Abstract
Purpose
Ectopic expression of GRM1 in murine melanocytes results in transformation into a form of melanoma, and more than 60% of human melanoma samples tested ectopically express GRM1. Stimulation of this receptor in vitro results in up-regulation of activated extracellular signal–regulated kinase (ERK). Furthermore, a xenograft model of melanoma treated with riluzole, an oral GRM1 blocking agent, showed decreased tumor growth compared with the untreated controls. We have now completed a phase 0 trial of riluzole in patients with melanoma.
Experimental Design
Patients enrolled on this trial underwent a pretreatment biopsy, took 200 mg of oral riluzole per day for 14 days, and then underwent resection of their remaining tumor. We compared the levels of pERK and pAKT in the pretreatment and post-treatment samples and assessed the metabolic activity of pretreatment and post-treatment tumors using fluorodeoxyglucose positron emission tomography (FDG-PET) scanning.
Results
We accrued 12 patients and all expressed GRM1. We found a significant decrease in pAKT and/or pERK in post-treatment tumor samples as compared with pretreatment samples in 4 (34%) patients. These four patients had a significant decrease in FDG-PET intensity post-treatment as well. Two other patients had a clinical response with no corresponding metabolic response; five patients had similar pretreatment and post-treatment FDG-PET scan findings; and one patient had progressive disease.
Conclusions
Our data show that glutamate blockade with riluzole can inhibit signaling through the mitogen-activated protein kinase and phosphatidylinositol 3-kinase/AKT pathways and suppress the metabolic activity of melanoma. The ectopic expression of metabotropic glutamate receptors may be important in the pathogenesis of human melanoma, and targeting this pathway may be an effective therapy.
Recently, our group described a heretofore unknown component of melanoma pathogenesis. A transgenic murine model of melanoma was developed by the ectopic expression of metabotropic glutamate receptor 1 (GRM1) in melanocytes (1–3). These mice spontaneously develop melanocytic lesions indistinguishable from human melanoma. We have expanded these original studies and have now shown that more than 60% of human melanomas express GRM1 and that activation of this receptor results in activation of the mitogen-activated protein kinase (MAPK) pathway in a B-Raf– and N-Ras– independent fashion (1). In preclinical studies, we have shown that the ectopic expression of GRM1 in melanocytes is transforming and that inhibition of GRM1 signaling in vitro and in vivo results in cell cycle arrest and subsequent apoptosis in human melanoma (2).
We have now translated our findings into the clinic and have completed a phase 0 trial of riluzole in patients with stage III and IV melanoma. Riluzole (2-amino-6-trifluoro-methoxybenzothiazole) is a noncompetitive GRM1 receptor antagonist that has been shown to be safe and effective in patients with amylotropic lateral sclerosis (ALS; refs. 4–7). Riluzole is the only Food and Drug Administration–appoved GRM1 blocking agent and is used to slow the progression of disease in patients with ALS. We now report that administration of oral riluzole resulted in suppression of MAPK pathway signaling and involution of tumor in 34% of patients. We also found suppression of signaling through the phosphatidylinositol 3-kinase (PI3K)/AKT pathway and an increase in the number of apoptotic cells in some post-treatment tumor samples. These results lead us to hypothesize that oral riluzole, alone or in combination with other compounds, may be an effective therapy for patients with melanoma.
Materials and Methods
We performed an Institutional Review Board–approved phase 0 trial of oral riluzole in patients with resectable stage III or IV melanoma, who were to undergo resection of their tumors. We chose a phase 0 trial design because only a few patients need to be exposed to the drug to determine if riluzole is capable of modulating GRM1 signaling in patient tumors. In addition, phase 0 trial designs have been recommended by the Food and Drug Administration as proof-of-mechanism studies for agents that modulate signaling pathways. Furthermore, because 200 mg daily seems to be the maximum tolerated daily dose of riluzole in humans (8), a dose-finding phase I trial in cancer patients was not necessary to proceed with a phase 0 trial although we will incorporate dose escalation into future therapeutic trials. The primary objective of this trial was to determine whether treatment with riluzole alters the levels of activated extracellular signal–regulated kinase (ERK) and AKT in biopsy specimens obtained before and after treatment. Sample size calculations based on examination of previous tumor biopsy specimens indicated that by enrolling 15 patients, we should have been able to detect a 25% decrease in the ratio of activated to total protein after riluzole treatment.
