Abstract
Background
Mixed pathologies are common in older persons with dementia. Little is known about mixed pathologies in probable AD and about the spectrum of neuropathology in mild cognitive impairment (MCI).
Objective
Investigate single and mixed common age-related neuropathologies in persons with probable AD and MCI.
Methods
The study included 483 autopsied participants from the Religious Orders Study and the Rush Memory and Aging Project with probable AD (NINCDS-ARDA criteria), MCI (amnestic and non-amnestic) or no cognitive impairment. We excluded 41 persons with clinically possible AD and 14 with other dementias. We documented the neuropathology of AD (NIA-Reagan Criteria), macroscopic cerebral infarcts, and neocortical Lewy body (LB) disease.
Results
Of 179 persons (average age = 86.9) with probable AD, 87.7% had pathologically-confirmed AD and 45.8% had mixed pathologies, most commonly AD with macroscopic infarcts (n= 54), followed by AD with neocortical Lewy body disease (n=19) and both (n=8). Of the 134 persons with MCI, 54.4% had pathologically-diagnosed AD, (58.7% in amnestic; 49.2% in non-amnestic); 19.4% had mixed pathologies (22.7% in amnestic; 15.3% in non-amnestic). Macroscopic infarcts without pathologically-diagnosed AD accounted for 4.5% of probable AD, 13.3% of amnestic and 18.6% of non-amnestic MCI. Pure neocortical LB disease was uncommon in all persons with cognitive impairment (<6%). Microscopic infarcts (without macroscopic infarcts) were common as a mixed pathology, but rarely accounted for a clinical diagnosis of probable AD (n=4) or MCI (n=3).
Interpretation
Clinically-diagnosed probable AD and MCI, even amnestic MCI, are pathologically heterogeneous disorders with many persons exhibiting mixed pathologies.
Introduction
Mixed dementia refers to cognitive impairment resulting from the co-occurrence of more than one neuropathology, most commonly Alzheimer's disease (AD) pathology and cerebral infarcts (1). It has become increasingly recognized that community-dwelling older persons with dementia commonly have mixed pathologies (2-5) and that this additional pathology can increase cognitive impairment and the odds of dementia (5-9). Probable AD is the most commonly diagnosed dementia in older persons and though the diagnosis can only be confirmed at autopsy, clinical-pathologic studies have shown good diagnostic sensitivity (10). However, only a few of these clinical-pathologic studies have focused on whether the AD pathology was “pure” or coexisted with other pathologies (5, 11). Both cerebral infarct and Lewy body pathology which may co-occur with AD pathology are common in older persons and may be clinically unsuspected. There is little information regarding mixed pathologies in community-dwelling older persons with probable AD.
Mild cognitive impairment (MCI), especially of the amnestic type, is often considered the earliest clinical sign of probable AD. Studies of the pathologic basis of MCI (12-17), have shown AD type pathology, including amyloid plaques, neurofibrillary tangles, and changes in choline acetyltransferase activity (18). Though these studies have noted non-AD (eg. infarcts, cortical Lewy body disease) and mixed pathologies, a role in non-amnestic type MCI or non-AD dementias is often highlighted (19). The spectrum of pathology and the role of mixed pathologies at the earliest clinically-diagnosed stage of cognitive impairment warrant further study.
The presence of additional pathologies in person with clinically-diagnosed probable AD and MCI is important to recognize for many reasons. Not only may additional pathologies contribute to cognitive impairment, they may influence assumptions regarding environmental and genetic risk factors, implementation and interpretations of clinical trials and ultimately recommendations for prevention and treatment of cognitive impairment and dementia. We previously investigated mixed pathologies in 51 persons with dementia from the Rush Memory and Aging Project (20), and 37 persons with MCI from the Religious Orders Study (15). In our prior work, we had too few persons with probable AD and MCI subtypes to draw meaningful conclusions regarding common and mixed pathologies. In this study, we combine data from both studies and examine common and mixed pathologies in 179 persons with probable AD (NINCDS-ARDA criteria)) and 134 persons with MCI, including 75 persons with amnestic MCI, compared to 170 without cognitive impairment.
Methods
Subjects
We included consecutive deceased and autopsied subjects from the Religious Orders Study (21) and the Rush Memory and Aging Project (22), both longitudinal clinical-pathologic studies of aging and dementia. Enrollment criteria for both studies are essentially identical. Subjects from both studies enroll without dementia and agree to annual clinical evaluations and brain donation at the time of death. The studies were approved by the Institutional Review Board of Rush University Medical Center and each participant signed an informed consent and an Anatomical Gift Act.
