxicity of cationized ribonuclease. J. Biochem. (Tokyo) 2002;132:223–228. doi: 10.1093/oxfordjournals.jbchem.a003214. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Kurten EL, Abel RL, Raines RT. KFERQ sequence in ribonuclease A-mediated cytotoxicity. J. Biol. Chem. 2002;277:11576–11581. doi: 10.1074/jbc.M112227200. [DOI] [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J. Cell. Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofsteenge J. Ribonuclease inhibitor. In: D'Alessio G, Riordan JF, editors. Ribonucleases: Structures and Functions. New York: Academic Press; 1997. pp. 621–658. [Google Scholar]
- Hudson TH, Grillo FG. Brefeldin-A enhancement of ricin A-chain immunotoxins and blockade of intact ricin, modeccin, and abrin. J. Biol. Chem. 1991;266:18586–18592. [PubMed] [Google Scholar]
- Irie M, Nitta K, Nonaka T. Biochemistry of frog ribonucleases. Cell. Mol. Life Sci. 1998;54:775–784. doi: 10.1007/s000180050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the trans- Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- Kelemen BR, Klink TA, Behlke MA, Eubanks SR, Leland PA, Raines RT. Hypersensitive substrate for ribonucleases. Nucleic Acids Res. 1999;27:3696–3701. doi: 10.1093/nar/27.18.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, Soucek J, Matousek J, Raines RT. Mechanism of ribonuclease cytotoxicity. J. Biol. Chem. 1995a;270:31097–31102. doi: 10.1074/jbc.270.52.31097. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Soucek J, Matousek J, Raines RT. Structural basis for the biological activities of bovine seminal ribonuclease. J. Biol. Chem. 1995b;270:10525–10530. doi: 10.1074/jbc.270.18.10525. [DOI] [PubMed] [Google Scholar]
- Kothandaraman S, Hebert MC, Raines RT, Nibert ML. No role for pepstatin-A-sensitive acidic proteinases in reovirus infections of L or MDCK cells. Virology. 1998;251:264–272. doi: 10.1006/viro.1998.9434. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell. 2001;7:661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- Leamon CP, Low PS. Membrane folate-binding proteins are responsible for folate-protein conjugate endocytosis into cultured cells. Biochem. J. 1993;291:855–860. doi: 10.1042/bj2910855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Shapiro R, Vallee BL. Tight-binding inhibition of angiogenin and ribonuclease A by placental ribonuclease inhibitor. Biochemistry. 1989;28:225–230. doi: 10.1021/bi00427a031. [DOI] [PubMed] [Google Scholar]
- Leland PA, Raines RT. Cancer chemotherapy--ribonucleases to the rescue. Chem. Biol. 2001;8:405–413. doi: 10.1016/s1074-5521(01)00030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leland PA, Schultz LW, Kim B-M, Raines RT. Ribonuclease A variants with potent cytotoxic activity. Proc. Natl. Acad. Sci. U.S.A. 1998;98:10407–10412. doi: 10.1073/pnas.95.18.10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leland PA, Staniszewski KE, Kim B, Raines RT. A synapomorphic disulfide bond is critical for the conformational stability and cytotoxicity of an amphibian ribonuclease. FEBS Lett. 2000;477:203–207. doi: 10.1016/s0014-5793(00)01804-4. [DOI] [PubMed] [Google Scholar]
- Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K. Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. J. Cell Biol. 1998;140:553–563. doi: 10.1083/jcb.140.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM, Roberts LM. Toxin entry: retrograde transport through the secretory pathway. J. Cell. Biol. 1998;140:733–736. doi: 10.1083/jcb.140.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. Endocytosis. In: Hames BD, Glover DM, editors. Frontiers in Molecular Biology. Oxford: Oxford University Press; 2001. [Google Scholar]
- Matousek J. Ribonucleases and their antitumor activity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001;129:175–191. doi: 10.1016/s1532-0456(01)90202-9. [DOI] [PubMed] [Google Scholar]
- McNiven MA, Cao H, Pitts KR, Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- Messmore JM, Fuchs DN, Raines RT. Ribonuclease A: revealing structure – function relationships with semisynthesis. J. Am. Chem. Soc. 1995;117:8057–8060. doi: 10.1021/ja00136a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulski SM, Costanzi JJ, Vogelzang NJ, McCachren S, Taub RN, Chun H, Mittelman A, Panella T, Puccio C, Fine R, et al. Phase II trial of a single weekly intravenous does of ranpirnase in patients with unresectable malignant mesothelioma. J. Clin. Oncol. 2002;20:274–281. doi: 10.1200/JCO.2002.20.1.274. [DOI] [PubMed] [Google Scholar]
