Abstract
Cervical cancer kills 260,000 women annually, and nearly 85% of these deaths occur in developing nations, where it is the leading cause of cancer deaths in women. Disparities of health and poverty play a large role in this high mortality rate. Whereas routine Papanicolaou and human papillomavirus (HPV) testing has dramatically reduced cervical cancer deaths in Western nations, without proper infrastructure, facilities, and medical training, the rates of cervical cancer in developing nations will remain high. Studies on HPV DNA testing and the low-technology method of “screen and treat” are promising. In addition, reducing the cost and increasing the availability of HPV vaccines in developing nations brings hope and promise to the next generation of women.
Key words: Cervical cancer, Human papillomavirus, Human papillomavirus DNA testing, Human papillomavirus vaccine
In the 1970s, Harald zur Hausen detected the human papillomavirus (HPV) in warts and cervical cancer, and he subsequently isolated and cloned different strains of HPV. His research concluded that patients infected with HPV types 16 and 18 were at an increased risk of developing cancer. In 2008, Dr. Hausen received the Nobel Prize for his groundbreaking work on HPV.1
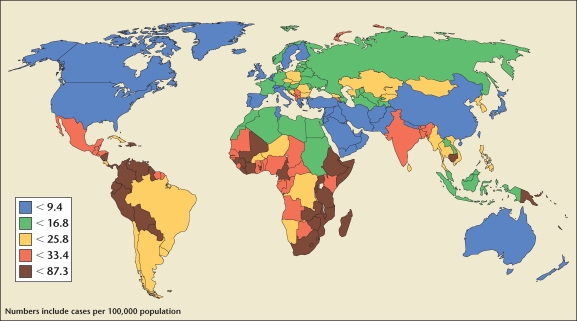
Human papillomavirus can cause cancer of the cervix, vagina, vulva, penis, and anus, as well as some head and neck cancers, anogenital warts, and recurrent respiratory papillomatosis. The World Health Organization (WHO) estimates that of the 500,000 new cases annually, 80% affect women between the ages of 15 and 45 years who live in developing nations. It predominantly impacts women living in Latin America and the Caribbean, sub-Saharan Africa, and Southeast Asia (Figure 1). Only 5% of women in these regions have been screened for cervical disease in the past 5 years.
Figure 1.
Global burden of cervical cancer greatest in developing countries. Reproduced with permission from Women Deliver. http://www.womendeliver.org/blog/wp-content/uploads/2009/08/cervicalcancer1.jpg.
Human Papillomavirus
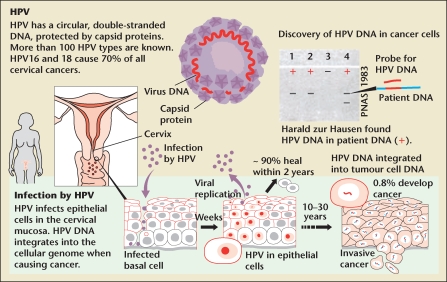
Human papillomavirus is a nonenveloped, double-stranded DNA virus. Its genome is enclosed in a capsid shell involving major and minor structural proteins (L1 and L2, respectively). It is predominantly spread through sexual contact. There are over 100 subtypes of HPV and the virus is found in 99.7% of women with cervical cancer. HPV types 16 and 18 cause over 70% of cervical cancer.
Once HPV enters the host, it develops an infection in the intraepithelial layer of the mucosa (Figure 2). Although 50% of patients develop serum antibodies, the antibodies are not effective unless they target the L1 protein. Seroconversion occurs in approximately 8 to 12 months. Once infected, the cells can develop precancerous properties that lead to cervical intraepithelial neoplasia (CIN) or adenocarcinoma in situ (AIS). Untreated, CIN grade II/III and AIS can develop into squamous cell carcinoma or adenocarcinoma, respectively.2
Figure 2.
The human papilloma virus (HPV). Reproduced with permission from The Nobel Committee for Physiology or Medicine 2008. http://img.thebody.com/press/2008/nobel_hpv.gif.