Participants of the Religious Orders Study are older catholic clergy without known dementia at the time of enrollment and are from the Chicago area and about 40 additional sites throughout the country. Since January of 1994, more than 1,100 persons have enrolled in the study and annual follow-ups have exceeded 95% of survivors. Details of the study have been previously reported (21, 23-24) Through July 23 2008, 448 participants died and 422 had undergone brain autopsy (94.2% autopsy rate). Complete neuropathologic data was available on 362. We excluded 43 subjects with a clinical diagnosis of possible AD or “other dementia” (see below). Thus, we included the first 319 consecutive autopsies for which there was complete neuropathologic data and a final clinical diagnosis of NCI (n=113), MCI (n=81), or probable AD (n=125).
Participants of the Memory and Aging Project are older Chicago area residents without known dementia at the time of enrollment, that come mostly from about 40 retirement centers and senior subsidized housing facilities. Details of the study have been previously reported (22, 25). Since November of 1997, more than 1,200 persons enrolled and completed their baseline evaluation. The overall annual follow-up rate of survivors exceeds 90%. Through July 23, 2008, 299 participants died and 253 had undergone brain autopsy (84.6% autopsy rate). Complete neuropathologic data was available on 152. We excluded 12 subjects with a clinical diagnosis of possible AD or “other dementia” (see below). We included the first 164 consecutive autopsies for which there was complete neuropathologic data and a final clinical diagnosis of NCI (n=57), MCI (n=53), or probable AD (n=54).
We excluded 55 autopsied subjects based on a final clinical diagnosis of possible AD (n=41) or “other dementia” (n=14). No subjects were excluded based on neuropathologic diagnoses. We specifically excluded 26 with possible AD and cognitive impairment due to stroke, 7 with possible AD and Parkinson's disease dementia, 4 with possible AD and Dementia with Lewy bodies, 4 with possible AD and cognitive impairment due to medical illness, 7 with vascular dementia, 3 with Dementia with Lewy bodies, 3 with dementia due to brain tumor, and 1 with possible paraneoplastic syndrome. In the 41 persons with possible AD, seventeen (30.9%) exhibited mixed pathology; in those with an “other” dementia, 3 persons (21.4%) exhibited mixed pathology.
Clinical Evaluations
Both studies included uniform and structured annual clinical evaluations with medical history questions, neurologic examination, and detailed cognitive testing [21,25]. Diagnostic classification followed a multi-step procedure as previously described [22,24]. Briefly, neuropsychologic tests encompassing a wide range of cognitive function were scored and adjusted for education by computer and reviewed by a neuropsychologist who rated the presence of cognitive impairment in multiple cognitive domains, including episodic memory. Participants were examined or records were reviewed by a clinician with expertise in the evaluation of older persons, and diagnostically classified using the recommendations of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) (26). The diagnosis of probable AD required a history of cognitive decline and evidence of impairment in memory and other cognitive abilities. Probable AD referred to persons meeting these criteria without a co-existing condition contributing to dementia and possible AD referred to persons meeting these criteria who had a co-existing condition (e.g., stroke) contributing to dementia. The diagnosis of MCI referred to persons with cognitive impairment by the neuropsychologist but without a clinical diagnosis of dementia by the examining clinician (24). MCI was considered of the “amnestic type” when the impairment included episodic memory. Other dementia diagnoses followed contemporary standards. Following death, clinical data were reviewed by a neurologist blinded to the neuropathology and the most likely clinical diagnoses at the time of death was rendered.
Brain Autopsy Procedures
Brain autopsies were performed at Rush and 11 predetermined sites across the United States for nearly all cases, as previously described (15,22). Brains were removed, weighed and the brainstem and cerebellum were removed. The hemispheres were cut into 1 cm coronal slabs in a Plexiglas jig. All fresh slabs were photographed and examined for visible pathology. One hemisphere (without visualized pathology) was frozen. The other hemisphere and slabs with visible pathology were fixed for 3-21 days in 4% paraformaldehyde after which there was a macroscopic review (including assessment of macroscopic infarctions) and the dissection of diagnostic blocks (midfrontal, middle temporal, inferior parietal, anterior cingulate, entorhinal cortex, hippocampus, basal ganglia, thalamus, and midbrain with substantia nigra). Blocks were embedded in paraffin, cut into 6 μm sections, and mounted on glass slides.
Pathologic Diagnoses
Neuropathologic diagnoses were made by a board-certified neuropathologist blinded to age and clinical data. For the pathologic diagnosis of AD, Bielschowsky silver stain was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in the frontal, temporal, parietal, entorhinal, and hippocampal cortices, as previously described (27). A neuropathologic diagnosis of “no AD,” “low likelihood AD,” “intermediate likelihood AD,” or “high likelihood AD” was rendered based on semiquantitative estimates of neuritic plaque density as recommended by CERAD [28] and the Braak score [29] as recommended by the National Institute on Aging (NIA) - Reagan criteria [30]. Details of the pathologic diagnoses have been described previously [15,20]. A final pathologic diagnosis of AD required either intermediate or high likelihood AD by these criteria. The minimum criteria for a diagnosis of intermediate likelihood AD required both a Braak score of III and moderate plaques; the minimum criteria for a diagnosis of high likelihood AD required both a Braak score of V and frequent plaques. Chronic macroscopic infarcts were summarized as present or absent as previously described (27). We used H&E stain to document chronic microscopic infarcts (27), and hippocampal sclerosis (severe neuronal loss and gliosis in CA1 or subiculum at level of lateral geniculate). Microscopic infarcts (without macroscopic infarcts) and hippocampal sclerosis are described in the text but not as a single or mixed pathology in analyses or tables.