Riluzole
Riluzole (2-amino-6-trifluoromethoxybenzothiazole) is a noncompetitive GRM1 receptor antagonist that has been shown to be safe and effective in patients with ALS (4–7). Riluzole is the only Food and Drug Administration–appoved GRM1 blocking agent and is used to slow the progression of disease in patients with ALS. Riluzole is a moderately potent noncompetitive GRM1 antagonist but is a very potent inhibitor of glutamate release by GRM1-expressing cells. How blockade of GRM1 results in slowing of the progression of ALS is unknown, but inhibition of signaling through GRM1 on binding of riluzole has been documented in neuronal cells. It has recently been suggested that riluzole is an inhibitor of protein kinase C, a central component of GRM1 signaling (6).
Patients and dosing
Patients with resectable stage III and IV melanoma were eligible for this trial. Patients enrolled on the trial under went pretreatment biopsy of their tumor; received 200 mg/d of oral riluzole, divided into two 100-mg doses given 12 h apart, for 2 wk (the maximal daily recommended dose in patients with ALS); and then underwent resection of their residual tumors. Patients with ALS receiving this dose had little toxicity (8, 9). Dosing the patients for 2 wk was chosen because this represents ~7 half-lives of the drug after it reaches steady state (8, 9). In a previously reported xenograft model, we found that 2 wk of drug administration was sufficient to significantly alter tumor growth (2). Notably, administration of this dose of riluzole was unlikely to be toxic; thus, whereas patients were unlikely to benefit in terms of antitumor activity from receiving the drug for this short of a time, the risk to the patients was quite low. Furthermore, 2 wk is a normal time period between initial evaluation of a patient in the clinic and getting the patient into the operating room for a procedure. Therefore, we did not delay treatment in patients enrolled on this trial.
Definitive surgical procedures and tumor harvest
During the definitive operations, we needed to obtain clearance of the tumor, harvest tumor for diagnostic purposes, and harvest remaining tumor for trial purposes. No particular algorithm was used to choose tumor samples for trial purposes. If the patient had one metastatic deposit, we performed the pretreatment biopsy on this deposit and removed a portion of the resected deposit 2 wk later for the post-treatment biopsy. If the patient had multiple metastatic deposits, we removed one accessible deposit for the pretreatment biopsy and removed a second deposit for the post-treatment biopsy. During the definitive operation, we needed to identify a deposit that contained enough tumor for diagnostic purposes as well as protocol experiments. This resulted in the resection of the largest remaining deposit at the time of the definitive operation for protocol purposes. This was the only way we could perform a phase 0 trial with minimal discomfort and potential harm to the patients. There were likely metabolic differences between tumor samples obtained from different tumor deposits but this is unavoidable.
Fluorodeoxyglucose positron emission tomography scanning
We also obtained pretreatment and post-treatment fluorodeoxyglucose (FDG) positron emission tomography (PET) scans or PET/computed tomography (CT) scans to evaluate the overall metabolic activity of the tumors and how this activity changes with inhibition of the GRM1 pathway. Melanomas are usually very FDG-avid and preoperative FDG-PET scans are routinely used in the evaluation of patients with melanoma (10–12). Standardized uptake value (SUV) was measured in matched pairs of tumors imaged in the same facility and instrument and interpreted by the same team of Nuclear Medicine Doctors in a blinded fashion.
Protein extraction and Western blotting
Tumor samples were homogenized in a Bio101/Savant FP120 Fastprep machine. Briefly, ~5-mm3 pieces of frozen tumor samples were added to lysing matrix A, and the ceramic bead was placed on top of the tumor sample. To this, 500 µL of a modified NP40 lysis buffer [25 mmol/L Tris-HCl (pH 7.4), 150 mm NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 0.5% NP40, 10% glycerol, complete protease inhibitor tablets (Roche), 1 mmol/L phenylmethylsulfonyl fluoride, 50 mmol/L β-glycerol phosphate, 10 mmol/L p-nitrophenyl phosphate, 2.5 mmol/L sodium pyrophosphate, 100 nmol/L okadaic acid, 10 mmol/L NaF, 1 mmol/L Na3VO4, and 10 µmol/L phenylarsine oxide] were added. The samples were then pulsed at speed 4 for 40 s, placed on ice for 5 min, and pulsed again. The samples were then centrifuged (5,000 × g, 4°C, 10 min) and the supernatant was collected. Fresh lysis buffer (500 µL) was then added to the pelleted lysing matrix, pulsed once more, and the supernatant collected and added to the previous collected sample. The lysates were then cleared by centrifugation (10,000 × g, 4° C, 10 m). The protein lysates were quantified by Bradford assay (Bio-Rad) and, routinely, 10 µg of total lysate were resolved on 10% SDS-PAGE gels. Resolved proteins were semi-dry blotted (Bio-Rad 170-3940) to polyvinylidene difluoride membranes at 25 V for 45 min and probed with antibodies as indicated in the figure legends.