- Mosimann SC, Ardelt W, James MNG. Refined 1.7 Å X-ray crystallographic structure of P-30 protein, an amphibian ribonuclease with anti-tumor activity. J. Mol. Biol. 1994;236:1141–1153. doi: 10.1016/0022-2836(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Newton DL, Hansen HJ, Liu H, Ruby D, Iordanov MS, Magun BE, Goldenberg DM, Rybak SM. Specifically targeting the CD22 receptor of human B-cell lymphomas with RNA damaging agents. Crit. Rev. Oncol. Hematol. 2001;39:79–86. doi: 10.1016/s1040-8428(01)00116-0. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11:406–412. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- Olsnes S, Kozlov JV. Ricin. Toxicon. 2001;39:1723–1728. doi: 10.1016/s0041-0101(01)00158-1. [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. Multiple targets for brefeldin A. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Anderson TG, McConnell HM. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12422–12427. doi: 10.1073/pnas.220418097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines RT. Ribonuclease A. Chem. Rev. 1998;98:1045–1065. doi: 10.1021/cr960427h. [DOI] [PubMed] [Google Scholar]
- Rapak A, Falnes PO, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Garred O, Prydz K, Kozlov JV, Hansen SH, van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J. Cell Biol. 1980;87:828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J. Biol. Chem. 1981;256:9068–9076. [PubMed] [Google Scholar]
- Sandvig K, Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J. Biol. Chem. 1982;257:7504–7513. [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943–5950. doi: 10.1093/emboj/19.22.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena SK, Rybak SM, Winkler G, Meade HM, McGray P, Youle RJ, Ackerman EJ. Comparison of RNases and toxins upon injection into Xenopus oocytes. J. Biol. Chem. 1991;266:21208–21214. [PubMed] [Google Scholar]
- Schmid SL, McNiven MA, De Camilli P. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Sciaky N, Presley J, Smith C, Zaal KJ, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M, Anfinsen CB, Harrington WF. The correlation of ribonuclease activity with specific aspects of tertiary structure. Biochim. Biophys. Acta. 1957;26:502–512. doi: 10.1016/0006-3002(57)90096-3. [DOI] [PubMed] [Google Scholar]
- Shapiro R. Cytoplasmic ribonuclease inhibitor. Methods Enzymol. 2001;341:611–628. doi: 10.1016/s0076-6879(01)41180-3. [DOI] [PubMed] [Google Scholar]
- Simpson JC, Smith DC, Roberts LM, Lord JM. Expression of mutant dynamin protects cells against diphtheria toxin but not against ricin. Exp. Cell. Res. 1998;239:293–300. doi: 10.1006/excr.1997.3921. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Saxena SK, Boix E, Prill RJ, Vasandani VM, Ladner JE, Sung C, Youle RJ. Engineering receptor-mediated cytotoxicity into human ribonucleases by steric blockade of inhibitor interaction. Nat. Biotechnol. 1999;17:265–270. doi: 10.1038/7010. [see comments] [DOI] [PubMed] [Google Scholar]
- Tagge E, Chandler J, Tang BL, Hong W, Willingham MC, Frankel A. Cytotoxicity of KDEL-terminated ricin toxins correlates with distribution of the KDEL receptor in the Golgi. J. Histochem. Cytochem. 1996;44:159–165. doi: 10.1177/44.2.8609372. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini AM, Kieffer B, Mathies R, Meyhack B, Hemmings BA, Stone SR, Hofsteenge J. Protein chemical and kinetic characterization of recombinant porcine ribonuclease inhibitor expressed in Saccharomyces cerevisiae. Biochemistry. 1990;29:8827–8834. doi: 10.1021/bi00489a046. [DOI] [PubMed] [Google Scholar]
- Wales R, Roberts LM, Lord JM. Addition of an endoplasmic reticulum retrieval sequence to ricin A chain significantly increases its cytotoxicity to mammalian cells. J. Biol. Chem. 1993;268:23986–23990. [PubMed] [Google Scholar]
- Wang E, Pennington JG, Goldenring JR, Hunziker W, Dunn KW. Brefeldin A rapidly disrupts plasma membrane polarity by blocking polar sorting in common endosomes of MDCK cells. J. Cell Sci. 2001;114:3309–3321. doi: 10.1242/jcs.114.18.3309. [DOI] [PubMed] [Google Scholar]
- Wlodawer A. Structure of bovine pancreatic ribonuclease by X-ray and neutron diffraction. In: Jurnak FA, McPherson A, editors. Biological Macromolecules and Assemblies, Vol. II, Nucleic Acids and Interactive Proteins. New York: Wiley; 1985. pp. 395–439. [Google Scholar]
- Wu Y, Mikulski SM, Ardelt W, Rybak SM, Youle RJ. A cytotoxic ribonuclease. J. Biol. Chem. 1993;268:10686–10693. [PubMed] [Google Scholar]
- Wu Y, Saxena SK, Ardelt W, Gadina M, Mikulski SM, De Lorenzo V, D’Alessio G, Youle RJ. A study of the intracellular routing of cytotoxic ribonucleases. J. Biol. Chem. 1995;270:17476–17481. doi: 10.1074/jbc.270.29.17476. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Chen CC, Zhang MS, Wu HC. Disruption of the Golgi apparatus by brefeldin A inhibits the cytotoxicity of ricin, modeccin, and Pseudomonas toxin. Exp. Cell Res. 1991;192:389–395. doi: 10.1016/0014-4827(91)90056-z. [DOI] [PubMed] [Google Scholar]
- Youle RJ, D'Alessio G. Antitumor RNases. In: D'Alessio G, Riordan JF, editors. Ribonucleases: Structures and Functions. New York: Academic Press; 1997. pp. 491–514. [Google Scholar]