We used alpha-synuclein immunohistochemistry (Zymed, 1:100) to detect Lewy bodies in the substantia nigra, entorhinal, cingulate, midfrontal, middle temporal, and inferior parietal cortex. Only neocortical LB disease was included in the estimates of mixed pathology in both the tables and in analyses.
Data Analysis
We investigated the proportion of persons in each group (probable AD, MCI, no cognitive impairment) with a pathologic diagnosis of AD, macroscopic cerebral infarct, and/or neocortical Lewy body disease. The three clinical groups were compared using ANOVA or chi-square tests as appropriate. We used ordinal multiple logistic regression models after controlling for age, sex and education to determine whether both the odds of AD and the odds of AD or MCI are associated with the presence or absence of each kind of pathology. Ordinal logistic regression models are appropriate when the odds of MCI is always the same multiple of the odds of a probable AD diagnosis, even as the odds of probable AD varies with age, years of education, sex and presence of AD pathology, infarcts, and Lewy bodies. The score tests for proportionality of the odds was used to check that the assumptions of ordinal logistic regression were not violated (31). Analyses were programmed in SAS® software Version 9.1.3 of the SAS system for UNIX (32).
Results
A total of 483 subjects were included in this study, including 179 with clinically probable AD, 134 persons with MCI (75 with amnestic MCI and 59 with non-amnestic MCI), and 170 persons with no cognitive impairment (table 1). The three diagnostic groups differed in age (F2, 481 = 39.65, p< 0.001) and in MMSE (KW X 22 > 316.65, p < 0.0001). Post hoc testing demonstrated that all pairs of groups differed in mean age and MMSE. The average clinical interval from last examination to autopsy was 6.5 months and the average post-mortem interval was 8.4 hours.
Table 1.
Demographics and pathology in 483 deceased community-dwelling older persons.
| Clinical Diagnosis | |||
|---|---|---|---|
| No cognitive impairment | Mild Cognitive Impairment | Probable Alzheimer's disease | |
| N=170 | N=134 | N=179 | |
|
| |||
| Age, years | 83.9 (SD 6.6) | 86.9 (SD 6.3) | 89.7 (SD 5.6) |
|
| |||
| Education, years | 17.0 (SD 3.8) | 16.8 (SD 3.7) | 16.7 (SD 3.6) |
|
| |||
| Gender, female | 96 (56.5%) | 77 (57.5%) | 107 (60%) |
|
| |||
| MMSE | 28.3 (SD 1.7) | 26.2 (SD 3.3) | 14.1 (SD 8.5) |
|
| |||
| AD path diagnosis | 65 (38.2%) | 73 (54.5%) | 157 (87.7%) |
| NIA- high | 2 (1.2%) | 11 (8.2%) | 71 (39.7%) |
| NIA- intermediate | 63 (37.0%) | 62 (46.3%) | 86 (48.0%) |
|
| |||
| Infarcts* | 39 (22.9%) | 44 (32.8%) | 71 (39.7%) |
|
| |||
| Lewy bodies** | 5 (2.9%) | 8 (6.0%) | 30 (16.8%) |
macroscopic infarcts only
neocortical-type only
Probable Alzheimer's disease
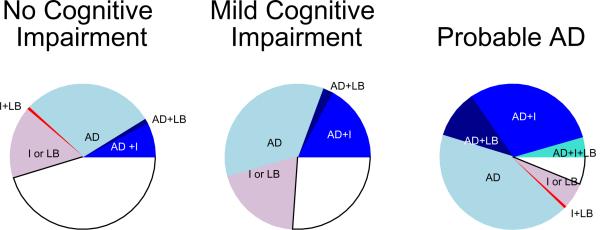
Of the 179 persons diagnosed with probable AD, almost 90% (157 cases) were confirmed to have AD by pathologic criteria (table 1, Figure). Seventy-one of the 157 (45.2%) persons fulfilled criteria for high likelihood AD (NIA-Reagan criteria); a high percentage compared to 15.1% of those with MCI and 3.1% of those with no cognitive impairment.
Figure.
Pathology by clinical status proximate to death.
