Abstract
The prevalence of polycystic ovary syndrome (PCOS) is estimated to be nearly 10% among reproductive-age women. PCOS may represent the largest underappreciated segment of the female population at risk of cardiovascular disease. Clinicians providing care to women of childbearing age must recognize the presenting clues, including irregular menses, hirsutism, alopecia, hyperandrogenemia, and obesity. The pathophysiology of PCOS is complex, involving the hypothalamus-pituitary-ovarian axis, ovarian theca cell hyperplasia, hyperinsulinemia, and a multitude of other cytokine- and adipocyte-driven factors. Cardiac risk factors associated with PCOS have public health implications and should drive early screening and intervention measures. There are no consensus guidelines regarding screening for cardiovascular disease in patients with PCOS. Fasting lipid profiles and glucose examinations should be performed regularly. Carotid intimal medial thickness examinations should begin at age 30 years, and coronary calcium screening should begin at age 45 years. Treatment of the associated cardiovascular risk factors, including insulin resistance, hypertension, and dyslipidemia, should be incorporated into the routine PCOS patient wellness care program.
Key words: Polycystic ovary syndrome, Cardiovascular disease, Diabetes, Obesity, Carotid intimal medial thickness, Coronary calcium screening
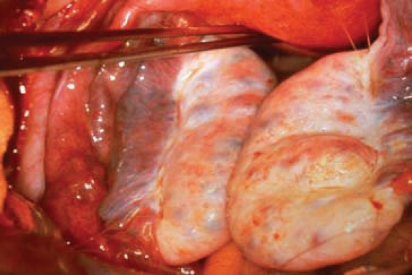
In 1935, Stein and Leventhal1 first described 7 female patients with unexplained chronic anovulation. During surgery, these women were found to have bilateral polycystic ovaries. Based on these observations, Stein and Leventhal characterized polycystic ovary syndrome (PCOS)-a symptom complex that includes amenorrhea, hirsutism, and enlarged polycystic ovaries (Figure 1). In the past decade, researchers have accumulated a wealth of clinical data that not only demonstrate the effects of PCOS on female reproductive function, but also its metabolic and cardiovascular implications. In 2003, the Rotterdam Consensus Workshop group proposed that the diagnosis of PCOS be made only after the exclusion of other medical conditions that cause irregular menstrual cycles and androgen excess. At least 2 of the following symptoms must be present for diagnosis: oligoovulation or anovulation (usually manifested as oligomenorrhea or amenorrhea), elevated levels of circulating androgens (hyperandrogenemia) or their clinical manifestations (hyperandrogenism), and polycystic ovaries as defined by ultrasonography (ultrasonographic criteria of a mean of 12 follicle numbers per ovary of both ovaries or ovarian volume of 10 mL [calculated using the equation 0.5 × length × width × thickness]).2 Both PCOS and the cardiometabolic syndrome share insulin resistance as a pathogenic feature. The incidence of impaired glucose tolerance, type 2 diabetes mellitus, obesity, hypertension, and dyslipidemia, as well as of coronary and vascular disease, may be higher in women with PCOS during their reproductive years than in patients with the cardiometabolic syndrome.
Figure 1.
Enlarged ovaries in a woman with polycystic ovary syndrome. (Photograph by Carolyn J. Alexander, MD.)
The published prevalence estimates of PCOS range from 4% to 7% among selected samples of women screened for this condition.3 The prevalence and characteristics of women with PCOS among broader and diverse populations in usual care settings is unknown and may be significantly higher.
Traits of PCOS
Hypothalamus-Pituitary-Ovarian Axis
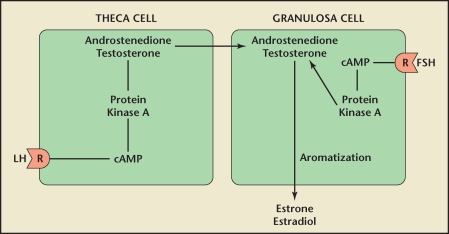
Although many experts would agree that anovulation and hyperandrogenemia represent fundamental cornerstones of PCOS, the actual pathophysiologic mechanisms are complex and often highly debated. In the hypothalamus-pituitary-ovarian axis, the hypothalamus secretes gonadotropin-releasing hormone in a pulsatile fashion. During the follicular phase of the menstrual cycle, ovarian theca cells respond to luteinizing hormone (LH) by increasing androgenic precursor output (Figure 2). Meanwhile, increasing levels of folliclestimulating hormone (FSH) stimulate ovarian granulosa cells to convert these androgens into estrogens (estradiol) that assist in follicular development.4
Figure 2.