Antibodies
Anti-GRM1 was purchased from Abcam; anti–phospho-MAPK (9101S), anti–phospho-AKT, anti–MAPK, anti-AKT, and anti– cleaved caspase-3 were purchased from Cell Signaling Technology.
Immunohistochemisty
Anti–cleaved caspase-3 (Cell Signaling) was first optimized on human tonsil slides using Ventana Medical Systems Discovery XT automated immunostainer. Then, melanoma case slides were deparaffinized and antigen retrieval was done using CC1 (Cell Conditioning Solution, Ventana Medical Systems). Anti–cleaved caspase-3 antibody was applied at a dilution of 1:300 and incubated at 37° C for 1 h. Ventana Universal Secondary antibody was applied for 12 min, followed by chromogenic detection kit DABMap (Ventana Medical Systems). Slides were counterstained with hematoxylin and dehydrated and cleared before coverslipping from xylene.
Translational Relevance
Our group made the initial discovery of the ectopic expression of metabotropic glutamate receptors in human cancer. We have performed extensive preclinical studies that show that one of these receptors, GRM1, is a potential therapeutic target in patients with melanoma. As a first step in translating these findings into the clinic, we have performed a phase 0 trial of a glutamate signaling inhibitor, riluzole, in patients undergoing resection of stage III and IV melanoma. Our results show that riluzole is a potential therapeutic agent in patients with melanoma, and we can now embark on therapeutic trials with this drug.
Results
Enrollment
We enrolled 12 patients on this phase 0 trial, with 11 successfully completing the protocol with little toxicity noted. The 10th patient was removed for grade 3 toxicity (dizziness) that resolved on stopping the drug. Our initial pre-trial statistical analysis suggested that we would need to enroll 15 patients to show a metabolic response to riluzole. However, after enrolling 12 patients, a metabolic and clinical response was evident. This was a phase 0 nontherapeutic trial that was designed to determine whether riluzole affects signaling in humans as it did in pre-human experiments. The patients did not derive direct therapeutic benefit while on this trial. Once we obtained data that showed that we were affecting signaling through the MAPK and PI3K pathways, it would have been unethical to continue to enroll patients on this trial because the question was answered. We therefore stopped accruing patients to this trial when patient 11 showed a clinical and metabolic response. Patient 12 was already accrued at this point and was allowed to complete the trial.
Clinical responses
Although it was not expected that we would have clinical responses with just 2 weeks of riluzole administration, three patients had obvious shrinkage of their tumors on physical exam. One patient had multiple regional dermal metastases of the lower extremity. After 2 weeks of therapy, one of these lesions involuted completely. A second patient presented with a painfully enlarged right cervical lymph node that shrunk by more than 50% with 1 week of riluzole administration. He remained asymptomatic for the remainder of the trial. A third patient had very large, painful dermal metastases of the right arm, flank, and chest wall. At the end of the 2 weeks of riluzole administration, many of these lesions had shrunk significantly and some had completely resolved. This last patient had been on very high levels of narcotic pain medications, but on the day of his final resection, he was down to a very small dose of oxycodone taken before bedtime. This last patient was among the four patients that had metabolic responses seen on Western blot analysis (see below). The first and second patients were technically nonresponders on this trial because they had no decrease in post-treatment signaling through either the MAPK or PI3K/AKT pathways. The patient with shrinkage of the cervical lymph node had increased FDG-PET signal in that area on the post-treatment scan. This is likely an example of a flare phenomenon (13–15), discussed below in the PET/CT section. The patient with complete resolution of one of his regional dermal metastases had a mixed response, with other of his lesions increasing in size. A fourth patient with left inguinal lymph node involvement was found to have no viable tumor left at the time of definitive resection. We cannot know for certain if this was involution of the tumor secondary to riluzole administration or a technical problem such as injury to the tumor blood supply during biopsy, which could have resulted in involution of the tumor unrelated to riluzole administration. This last patient is not included as one of the patients who responded to riluzole administration.