Blue shades: Pathologic diagnosis of Alzheimer's disease. Clockwise: light blue - pathologic diagnosis of Alzheimer's disease only (AD); dark blue - pathologic diagnosis of Alzheimer's disease and neocortical Lewy bodies (AD+LB); medium blue - pathologic diagnosis of Alzheimer's disease and cerebral infarcts (AD+I); aqua - pathologic diagnosis of Alzheimer's disease, cerebral infarcts and neocortical Lewy bodies (AD+I+LB).
Red shades: Cerebral infarcts and/or neocortical Lewy bodies (with no pathologic diagnosis of AD). Clockwise: pink - cerebral infarcts or neocortical Lewy bodies (I ± LB); red - cerebral infarcts and neocortical Lewy bodies (I+LB).
White: No pathologic diagnosis of Alzheimer's disease, no cerebral infarcts, no neocortical Lewy bodies.
Cerebral infarcts and neocortical Lewy bodies were also common, with more than one-third (n=71) having macroscopic infarcts and approximately one-fourth (n=46) having Lewy bodies.
We diagnosed mixed pathologies in 45.8% of persons with clinically probable AD; the most common mixed pathology was pathologically-diagnosed AD and infarcts, followed by mixed AD and neocortical Lewy bodies, and then all three pathologies, i.e. AD, infarcts, and Lewy bodies (table 2, Figure). Interestingly, AD mixed with another pathology (n=81) was present in a greater proportion of persons with probable AD than AD pathology alone (n=76).
Table 2.
Number (%) of subjects with one, mixed, and no (AD or macrocopic infarcts or neocortical Lewy body) pathology with no cognitive impairment, MCI, and probable AD.
| Clinical Diagnosis | ||||
|---|---|---|---|---|
| No cognitive impairment | Mild Cognitive Impairment | Probable Alzheimer's disease | ||
| N=170 | N=134 | N=179 | ||
|
| ||||
| One pathology | 77 (45.3%) | 73 (54.5%) | 86 (48.0%) | |
|
| ||||
| AD path diagnosis | 50 (29.4%) | 47 (35.1%) | 76 (42.4%) | |
| NIA- high | 2 (1.2%) | 10 (7.5%) | 36 (20.1%) | |
| NIA- intermediate | 48 (28.2%) | 37 (27.6%) | 40 (22.3%) | |
|
| ||||
| Infarcts | 25 (14.7%) | 21 (15.7%) | 8 (4.5%) | |
|
| ||||
| LB | 2 (1.2%) | 5 (3.7%) | 2 (1.1%) | |
|
| ||||
| Mixed pathology | 16 (9.4%) | 26 (19.4%) | 82 (45.8%) | |
|
| ||||
| AD + Infarcts | 13 (7.6%) | 23 (17.2%) | 54 (30.2%) | |
|
| ||||
| AD + LB | 2 (1.2%) | 3 (2.2%) | 19 (10.6%) | |
|
| ||||
| AD + Infarcts + LB | 0 | 0 | 8 (4.5%) | |
|
| ||||
| Infarcts + LB | 1 (0.6%) | 0 | 1 (0.6%) | |
|
| ||||
| No AD, infarcts or LB | 77 (45.3%) | 35 (26.1%) | 11* (6.2%) | |
see text for pathologic diagnoses in those with probable AD without a pathologic diagnosis of AD, macroscopic infarcts or Lewy bodies
Less than half (n=76) of the 179 persons with probable AD, had “pure” AD pathology (AD without infarcts or LB). Only, 8 persons (4.5%) with probable AD had macroscopic infarcts without a pathologic diagnosis of AD; and only 2 persons had “pure” neocortical Lewy body disease (without AD or infarcts). One person had both infarcts and neocortical Lewy body disease. Overall macroscopic infarcts alone and “pure” Lewy body disease uncommonly accounted for a clinical diagnosis of probable AD.
Mild Cognitive Impairment
More than half of persons with MCI had a pathologic diagnosis AD; about 1/3 had macroscopic infarcts (table 1). About half of subjects with MCI had a single pathology (54.5%), most commonly AD pathology alone (n=47) or infarcts alone (n=21) (table 2). Mixed pathologies were about half as common in persons with MCI (19.4%) compared to probable AD (45.8%); but similar to probable AD the most common mixed pathology was AD with infarcts (n=23). Eight (6.0%) persons with MCI had neocortical Lewy bodies, half of whom also had AD pathology (table 2, figure). No person with MCI exhibited a combination of a pathologic diagnosis of AD, macroscopic infarcts, and neocortical Lewy bodies.
Because amnestic MCI is thought to be a relatively specific early sign of probable AD (19), we also investigated the common pathologies underlying the subtypes of amnestic (n=75) and non-amnestic MCI (n=59) (table 3). A pathologic diagnosis of AD was present in more persons with amnestic (59%) compared to non-amnestic MCI (49%) (table 3). Interestingly, in both subgroups, “pure AD” was the most frequent underlying pathology (36% amnestic and 33.9% in non-amnestic MCI). In both groups over 75% had an intermediate rather than high likelihood by NIA-Reagan criteria.