During the follicular phase of the menstrual cycle, ovarian theca cells respond to the luteinizing hormone (LH) by increasing androgenic precursor output. Follicle-stimulating hormone (FSH) stimulates granulose cells to aromatize androgens into estrogens, which ultimately end up in the blood stream. cAMP, cyclic adenosine monophosphate. Reprinted with permission from Speroff L and Fritz MA.46
In the setting of chronic anovulation, the characteristic ebb and flow of LH and FSH does not occur. Instead, a “steady state” of LH and sex steroids rises in the blood stream.5 Although this observation is not part of the current diagnostic criteria, it has been found that LH levels are high and FSH levels are normal to low. High estrogen levels exert negative feedback on the pituitary gland, and, ultimately, on FSH secretion. Theca cell hyperplasia ensues, thereby leading to a hyperandrogenic state that presents clinically as oligomenorrhea and chronic anovulation.
Many of the traits found in PCOS overlap with those in the metabolic syndrome, such as dyslipidemia, obesity, insulin resistance, and hypertension.6 Glueck and colleagues7 concluded that 46% of women with PCOS also have the metabolic syndrome. Taken together, these factors affect the lipid profile of the PCOS patient. It is well known that the metabolic syndrome is associated with an increased risk of developing diabetes mellitus8 and cardiovascular disease.9
Obesity
Additionally, studies have shown that the increased waist-to-hip ratio associated with PCOS places these patients at a higher risk of dyslipidemia due to the adverse effect on blood lipids of central adipocytes.10 Central fat is more insulin resistant than is peripheral fat.11 The hyperandrogenemia associated with PCOS also appears to be related to lipid metabolism. According to a study examining the direct effect that testosterone plays on adipocytes, the investigators demonstrated induction of androgen receptor-mediated insulin resistance via testosterone.12
Hyperandrogenemia, Hypertriglyceridemia, Hyperinsulinemia
Approximately 70% of PCOS patients exhibit an abnormal serum lipid profile.13 The lipid abnormalities present are similar to those observed in diabetic patients who have elevated levels of low-density lipoprotein cholesterol (LDL-C) and triglycerides and lower levels of high-density lipoprotein cholesterol (HDL-C). Hyperinsulinemia and hyperandrogenemia cause adipocytes to undergo increased catecholamine-induced lipolysis and release of free fatty acids into the circulation. Increased free fatty acids in the liver stimulate secretion of very low-density lipoprotein (VLDL), which ultimately leads to hypertriglyceridemia.14
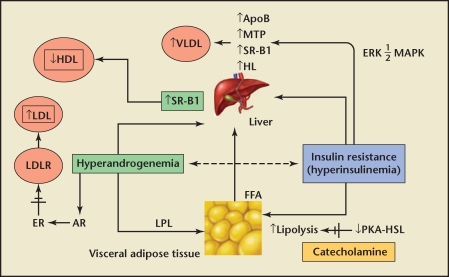
A fundamental element surrounding PCOS is insulin resistance. Legro and colleagues15 found that 81% of insulin-resistant PCOS patients demonstrated lipid abnormalities, compared with 65% of those with normal insulin sensitivity. It has been postulated by Wetterau and coworkers16 that insulin inhibits the expression of the microsomal triglyceride protein, which is responsible for the secretion of apolipoprotein B (apoB) and VLDL. Insulin resistance leads to hepatic overproduction of apoB and VLDL and, ultimately, to hypertriglyceridemia (Figure 3). PCOS patients share many characteristics with patients who have the metabolic syndrome, and thus PCOS patients have increased risk factors for cardiovascular disease-although this risk profile may not contribute to increased mortality.
Figure 3.