N-Ras and B-Raf mutational status
We used standard cDNA sequencing to determine if the tumors from patients on this trial harbored B-Raf or N-Ras mutations. We found that the tumor from one patient harbored the V600E B-Raf mutation, a second patient's tumor harbored a V600K B-Raf mutation, and one patient's tumor harbored a Q61K N-Ras mutation. All of the other patients had tumors wild type in both of these genes. Of the four patients that had FDG-PET scan and metabolic evidence of response to riluzole, one had the V600E B-Raf mutation, one had the V600K mutation, one had the Q61K N-Ras mutation, and one was wild type in these genes (Table 1).
Table 1.
A list of the 12 patients receiving riluzole on the trial
| Patient no. |
Toxicity | Pre- and post-tumor |
B-Raf or N-Ras |
Clinical or radiologic response |
Suppression of MAPK |
Suppression of PI3K/AKT |
|---|---|---|---|---|---|---|
| 1 | None | Pre only | WT | ?? | NA | NA |
| 2 | Dry mouth | Yes | V600E | Yes | Yes | Yes |
| 3 | None | Yes | WT | No | No | No |
| 4 | Dry mouth | Yes | WT | No | No | No |
| 5 | Dizziness | Yes | Q61K | Yes | Yes | Yes |
| 6 | None | Yes | WT | No | No | No |
| 7 | None | Yes | WT | Yes | No | No |
| 8 | None | Yes | WT | Yes | No | No |
| 9 | None | Yes | WT | Yes | Yes | Yes |
| 10 | Dizziness | Pre only | WT | NA | NA | NA |
| 11 | Dizziness | Yes | V600K | Yes | Yes | No |
| 12 | None | Yes | WT | No | No | No |
NOTE: Patient no. 1 had no viable tumor on final resection and patient no. 10 was removed from study for grade 3 toxicity (dizziness); thus, only 10 pairs of tumor specimens were available for study. Patients 2, 5, 9, and 11 had clinical or radiologic evidence of response to riluzole administration as well as metabolic evidence of suppression of the MAPK (all four) and PI3K/AKT (all but patient 11) pathways. Patients 7 and 8 had clinical or radiologic evidence of response to riluzole administration but no suppression of the MAPK or PI3K/AKT pathways. Patient 10 was the only patient to be removed from study for toxicity.
Abbreviation: NA, not applicable.
Immunohistochemical staining
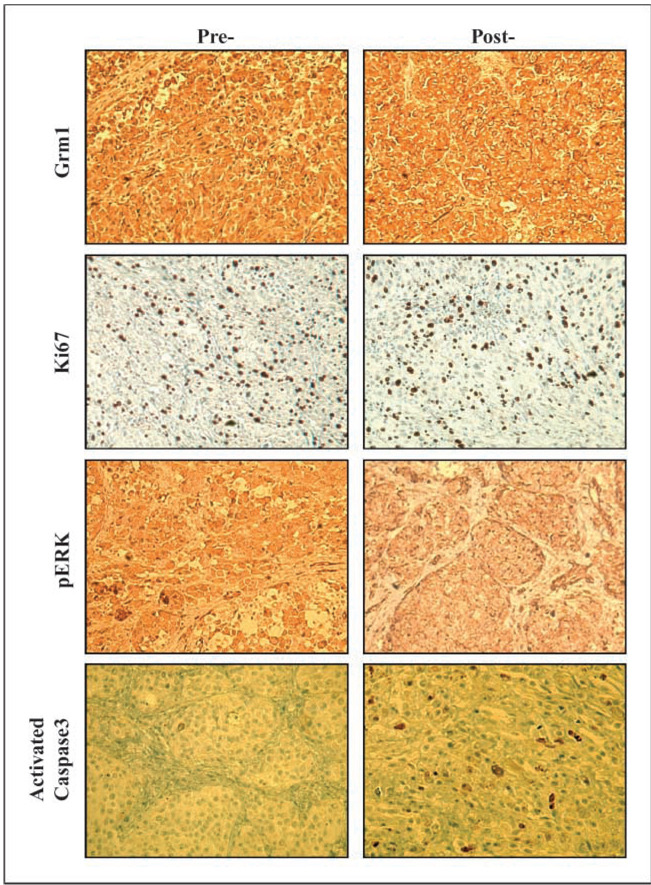
All 12 patients were found to express GRM1 by immunohistochemical staining. One patient had a 25% increase in the number of apoptotic cells in the post-treatment tumor sample as compared with the pretreatment sample, as determined by activated caspase-3 staining (Fig. 1). All other patients had similar pretreatment and post-treatment activated caspase-3 staining. The patient with increased levels of apoptotic cells was one of the two patients that had a response by PET/CT or clinical evaluation but not by metabolic analysis. We also evaluated pretreatment and post-treatment tumor samples for Ki-67 staining intensity to see if we could document a decrease in metabolic activity of the tumors with riluzole administration (16). Unfortunately, we found no consistent difference in Ki-67 staining intensity between pretreatment and post-treatment tumors samples. This finding could be related to the fact that we harvested the largest and most accessible tumor post-treatment for our studies and thus examined the post-treatment tumor sample that responded least to riluzole administration.