Table 3.
Number (%) of subjects with one, mixed, and no (AD or macroscopic infarcts or neocortical Lewy body) pathology with amnestic and non-amnestic MCI.
| Amnestic MCI | Non-Amnestic MCI | ||
|---|---|---|---|
| N=75 | N=59 | ||
|
| |||
| One pathology | 41 (54.7%) | 32 (54.2%) | |
|
| |||
| AD path diagnosis | 27 (36%) | 20 (33.9%) | |
| NIA- high | 6 (8.0%) | 4 (6.8%) | |
| NIA- intermediate | 21 (28.0%) | 16 (27.1%) | |
|
| |||
| Infarcts | 10 (13.3%) | 11 (18.6%) | |
|
| |||
| LB | 4 (5.3%) | 1 (1.7%) | |
|
| |||
| Mixed pathology | 17 (22.7%) | 9 (15.3%) | |
|
| |||
| AD + Infarcts | 15 (20.0%) | 8 (13.6%) | |
|
| |||
| AD + LB | 2 (2.7%) | 1 (1.7%) | |
|
| |||
| AD + Infarcts + LB | 0 | 0 | |
|
| |||
| Infarcts + LB | 0 | 0 | |
|
| |||
| No AD, infarcts or LB | 17 (22.7%) | 18 (30.5%) | |
Macroscopic cerebral infarcts without a pathologic diagnosis of AD, were more common in non-amnestic MCI (18.6%) compared to amnestic MCI (13.3%). Conversely, mixed pathologies were more frequent in amnestic (22.7%) vs. non-amnestic (15.3%); probably due to a higher percentage of persons with AD in the amnestic MCI group (table 3). There were too few persons in these groups for meaningful analyses.
No cognitive impairment
In the 170 persons without cognitive impairment, more than 50% had one or more of the three common pathologies, AD pathology, macroscopic infarcts, or neocortical Lewy Bodies (table 2, figure). Single pathologies (45.3%) were much more frequent than mixed pathologies (9.4%); and included a pathologic diagnosis of AD, followed by cerebral infarcts, and rarely neocortical Lewy bodies. In the subjects with multiple pathologies most had AD with infarcts. AD with neocortical LB, and infarcts and neocortical Lewy bodies were uncommon. Similar to the MCI group, none of the persons with no cognitive impairment exhibited all 3 pathologies.
Other Pathology
Other pathologies may also be combined with pathologic AD, macroscopic infarcts, and neocortical Lewy bodies, however their role in cognitive impairment is less clear.
Microscopic infarcts (without macroscopic infarcts) were present in 126 of 483 persons (26%); however, almost half (n=56) also had macroscopic infarcts. Microscopic infarcts without macroscopic infarcts were present in 28 persons with probable AD (15.6%), 13 persons with MCI (9.7%), and 29 persons with no cognitive impairment (17.1%). Microscopic infarcts were the only pathology to account for the cognitive impairment in four persons with probable AD (2.2%); and 3 persons with MCI (2.2%). Microscopic infarcts mixed with other pathologies increased the proportion of mixed pathologies in probable AD to 60.1% and in MCI to 26%.
Nigral-predominant or limbic-type Lewy body disease (without neocortical Lewy bodies) were present in 47 additional persons; 16 with probable AD, 14 MCI, and 17 no cognitive impairment. They were mixed with AD and/or infarcts in 15 of those with probable AD, 9 with MCI and 4 with no cognitive impairment. In one person with a clinical diagnosis of probable AD, limbic Lewy bodies accompanied a tauopathy (see below).
Hippocampal sclerosis was present in 23 persons (4.8%); 17 with probable AD, 4 with MCI and two with no cognitive impairment. In persons with a clinical diagnosis of probable AD, hippocampal sclerosis accompanied AD pathology alone (n=5), AD with infarcts (n=1), AD with Lewy bodies (n=2), AD with infarcts and Lewy bodies (n=6), infarcts alone (n=1), tauopathy (n=1), and frontotemporal dementia (n=1). In 3 persons with MCI, hippocampal sclerosis was mixed with “pure” AD pathology; in one person, it was the only pathology to explain the impairment.
Eleven persons with clinically probable AD, did not have pathologic AD, macroscopic infarcts, or neocortical Lewy bodies; pathologic diagnoses included frontotemporal dementia with ubiquitin-positive inclusions and hippocampal sclerosis (1), tauopathy, hippocampal sclerosis, and limbic Lewy bodies (1), multisystem atrophy (1), progressive supranuclear palsy (1), microscopic infarcts (4), dementia lacking distinctive histology (3).