In Western nations, the cytologybased Papanicolaou test and HPV DNA tests are used for diagnosing precancerous and cancerous cells. Subsequent follow-up involves the use of colposcopy to evaluate the cervix and obtain tissue samples for biopsy. As a result, developed nations have seen a major decline in cervical cancer deaths. In contrast, women in low-resource settings are not benefiting from these technological advances. The screening and diagnosis of cervical cancer is a challenge given the lack of funding, infrastructure, and trained pathologists. As a result, other innovative approaches that are affordable, efficient, and effective have been studied in an attempt to improve cervical cancer outcome in developing nations.3–8
Risk Factors
Because cervical cancer is caused by a sexually transmitted virus, the risk factors are the same as other sexually transmitted infections: early age at first sexual activity, multiple sexual partners, early age at first delivery, increased number of pregnancies, smoking, immunosuppression (eg, human immunodeficiency virus [HIV] or medication), and long-term oral contraceptive use. Preventive health measures should ensure education on safe sexual behavior. However, in some regions, social customs can place women at an increased risk of contracting HPV; it has become endemic in regions that support child marriages, polygamy, high parity, and long-term oral contraceptive use. Women are at high risk of cervical cancer if they are of low socioeconomic status, have poor access to health care, and have husbands with a history of multiple sexual partners.9–13 The low status of women and their lack of empowerment play a significant role in the high rate of cervical cancer. These factors impose challenges on health providers who seek to educate, and on the women themselves, who may hesitate to seek adequate health care.
Preventive Measures
Screen and Treat
Studies are proving that screen-and-treat programs are effective. The steps are simple: women are initially counseled regarding the risks and benefits of screen-and-treat programs. They undergo a speculum examination, and acetic acid (3%–5%) is applied to the cervix. The cervix is visually inspected (at times with only a flashlight). If acetowhite lesions are noted, the findings are discussed with the patient and she is offered immediate treatment with cryotherapy. Visual inspection with acetic acid (VIA) has a sensitivity of 77% and a specificity of 86%.
A study in Thailand recruited 5999 women, of whom 789 (13.3%) tested positive after VIA; 738 (92.5%) agreed to undergo cryotherapy. There was a very high correlation between the VIA findings of nurses and gynecologists (92%) and their decision to treat (93%), which demonstrated the ease with which health providers could be trained to perform the screen-andtreat technique.3 Another study randomized clusters into VIA and control groups (standard care) in India. Those who tested positive underwent colposcopy, directed biopsy, and cryotherapy by nurses during the same visit. Of the 31,343 who underwent VIA, 3088 (9.9%) screened positive, 3052 had colposcopy (9.7%), and 2539 (8.1%) had directed biopsies. There were 97 and 1303 deaths in the VIA group and the control group, respectively. There was a significant decline in cervical burden detected within 5 to 7 years. The study stressed the impracticality of multiple visits for diagnosis and treatment and that a single VIA screening could effectively reduce cervical cancer incidence and death.14
HPV DNA Testing
A recent study compared HPV DNA testing, cytologic testing, VIA, and standard care on the incidence of cervical cancer in India. Of the 131,749 eligible women in this study, the HPV-tested group showed a significant reduction in the number of advanced cervical cancer and cervical cancer deaths when compared with the standard care group. Interestingly, there was no reduction in advanced cervical cancers or cervical cancer deaths when compared with the control group in either the VIA or the cytologic testing group.15 No cancer deaths were found in HPV-negative women after 8 years. The high negative predictive value indicated that if a woman tested negative, she would not need to be screened for many years. The authors concluded that HPV screening could potentially lower the mortality rate within 5 to 10 years. However, they could not explain the discrepancies between this and their earlier study.14 Trials in Europe and North America have demonstrated that HPV screening is more sensitive at detecting precancerous abnormalities than cytologic testing.16
The HPV DNA test that was used for this study (Hybrid Capture II; Digene Corporation, Gaithersburg, MD) costs $20 to $30 per test. This makes HPV DNA tests unavailable in resourcepoor settings. However, the authors stated that a “simple, affordable, and accurate” HPV test with sensitivity of 90.2% and specificity of 84.2% was expected to be commercially available. This test (careHPV, Qiagen, Venlo, The Netherlands) will run on batteries and does not require water or refrigeration. It may cost $5 or less and produces results in 3 hours, enabling treatment during a single visit.17 This technique, if affordable, would not only make a significant impact on disease burden, but might be more preferable to women themselves. For women who are concerned about male providers or are simply nervous about pelvic examinations, performing their own vaginal swabs might encourage them to come to health centers and thus increase the number of women screened.