Fig. 1.
Pretreatment and post-treatment tumor specimens stained for GRM1, Ki-67, pERK, and activated caspase-3 from one of the patients who had a metabolic and radiologic response to riluzole administration (patient 5). There seems to be no change in the pre- and post-staining intensity for GRM1 and Ki-67. There is a decrease in staining intensity for pERK in the post-treatment specimen as compared with the pretreatment specimen. Finally, ~25% of the cells in the post-treatment sections are positive for activated caspase-3 whereas few of the pretreatment tumor cells stain for activated caspase-3.
PET/CT scanning
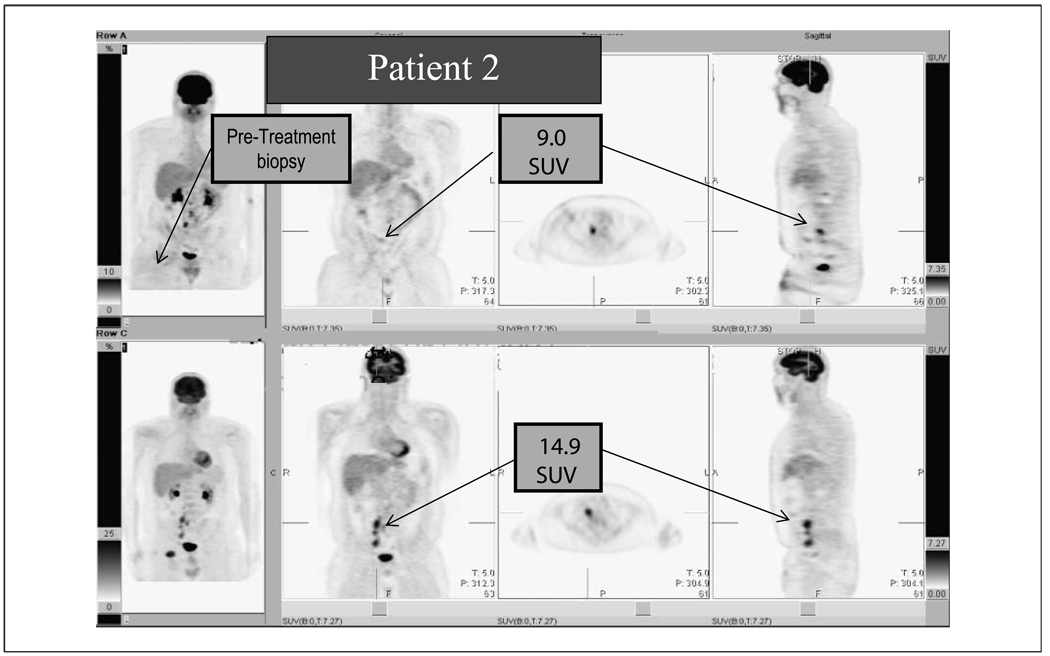
Five patients had apparent responses to riluzole administration on radiologic examination, including two of the three patients that also had clinical evidence of response. Three patients had significant decreases in SUV intensity on post-treatment FDG-PET scans as compared with the pretreatment scans, and two patients had a decrease in the size of the tumors on post-treatment CT scans but an increase in the intensity of the tumors on FDG-PET. In three of these five cases, some of the multiple nodal and cutaneous metastases completely resolved. One of these patients had six different tumors measured by PET/CT and all shrunk in size and decreased in FDG-PET intensity by 10% to 20%. A second patient had multiple cutaneous metastases in the right arm and chest wall with SUV intensities of 35 to 48. Many of these lesions completely resolved on post-treatment scans and some dropped in intensity by 90%. Representative pretreatment and post-treatment scans are shown in Fig. 2. Four of the remaining six patients had stable disease on post-treatment scans and the remaining two patients had progressive disease. Patient 10 was removed from the study before the second FDG-PET scan, which is why we have results from only 11 patients. As noted above, two patients had shrinkage of their tumors on CT although they had an increase in FDG-PET intensity in these same tumors. This may represent a phenomenon known as a flare, an increase in FDG-PET intensity on tumor inflammation and not actual progression of disease (13–15). All SUVs were normalized to liver intensity.