Insulin resistance leads to hepatic overproduction of apoB and VLDL (which ultimately leads to hypertriglyceridemia). HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; ER, estrogen receptor; AR, androgen receptor; VLDL, very low-density lipoprotein; SR-B1, scavenger receptor B1; LPL, lipoprotein lipase; ApoB, apolipoprotein B; MTP, microsomal triglyceride protein; HL, hepatic lipase; FFA, free fatty acid; ERK ½ MAPK, extracellular signal regulated kinase ½ mitogen-activated protein kinase; PKA-HSL, protein kinase A hormone sensitive lipase complex. Reprinted from TRENDS in Endocrinology and Metabolism, Volume 18, Diamanti-Kandarakis E et al. Pathophysiology and types of dyslipidemia in PCOS. Pages 288–285.14 Copyright © 2007, with permission from Elsevier.
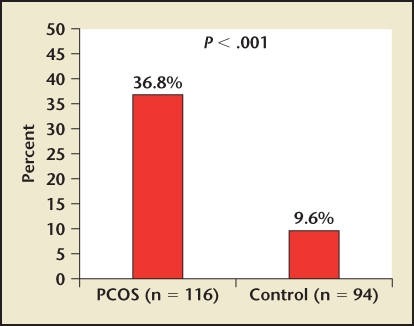
PCOS has also been found on multivariate analysis to be an independent risk factor for the development of diabetes, dyslipidemia, obesity, and hypertension.17 C-reactive protein (CRP), a marker of inflammation, has been found to correlate with rates of cardiovascular events, including myocardial infarction, stroke, sudden cardiac death, and peripheral vascular disease.18 Speculation exists as to whether CRP, which is a product of activated macrophages, is solely a marker of the inflammatory process associated with atherosclerosis or if it plays an active role in the development of this vascular disease. A recent study has confirmed the increased prevalence (> 4 times) of elevated CRP levels (> 5 mg/L) in patients with PCOS (Figure 4).19
Figure 4.
Patients with PCOS have an increased prevalence (> 4 times) of elevated C-reactive protein levels (> 5 mg/L). PCOS, polycystic ovary syndrome. Reprinted with permission from Boulman N et al.19
The recently reported Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) showed that patients with no apparent cardiovascular disease and lower cholesterol levels but with elevated levels of high-sensitivity CRP (hs-CRP) benefited from the use of statins in reducing cardiovascular event rates.20 Matrix metalloproteinases (MMPs) are a family of zincbinding proteolytic enzymes that normally remodel the extracellular matrix. Increased activity of MMPs has been reported in numerous disease processes, including atherosclerosis and cardiovascular disease. Increased expression of selected MMPs, particularly MMP-9, has been demonstrated in the vulnerable regions of human atherosclerotic plaques. MMP-9 belongs to the family of gelatinases that attack type IV collagen, laminin, and fibronectin.21 After these vascular proteins are attacked, it is believed that the coronary plaques are more vulnerable to rupture and thrombosis, which leads to acute vascular syndromes. Interestingly, MMP-9 is also expressed in the human ovary, and it is necessary for follicular rupture and oocyte release.22 These observations may be particularly relevant to PCOS patients.
Abnormalities of coagulation that may contribute to an increase in atherosclerotic events have also been studied in the PCOS population. A recent report has shown that in PCOS patients without diabetes, global fibrinolytic activity was reduced compared with control subjects matched for age and body mass index (BMI).23 Studies evaluating the levels of specific proteins have not consistently shown them to be abnormal in PCOS.