Mixed pathologies and odds of MCI or probable AD
Ordinal logistic regression was used to determine how one or more pathologies influences the odds of having cognitive impairment. The proportional odds assumption required for ordinal logistic regression was not rejected (X62=8.64, p=0.19). Each of the pathologies contributed independently to the odds of probable AD, including a pathologic diagnosis of AD by 4.7 fold, infarcts by 64%, and Lewy bodies by 2.1 fold. (table 4). The addition of infarcts and/or Lewy bodies to a pathologic diagnosis of AD increased the odds of probable AD (table 4). For MCI the estimated odds ratio was the same multiple of the corresponding odds for probable AD. Therefore, having multiple pathologies was associated with a very high likelihood of impairment. Indeed, all three pathologies were only present in persons with probable AD.
Table 4.
Odds ratios (OR) and 95% confidence intervals (CI) for clinically probable AD*
| Neuropathologic diagnoses | OR | 95% CI |
|---|---|---|
| AD pathologic diagnosis | 4.7 | (3.2, 6.9) |
| Macroscopic infarct (s) | 1.6 | (1.1, 2.4) |
| Neocortical Lewy bodies | 2.1 | (1.3, 3.4) |
| AD pathologic diagnosis & macroscopic infarcts | 7.4 | (4.3, 13.6) |
| AD pathologic diagnosis & neocortical Lewy bodies | 9.9 | (5.2, 18.9) |
| AD pathologic diagnosis & macroscopic infarcts & neocortical Lewy bodies | 16.2 | (7.4, 35.7) |
odds ratios are multiplicative for odds of MCI or probable AD. See text for derivation & statistical models.
Discussion
In this longitudinal community-based clinical-pathologic study of 483 older persons we found that clinically probable AD is a heterogeneous and often mixed disorder. While nearly all persons with clinically probable AD were pathologically-confirmed to have AD at autopsy, almost half had AD pathology mixed with cerebral infarcts or neocortical Lewy bodies. The pathology underlying MCI is also heterogeneous; though AD is the most common pathology; infarcts and mixed pathologies also occur in many persons with MCI. We found that the cooccurrence of infarcts and neocortical Lewy bodies with AD pathology is not trivial but rather significantly increases the likelihood of a diagnosis of MCI or probable AD. Finally, we found that AD was the most frequent pathology in both amnestic and non-amnestic MCI; whereas “pure” infarct pathology (without a concomitant pathologic diagnosis of AD) was less frequent even in non-amnestic MCI.
Mixed pathologies have been identified as the most common substrate for dementia in several community-dwelling cohorts (2-4, 20). Because AD is the most common clinical diagnosis in older persons with dementia it is not unexpected that mixed pathologies would also be common in this group. Indeed, in this study, though nearly 90% of persons with clinically probable AD met pathologic criteria for AD, mixed pathologies were as common as “pure” AD pathology. Furthermore, mixed pathologies were associated with a marked increase in the odds of cognitive impairment. In other words, these additional pathologies were not innocent bystanders in the aging brain, but rather added to the likelihood that someone meeting pathologic criteria for AD would also meet clinical criteria for probable AD.
There have been few reports on the neuropathology of MCI (12-17). Though the operational definitions of MCI between studies differed, they similarly showed that persons with MCI often meet the minimal pathologic criteria for AD (12,14-16) and their level of AD pathology is typically intermediate between persons with and without clinical AD (12,13,15-17). While most studies have focused on AD pathology, additional pathologies, particularly vascular pathologies, appear to be common (11-16). Prior studies had relatively few persons with MCI, with the largest study including 57 subjects (16); further, they either included both amnestic and non-amnestic MCI as a single group (14-17) or were restricted to amnestic MCI (12-13). Two additional studies focused on the neuropathology of MCI after conversion to dementia; these showed the pathology of AD (33, 34) and mixed pathologies (34). Thus, the current study markedly extends and adds to these prior observations. Overall, there was substantial pathologic heterogeneity in MCI. In contrast to probable AD which was found to be almost always associated with a pathologic diagnosis of AD, about half of persons with MCI met pathologic criteria for AD, and few had high levels of pathology as reflected by a low proportion with high likelihood AD by NIA-Reagan criteria compared to probable AD. Interestingly, a pathologic diagnosis of AD was the most common pathology in both amnestic MCI and non-amnestic MCI; whereas, “pure” infarct pathology or Lewy body pathology accounted for less than a quarter of amnestic and non-amnestic MCI cases. This lack of specificity may be partly due to the observation that infarcts can damage brain regions associated with episodic memory while AD pathology frequently accumulates in non-memory regions. Indeed, we (35) and others (36-37) have shown that infarcts can affect episodic memory; and that AD pathology may affect non-memory functions (38).