The HPV Vaccine Controversy
In June 2006, the US Food and Drug Administration (FDA) approved the Gardasil® vaccine (Merck & Co., Inc., Whitehouse Station, NJ), which provides 100% protection against HPV type 16 and 18, and protects against HPV type 6 and 11. Bivalent Cervarix ® (GlaxoSmithKline, Research Triangle Park, NC) against HPV type 16 and 18 was first licensed in 2007 and was initially approved only in Europe. In October 2009, the FDA approved its use in the United States. Studies show that Cervarix is 93% effective in preventing cervical precancerous changes. Both vaccines require a series of 3 0.5-mL intramuscular injections. The vaccines form virus-like particles, are noninfectious, and are designed for prophylaxis only. Western nations have been tackling questions that address who requires the vaccine and at what age girls should receive it. The US Advisory Committee on Immunization Practices recommends that girls and women between the ages of 9 and 26 years should receive the quadrivalent vaccine. The WHO position paper on the HPV vaccine recommends that it should be a part of national immunization programs. WHO recommends the vaccine be given between the ages of 9 and 13 years, prior to a girl’s first coitus. However, they acknowledged nationwide administration of HPV vaccine would only be cost effective in countries that have high gross domestic products.2
Gardasil costs approximately $90 per dose ($270 for 3 doses). Several models have calculated that vaccinating girls in resource-poor settings would be cost effective only if the 3-dose vaccine (including vaccination delivery and education) was between $10 and $25.18 Tiered pricing with the help of WHO, international organizations, and other funding sources might make this possible. However, the HPV vaccine is not an immediate panacea. Even if the vaccine were affordable and widely available in resource-poor settings, the rate of cervical cancer would not decline for decades as a result of the latency phase between infection and cancer.16
Conclusions
Cervical cancer prevention in resource-poor settings requires affordable and effective screening programs that are designed to incorporate communities and their needs. It is evident that regular Papanicolaou tests are impractical. The screen-and-treat technique has several advantages, including its simplicity and sensitivity, lack of intensive training requirements, and immediate results with treatment. HPV DNA testing is currently impractical. If careHPV becomes available and affordable, its simplicity, sensitivity, and speed in obtaining results could greatly impact the future of cervical cancer. Unless funding becomes available to bear the cost of the HPV vaccine, it will not realistically be available to women in developing nations. However, none of these technologies will truly be effective without outreach programs that educate women and their communities on the prevention of cervical cancer. Unless we are able to address the marginalization of women in these regions and empower them to make decisions about their lives and health, introducing simple or complex technologies will not reach girls and women in developing nations.
Main Points.
The World Health Organization estimates that of the 500,000 new cases of cervical cancer annually, 80% affect women between the ages of 15 and 45 years who live in developing nations.
Because cervical cancer is caused by a sexually transmitted virus, the risk factors are the same as other sexually transmitted infections: early age at first sexual activity, multiple sexual partners, early age at first delivery, increased number of pregnancies, smoking, immunosuppression (eg, human immunodeficiency virus or medication), and long-term oral contraceptive use. Social customs can place women at an increased risk of contracting human papilloma virus (HPV); it has become endemic in regions that support child marriages, polygamy, high parity, and long-term oral contraceptive use.
Papanicolaou testing, HPV DNA testing, and the HPV vaccine are not realistically available to women in developing nations.
The screen-and-treat technique has several advantages, including its simplicity and sensitivity, lack of intensive training requirements, and immediate results with treatment.