The clustering of cardiac risk factors with PCOS, one of the most common reproductive abnormalities in young women, would seem to have public health implications if it were to be associated with an increased risk for the development of cardiovascular disease. Studies using noninvasive vascular assessment of atherosclerotic plaque, such as coronary calcium and carotid intimal medial thickness (cIMT) assessments, have confirmed a greater prevalence of disease in PCOS patients relative to the general population. In a recent evaluation of premenopausal women ages 30 to 45 years, the incidence of coronary calcium was significantly higher in women with PCOS, at 39%, compared with age- and weight-adjusted controls (21%) and community-dwelling women of similar age (only 9.9%).24 Mean calcium scores were higher in the PCOS cohort compared with the age- and weight-adjusted controls. PCOS patients have also been found to have a higher prevalence of aortic calcification, another marker of atherosclerotic disease.25 Increases in cIMT thickness have been associated with an increase in cardiovascular events, in particular, stroke. Multiple investigators have found that patients with PCOS have a greater prevalence of abnormal cIMTs than the general population.26,27 In addition, Talbott and colleagues28 found a relationship between the degree of cIMT thickening in PCOS patients and levels of CRP. In a cohort of women with a mean age of 33 years, although there were no differences in cIMT, earlier manifestations of vascular disease, including abnormalities in pulsewave velocity and brachial arterial flow-mediated vasodilation, were found.29
Evaluation
Laboratory Studies
It is essential when diagnosing PCOS to establish that the patient is euthyroid, has normal levels of prolactin and 17-hydroxyprogesterone, and has no manifestations of Cushing’s syndrome. Although PCOS patients present with irregular periods and hirsutism or alopecia, laboratory studies are necessary to exclude other endocrinologic abnormalities or even life-threatening adrenal or ovarian tumors. It is important to note that a normal LH:FSH ratio does not exclude PCOS as a possible diagnosis. An elevated FSH (particularly on day 3) may help ascertain premature ovarian failure as a cause of amenorrhea. To rule out androgen-producing tumors, total and free testosterone, 17-hydroxyprogesterone, and dehydroepiandrosterone sulfate (DHEAS) are evaluated. Total testosterone reference values range from 6 ng/dL to 86 ng/dL; levels greater than 200 ng/dL favor a virilizing tumor over PCOS. The differential diagnoses of a significantly elevated testosterone level include Sertoli-Leydig tumor, hilar cell tumor, and luteoma of pregnancy. The primary source of DHEAS comes from the adrenal glands; levels greater than 700 µg/dL suggest a virilizing adrenal tumor. In screening for late-onset congenital adrenal hyperplasia, 17-hydroxyprogesterone levels may be drawn. A level exceeding 5 ng/mL is diagnostic for late-onset congenital adrenal hyperplasia. For intermediate values between 2 ng/dL and 5 ng/dL, an adrenocorticotropic hormone stimulation test should be performed. For patients in whom Cushing’s syndrome is suspected, a 24-hour urine collection for free cortisol or, alternatively, a 1-mg dexamethasone suppression test can be done.
2-Hour Glucose Tolerance Test With Glucose and Insulin Measurements
In addition to endocrine laboratory tests, glucose testing is also imperative in the evaluation of patients with PCOS. It is well documented that insulin resistance and resultant hyperinsulinemia affects approximately 65% to 70% of PCOS patients.13 According to a study by Legro and colleagues,15 a ratio of fasting glucose to fasting insulin that is less than 4.5 predicts insulin resistance. However, because of the large range of variability in insulin levels (particularly among different ethnicities), the 2-hour oral glucose tolerance test is the preferred method of assessment. In this test, a 75-g oral load of glucose is administered (Table 1). All anovulatory women who are hyperandrogenic should be tested for glucose tolerance and insulin resistance with the 2-hour glucose tolerance test.5
Table 1.
Interpretation of 2-Hour Glucose Test
| 2-Hour Glucose Response | |
| Normal | < 140 mg/dL |
| Impaired | 140–199 mg/dL |
| Type 2 diabetes (non-insulin dependent) | >200 mg/dL |
| 2-Hour Insulin Response | |
| Insulin resistance very likely | 100–150 µU/mL |
| Insulin resistance | 151–300 µU/mL |
| Severe insulin resistance | 300 µU/mL |
Reprinted with permission from Speroff L and Fritz MA.47
Pelvic Ultrasound Imaging
Historically, due to the heterogeneous nature of PCOS, experts have debated the way that ultrasound may be used in PCOS diagnosis. Currently, no universal agreement on the specific criteria for diagnosis of PCOS ovaries on ultrasound exists; however, it is generally accepted that the ovary increases in size due to the increased number of follicles.30,31 According to the 2003 Rotterdam criteria, ultrasound characteristics must include either more than 12 follicles measuring 2 mm to 9 mm in diameter or increased ovarian volume exceeding 10 cm3. These specifications were agreed upon at the Rotterdam Consensus Workshop on PCOS in 2003.32 Figure 5 shows 12 ovarian cysts of 2 mm to 5 mm in diameter spanning the perimeter of the left and right ovaries.