Fig. 2.
A representative FDG-PET scan of one of the four patients that had a radiologic and metabolic response to 2 wk of riluzole administration. Pretreatment FDG-PET scan (bottom) and post-treatment scan (top) done 2 wk after the start of riluzole. There is a large decrease in the number of FDG-avid lymph nodes seen in the post-treatment scan, and the remaining node seen in the top image has decreased in intensity by >40%. The three other patients who had metabolic responses had similar FDG-PET scan results. The far left images show where the pretreatment biopsy was taken.
Western blotting
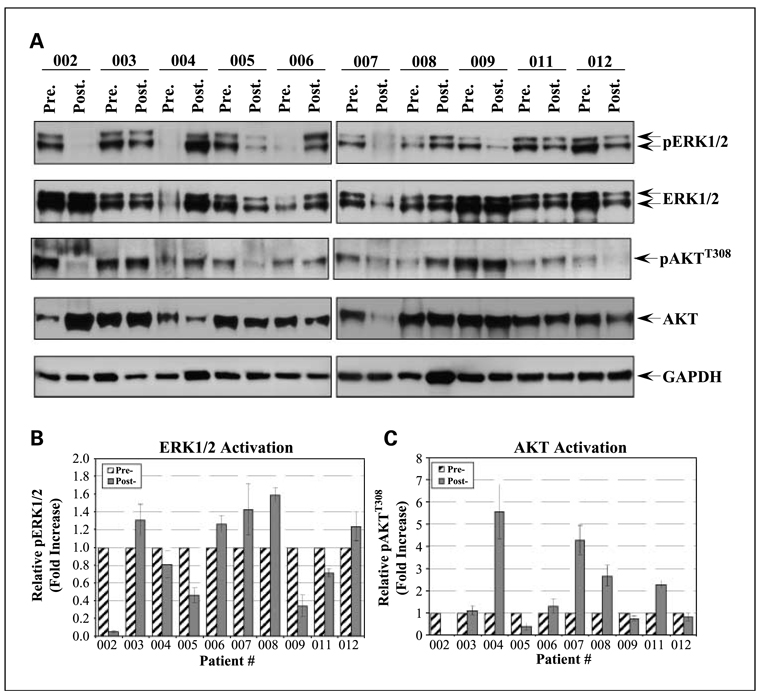
We performed Western blotting on the pretreatment and post-treatment tumor samples to detect the activated (phosphorylated) forms of ERK and AKT (Fig. 3). We only had 10 tumor pairs for analysis because one patient was removed from the trial for toxicity and one patient had no viable tumor on final resection. We found a significant decrease in pERK levels in four patients (four of the patients that had responses by FDG-PET or CT) and a decrease in the level of pAKT in three of these patients. These responses were measured by comparing pERK and pAKT to total ERK and AKT and represent a 50% to 90% decrease in the activated forms post-treatment. One patient who had stable disease on post-treatment PET/CT had a less dramatic decrease in pERK and pAKT. The remaining five patients had increases in post-treatment levels of pERK and pAKT.
Fig. 3.
Total protein lysates were prepared as indicated in Materials and Methods. A, total protein lysate (10 µg) was resolved by SDS-PAGE, electroblotted onto polyvinylidene difluoride membranes, and probed with the indicated phospho-antibodies. The membranes were then probed with goat anti-rabbit IgG conjugated to horseradish peroxidase. The blots were developed using ECL Advance and exposed to X-ray film. The membranes were then subsequently stripped using Western Reprobe according to the manufacturer's protocol. The membranes were then probed with the indicated protein antibodies and developed as above. The blots are representative of three independent experiments. The band intensities from three independent experiments were quantified using ImageJ 1.41L. Phospho-ERK1/2 (B) and phospho-AKT (C) levels were normalized to their respective protein expression levels. Phosphorylation levels of the posttreatment tumor samples were then compared with the matched pretreatment samples to generate the relative fold increase in phosphorylation.