We found that mixed pathologies are not an infrequent finding in MCI, particularly amnestic MCI. It is interesting to note the conceptual similarities between amnestic MCI and probable AD. A pathologic diagnosis of AD is common in both amnestic MCI and probable AD, and additional pathology increases the likelihood of cognitive impairment. Indeed, the proportion of persons with a single pathology in MCI differs little from persons with no cognitive impairment; yet, the number of persons with mixed pathology was twice that in comparison to those no cognitive impairment. These findings support the contention that MCI, especially amnestic MCI is often a synonym for early clinical AD. Finally, compared to amnestic MCI, non-amnestic MCI has a somewhat lower proportion of mixed pathologies; this is likely due to the fact that somewhat fewer persons had a pathologic diagnosis of AD. Larger numbers will be needed to determine the significance of these findings.
The observation that clinical AD is a pathologically heterogeneous and often mixed disorder has implications for the public health and medical research of our aging population. First, because infarcts are the most common unrecognized contributing pathology in clinical AD, prevention of infarcts is likely to be important for delaying onset and slowing cognitive decline in persons with AD pathology. From a public health perspective, measures that can improve vascular health, such as life style changes, and prevention and treatment of hypertension and diabetes, could decrease both the incidence and severity of clinical AD in the population.
Second, the prominent role of vascular disease in the clinical expression of MCI and AD means that inferences from epidemiologic studies that find associations between vascular risk factors and clinical AD need to be made with caution. In particular, one cannot assume that risk factors for clinical AD are also risk factors for AD pathology. For instance, diabetes, a known risk factor for stroke, has been linked to clinical AD (39-40); yet a relationship between diabetes and the pathology of AD remains unclear (41-42). Indeed, we previously found that diabetes is related to post-mortem cerebral infarctions but not to AD pathology (43). The biological mechanisms of other diverse posited risk factors for clinical AD such as hypertension, diet, and estrogen supplementation raises similar questions. To separate the vascular and degenerative aspects of the disease process will likely require studies that include both structural and amyloid neuroimaging, in addition to clinical-pathologic studies.
Finally, mixed pathologies in clinically probable AD and amnestic MCI also have implications for the implementation and interpretation of clinical trials. Given that many persons with clinically probable AD have unrecognized mixed pathologies, and a clinical diagnosis of probable AD does not ascertain pathologically “pure” disease, it's not clear that persons with known vascular disease should be excluded from AD clinical trials. Moreover, if a drug trial shows a clinical effect, interpretation of the biological mechanism could potentially be through a vascular mechanism. Indeed, if the conversion of MCI to AD and the progression of clinical AD in some individuals include vascular insults, then drugs with primarily vascular mechanisms could be beneficial for the prevention, slowing, and treatment of a subset of cognitive decline in persons with clinical AD, i.e. those with unrecognized mixed pathologies.
This study has important strengths. We used two community based studies that use standard, uniform clinical and pathologic procedures. Participants had high follow-up rates, high autopsy rates and were evaluated proximate to death. Clinical diagnoses were made blinded to pathology results, while final pathologic diagnoses were made without knowledge of clinical diagnoses.
There are also limitations. Selection of subjects who volunteer may introduce bias by effectively decreasing pathology; this, however, is partially mitigated by high follow-up and autopsy rates. Another potential source of bias is inherent to autopsy studies; subjects who are impaired and have pathology are at greater risk of death and deceased subjects are a nonrandom subset of the cohort. Thus, it is possible that persons with multiple brain pathologies may be more prone to come to autopsy than persons with only a single or no pathology. Thus, one should use caution when extrapolating findings from autopsy studies to the general population. Another limitation is the lack of routine neuroimaging. While neuroimaging would not likely have affected the classification of probable AD since most criteria for vascular cognitive impairment require a temporal relation between a clinical vascular event and cognitive decline (44), it is likely that some persons with possible AD would not have been found to have vascular disease and would have been included in the probable AD group. While the numbers are likely to be small, it's not clear how this would have affected our findings. Conversely, we did not include microscopic infarcts in our tables or analyses; and these would have further increased the numbers with mixed pathologies. Moreover, imaging studies suggest that white matter changes, some of which appears to be of vascular origin (45), also contribute to cognitive impairment. Finally, we included neocortical Lewy bodies, but not limbic or nigral Lewy bodies as a mixed pathology. Together, these data suggest that we may be underestimating the extent to which co-existing conditions contribute to probable AD and MCI.
Acknowledgements
The authors thank the more than 1,100 nuns, priests, and brothers from across the country participating in the Religious Orders Study, and the more than 1,200 older persons from across northeastern Illinois participating in the Rush Memory and Aging Project. The authors thank the staff of the Rush Alzheimer's Disease Center.
Supported by the National Institute on Aging (R01AG15819, R01AG17917, P30AG10161, K08AG00849, K23AG23675)
Footnotes
Disclosure: The authors have reported no conflicts of interest.