References
- 1.The Nobel Prize in Physiology or Medicine 2008 [press release] Stockholm, Sweden: The Nobel Assembly at Karolinska Institutet; 2008. Oct 6, [Accessed November 20, 2009]. http://nobelprize.org/nobel_prizes/medicine/laureates/2008/press.html. [Google Scholar]
- 2.World Health Organization, authors. Weekly Epidemiological Record. Geneva;: World Health Organization; 2009. [Accessed November 20, 2009]. Human papillomavirus vaccines. http://www.who.int/wer/2009/wer8415.pdf. [Google Scholar]
- 3.Gaffikin L, Blumenthal PD, Emerson M, Limpaphayom K Royal Thai College of Obstetricians and Gynaecologists (RTCOG)/JHPIEGO Corporation Cervical Cancer Prevention Group (JCCCPG), authors Safety, acceptability, and feasibility of a singlevisit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet. 2003;361:814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- 4.Sankaranarayanan R, Nene BM, Dinshaw KA, et al. Osmanabad District Cervical Screening Study Group, authors. A cluster randomized controlled trial of visual, cytology and human papillomavirus screening for cancer of the cervix in rural India. Int J Cancer. 2005;116:617–623. doi: 10.1002/ijc.21050. [DOI] [PubMed] [Google Scholar]
- 5.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Alliance for Cervical Cancer Prevention Cost Working Group, authors. Cost-effectiveness of cervicalcancer screening in five developing countries. N Engl J Med. 2005;353:2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 6.Denny L, Kuhn L, De Souza M, et al. Screen-andtreat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294:2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 7.Sankaranarayanan R, Gaffikin L, Jacob M, et al. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89(suppl 2):S4–S12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Jacob M, Broekhuizen FF, Castro W, Sellors J. Experience using cryotherapy for treatment of cervical precancerous lesions in low-resource settings. Int J Gynaecol Obstet. 2005;89(suppl 2):S13–S20. doi: 10.1016/j.ijgo.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Schmauz R, Okong P, de Yilliers E, et al. Multiple infections in cases of cervical cancer in tropical Africa. Int J Cancer. 1989;43:805–809. doi: 10.1002/ijc.2910430511. [DOI] [PubMed] [Google Scholar]
- 10.Serwadda D, Wawer MJ, Shah KV, et al. Use of a hybrid capture assay of self-collected swabs in rural Uganda for detection of human papillomavirus. J Infect Dis. 1999;180:1316–1319. doi: 10.1086/315026. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn L, Denny L, Pollack A, et al. Human papillomavirus DNA testing for cervical cancer screening in low-resource settings. J Natl Cancer Inst. 2000;92:818–825. doi: 10.1093/jnci/92.10.818. [DOI] [PubMed] [Google Scholar]
- 12.Bayo S, Boxch FX, de Sanjose S, et al. Risk factors of invasive cervical cancer in Mali. Int J Epidemiol. 2002;31:202–209. doi: 10.1093/ije/31.1.202. [DOI] [PubMed] [Google Scholar]
- 13.Chaouki N, Boschi FX, Muñoz N, et al. The viral origin of cervical cancer in Rabat, Morocco. Int J Cancer. 1998;75:546–554. doi: 10.1002/(sici)1097-0215(19980209)75:4<546::aid-ijc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Sankaranarayanan R, Esmy PO, Rajkumar M, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med. 2009;360:1453–1455. doi: 10.1056/NEJMe0901167. [DOI] [PubMed] [Google Scholar]
- 17.Qiao YL, Sellors JW, Eders PS, et al. A new HPVDNA test for cervical-cancer screening in developing regions: a cross sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 18.Goldie SJ, O’Shea M, Diaz M, Kim SY. Benefits, cost requirements and cost-effectiveness of the HPV16, 18 vaccine for cervical cancer prevention in developing countries: policy implications. Reprod Health Matters. 2008;16:86–96. doi: 10.1016/S0968-8080(08)32409-4. [DOI] [PubMed] [Google Scholar]