Statistical analysis
The purpose for performing a phase 0 trial was to determine if riluzole could affect signaling through the MAPK and PI3K/AKT pathways. This is a purely observational aim, and we were able to show a decrease in signaling through these pathways in 4 of the 10 patients that had pretreatment and post-treatment tumor specimens for examination. However, because we also found that five patients had clinical and radiologic responses, we can compare these clinical responses to the metabolic responses. The proportion of short-term response rate was estimated and tested against the null hypothesis of zero response rate by the exact binomial distribution. For this phase 0 trial, with only limited number of patients, the association of biomarkers MAPK and AKT to the short-term response was assessed by comparing their mean changes (fold increases) between the clinical or radiologic responders and nonresponders (as opposed to the more appropriate sensitivity and specificity in larger studies). It was shown that MAPK was significantly inhibited in the responders compared with the nonresponders (P < 0.005), whereas for AKT there were borderline differences between responders and nonresponders (P = 0.06). This last result is mainly a product of one of the patients with significant decrease in signaling through the MAPK pathway who had no change in signaling through the PI3K/AKT pathway. This patient had tumor that was wild type for the B-Raf and N-Ras genes. The test was based on nonparametric (rank) ANOVA with three observations from each subject.
Discussion
We have now completed a first-in-humans phase 0 trial of riluzole in patients with stage III and IV melanoma and have shown a significant short-term response rate (4 of 12 patients, 34%) and inhibition of signaling through the MAPK and PI3K/AKT pathways in tumors from responding patients. There was one significant toxicity on this trial; patient 10 had grade 3 dizziness that resulted in discontinuation of riluzole. Two other patients had grade 3 dizziness that occurred after the initial dose of riluzole but resolved with subsequent doses, and these patients were able to remain on trial. Dizziness is a known toxic side effect of riluzole in patients with ALS and is treated by dose reduction (9), which will be incorporated in future trials. Two other patients complained of dry mouth but this was considered very mild by these patients. Overall, 200 mg/d of riluzole was well tolerated by this cohort of patients.
The most surprising finding in this trial was the clinical and PET/CT documented responses to just 2 weeks of riluzole. Five patients had shrinkage of at least some of their tumors, with four patients showing decreased FDG-PET intensity and/or decrease in tumor size on CT along with evidence of suppression of the MAPK pathway, and three of these four patients had suppression of the PI3K/AKT pathway. We did not expect to see such dramatic responses to such a short course of riluzole, and we do not know if these responses will prove durable. Of note is that at the time of the final operations, we harvested obviously involved tumors for the Western blot experiments. Obviously involved means that tumor was visible to the naked eye in the operating room. We harvested these specimens and sent a small portion to the Pathology Department to confirm that the specimen contained melanoma. These large tumors were easily identified and contained abundant sample for the experiments. In retrospect, these were the tumor deposits that responded least to the treatment, and it is surprising that we obtained the results that we did. In future trials, we will attempt to harvest both responding and nonresponding tumor samples for correlative studies. It was also unexpected that patients with both V600E B-Raf and Q61K N-Ras mutations as well as patients with tumors wild type in these two genes would respond to therapy. It was also interesting that all 12 patients expressed GRM1 by immunohistochemical staining. Only 60% of melanoma tumor samples tested thus far in our laboratory have expressed GRM1, and we did not consciously exclude patients from the trial based on GRM1-expression status. It is possible that more advanced tumors have a higher incidence of GRM1 expression, and we are currently examining a larger set of banked melanoma tumor specimens to test this hypothesis. We also found that few of the post-treatment tumor samples showed an increase in the activated form of caspase-3 (Fig. 2). We expected an increase in the number of apoptotic cells in the post-treatment samples from patients responding to riluzole administration because our preclinical studies suggested that riluzole treatment results in a G2 cell cycle arrest followed by apoptosis of the cells (2). However, it is possible that after 2 weeks of administration of riluzole, the majority of apoptotic cells may have been cleared. We may be obtaining our post-treatment biopsies too long after the administration of riluzole to show apoptosis in responding tumors.
Another interesting finding was that only three patients had tumors that harbored B-Raf or N-Ras mutations. We would have expected more of these tumors to have the V600E B-Raf mutation, which is found in ~60% of cutaneous melanomas (17). However, it turned out that each of our four responders had a different mutational status, showing that mutations in B-Raf or N-Ras do not block response to riluzole.