References
- 1.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–87. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 2.White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in later life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18(4):224–7. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- 3.Neuropathology Group of the Medical Research Council Cognitive Function and Aging Study (MRC CFAS) Pathologic correlates of late onset dementia in a multicentre, community based population in England and Wales. Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 4.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007 Oct;62(4):406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 5.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007 Dec;66(12):1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esiri MM, Nagy Z, Smith MZ, et al. Cerebrovascular disease and the threshold for dementia in the early stages of Alzheimer's disease [letter] Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 7.Zekry D, Duyckaerts C, Moulias R, et al. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol. 2002;103:481–487. doi: 10.1007/s00401-001-0493-5. [DOI] [PubMed] [Google Scholar]
- 8.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of AD. The nun study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 9.Riekse RG, Leverenz JB, McCormick W, et al. Effect of vascular lesions on cognition in Alzheimer's disease: a community-based study. J Am Geriatr Soc. 2004 Sep;52(9):1442–8. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massoud F, Devi G, Stern Y, et al. A clinicopathological comparison of community-based and clinic-based cohorts of patients with dementia. Arch Neurol. 1999;56(11):1368–73. doi: 10.1001/archneur.56.11.1368. [DOI] [PubMed] [Google Scholar]
- 11.Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer's disease in a community-based case series. J Am Geriatr Soc. 1999 May;47(5):564–9. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 12.Markesbery WR, Schmitt FA, Kryscio RJ, et al. Neuropathologic Substrate of Mild Cognitive Impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63(5):665–72. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 14.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol. 2005;62(5):758–65. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005 Mar 8;64(5):834–41. 13. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 16.Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007 Dec;27(6):578–84. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 17.Sabbagh MN, Shah F, Reid RT, et al. Pathologic and nicotinic receptor binding differences between mild cognitive impairment, Alzheimer disease, and normal aging. Arch Neurol. 2006 Dec;63(12):1771–6. doi: 10.1001/archneur.63.12.1771. [DOI] [PubMed] [Google Scholar]
- 18.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002 Feb;51(2):145–55. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004 Sep;256(3):183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed Brain Pathologies Account for Most Dementia Cases in Community-Dwelling Older Persons. Neurology. 2007 Dec 11;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Beckett LA, Barnes LL, et al. Individual Differences in Rates of Change in Cognitive Abilities of Older Persons. Psychology and Aging. 2002;17(2):179–93. [PubMed] [Google Scholar]
- 22.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–75. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident AD. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 24.Bennett DA, Wilson RS, Schneider JA, et al. The natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RS, Barnes LL, Kreuger KR, et al. Early and late life cognitive activity and cognitive systems in old age. JINS. 2005;11:400–407. [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer's disease pathology. Neurology. 2004;62:1148–1156. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 28.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 30.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18(4 Suppl):S1–2. [PubMed] [Google Scholar]
- 31.Agresti A. Categorical Data Analysis. 2nd edition Wiley; 2002. [Google Scholar]
- 32.SAS Institute Inc. SAS OnlineDoc® 9.1.3. SAS Institute Inc.; Cary, NC: 2002-2005. [Google Scholar]
- 33.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001 Mar;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 34.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006 May;63:674–81. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JA, Boyle PA, Arvanitakis Z, et al. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007 Jul;62(1):59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 36.Reitz C, Luschsigner JA, Tang M-X, et al. Stroke and Memory Performance in Elderly Persons without dementia. Arch Neurol. 2006;63:571–576. doi: 10.1001/archneur.63.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Werf YD, Scheltens P, Lindeboom J, et al. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41(10):1330–44. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 38.Buchman AS, Schneider JA, Wilson RS, et al. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006 Dec 12;67(11):1949–54. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 39.Irie F, Fitzpatrick AL, Lopez OL, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol. 2008 Jan;65(1):89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rönnemaa E, Zethelius B, Sundelöf J, et al. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008 Apr 9; doi: 10.1212/01.wnl.0000310646.32212.3a. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Peila R, Rodriguez BL, Launer LJ, et al. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002 Apr;51(4):1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 42.Beeri MS, Silverman JM, Davis KL, et al. Type 2 diabetes is negatively associated with Alzheimer's disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005 Apr;60(4):471–5. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arvanitakis A, Schneider JA, Wilson RS, et al. Diabetes is related to Cerebral infarction but not to AD pathology in older persons. Neurology. 2006 Dec 12;67(11):1960–5. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 44.Chui H, Mack W, Jackson J, et al. Clinical criteria for the diagnosis of vascular dementia: A mulitcenter study of comparability and interrater reliability. Arch Neurol. 2000;57:191–196. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- 45.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008 Aug 6; doi: 10.1212/01.wnl.0000319691.50117.54. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]