Riluzole seems to have some activity in patients with advanced melanoma. Our phase 0 trial was designed to determine if blockade of glutamate signaling with riluzole would suppress metabolic activity and inhibit signaling through the MAPK and PI3K/AKT pathways in vivo. Our results show that this is indeed the case in a significant proportion of the patients tested. Another reason for performing a phase 0 trial is to determine the best methods to use in future trials. We have learned three lessons from this trial: (a) Because of possible flare phenomenon with FDG-PET scans, it is probably better to use CT scans to evaluate response to riluzole therapy. Different PET tracers such as FLT might be better suited for use in future trials incorporating GRM1 blockade to minimize flare. (b) Examination of signaling through the MAPK and PI3K/AKT pathways is a good way to judge initial metabolic response. (c) Ki-67 and activated caspase-3 staining is not very helpful in determining response in patients receiving riluzole. We will now embark on therapeutic trials of riluzole in patients with stage IV melanoma. These future trials will be designed to determine response rate, durability of response, and long-term toxicity of this compound in patients with melanoma.
Acknowledgments
Grant support: National Cancer Institute grant 1 R21 CA126148-01 (J.S. Goydos).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Marin YE, Namkoong J, Cohen-Solal K, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCε. Cell Signal. 2006;18:1279–1286. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Namkoong J, Shin SS, Lee HJ, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 3.Pollock PM, Cohen-Solal K, Sood R, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 4.Miller R. Riluzole for ALS: what is the evidence? Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:135. [PubMed] [Google Scholar]
- 5.Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:191–206. [PubMed] [Google Scholar]
- 6.Noh KM, Hwang JY, Shin HC, Koh JY. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis. 2000;7:375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- 7.Swash M. New ideas for therapy in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:3–4. doi: 10.1080/14660820510035379. [DOI] [PubMed] [Google Scholar]
- 8.Le Liboux A, Cachia JP, Kirkesseli S, et al. A comparison of the pharmacokinetics and tolerability of riluzole after repeat dose administration in healthy elderly and young volunteers. J Clin Pharmacol. 1999;39:480–486. [PubMed] [Google Scholar]
- 9.Groeneveld GJ, Van Kan HJ, Kalmijn S, et al. Riluzole serum concentrations in patients with ALS: associations with side effects and symptoms. Neurology. 2003;61:1141–1143. doi: 10.1212/01.wnl.0000090459.76784.49. [DOI] [PubMed] [Google Scholar]
- 10.Brady MS, Akhurst T, Spanknebel K, et al. Utility of preoperative [18F]fluorodeoxyglucose-positron emission tomography scanning in high-risk melanoma patients. Ann Surg Oncol. 2006;13:525–532. doi: 10.1245/ASO.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen AT, Akhurst T, Larson SM, Coit DG, Brady MS. PET scanning with 18F 2-f luoro-2-deoxy-d-glucose (FDG) in patients with melanoma. benefits and limitations. Clin Positron Imaging. 1999;2:93–98. doi: 10.1016/s1095-0397(99)00006-0. [DOI] [PubMed] [Google Scholar]
- 12.Tyler DS, Onaitis M, Kherani A, et al. Positron emission tomography scanning in malignant melanoma. Cancer. 2000;89:1019–1025. [PubMed] [Google Scholar]
- 13.Dehdashti F, Flanagan FL, Mortimer JE, Katzenellenbogen JA, Welch MJ, Siegel BA. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51–56. doi: 10.1007/s002590050359. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 15.Wade AA, Scott JA, Kuter I, Fischman AJ. Flare response in 18F-fluoride ion PET bone scanning. AJR Am J Roentgenol. 2006;186:1783–1786. doi: 10.2214/AJR.05.0225. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Izhak O, Bar-Chana M, Sussman L, et al. Ki67 antigen and PCNA proliferation markers predict survival in anorectal malignant melanoma. Histopathology. 2002;41:519–525. doi: 10.1046/j.1365-2559.2002.01444.x. [DOI] [PubMed] [Google Scholar]
- 17.Goydos JS, Mann B, Kim HJ, et al. Detection of B-RAF and N-RAS mutations in human melanoma. J Am Coll Surg. 2005;200:362–370. doi: 10.1016/j.jamcollsurg.2004.10.032. [DOI] [PubMed] [Google Scholar]