Figure 5.
Shown here are 12 ovarian cysts of 2 mm to 5 mm in diameter spanning the perimeter of the left and right ovaries. Reprinted from Balen AH et al. Ultrasound assessment of the polycystic ovary: international consensus definitions. Human Reproduction Update. 2003;9(6):505–514,30 by permission of the European Society of Human Reproduction and Embryology.
Cardiovascular Screening
Although there are no established consensus guidelines regarding the screening of patients with PCOS, the finding of a high prevalence of atherosclerosis would seem to dictate early screening. During the reproductive years, to minimize radiographic exposure, it would be reasonable to perform cIMT examinations in patients at age 30 years and, if normal, to repeat them every 3 to 5 years. cIMT screening can be supplemented by computed tomography coronary calcium assessments by the age of 45 years. The presence of carotid intimal thickening of greater than 75% or of any coronary calcium indicates the presence of atherosclerosis and should initiate treatment of all modifiable cardiovascular risk factors to secondary prevention levels. Secondary prevention lipid goals would be an LDL-C of less than 100 mg/dL as a primary goal and a non-HDL-C of less than 130 mg/dL.
Blood Pressure
Careful attention to blood pressure measurement techniques is important in the PCOS population so that accurate measures are obtained. Blood pressure should be taken and recorded at every clinic visit. If there is a wide variance between blood pressures measured in the clinic versus at home, 24-hour blood pressure monitoring can be quite useful. Normal mean 24-hour blood pressure is less than 130/80 mm Hg. In patients with complex hypertension (those who also have diabetes, cardiovascular disease, or kidney disease), a blood pressure goal of less than 130/80 mm Hg is recommended. Echocardiography can be useful in determining the presence of hypertensive heart disease in certain patients.
Lipid Panel
In the setting of hyperandrogenism, PCOS patients are at increased risk of cardiovascular disease. It is imperative that patients are screened for abnormal HDL-C, LDL-C, VLDL cholesterol, and triglycerides starting at the age of 30 years, with repeat measurements every 3 to 5 years (if values are normal). Aggressive testing for lipid aberrations early on ensures that these abnormalities are treated early, thus reducing the overall risk of cardiovascular disease.
Management
Oral Contraceptives
Oral preparations of estrogen and progesterone are frequently used to control symptoms of PCOS-in particular, hirsutism, acne, and irregular menstruation.33 The mechanisms of action of oral contraceptives (OCPs) occur via a reduction in ovarian androgen production (by suppressing pituitary gonadotrophin secretion) and stimulation of hepatic sex hormone-binding globulin, which has a reciprocal effect on circulating free testosterone. For dermatologic symptoms (acne, hirsutism), OCPs are very effective. To date, there is a paucity of data regarding the longterm effects of OCPs for modifying risk of coronary artery disease or diabetes mellitus either positively or negatively. The recommendation, then, is to tailor the OCP treatment regimen to the patient. In the presence of obesity, strong family history of type 2 diabetes mellitus, or personal history of gestational diabetes mellitus, the potential risk for increasing carbohydrate disturbance by OCP may outweigh the benefits. Therefore, for these patients, it may be preferable to explore other treatment options.34
Metformin
Metformin is an insulin-sensitizing agent that has been proven to treat anovulation in the infertile PCOS patient.35 PCOS patients can benefit from metformin’s ability to decrease insulin levels and alter the effect of insulin-stimulated ovarian androgen synthesis, theca cell proliferation, and endometrial cell growth.36 In addition, ovarian gluconeogenesis may be directly reduced, thereby further decreasing ovarian androgen production. 27 In a double-blind study conducted by Moghetti and colleagues, 37 treatment of 18 PCOS women with 1 year of metformin resulted in a statistically significant decrease in menstrual irregularities (P = .0002), reduced fasting plasma insulin (P = .057), and increased insulin sensitivity (P < .05). The authors concluded that metformin is effective for attenuating insulin resistance, hyperandrogenemia, and the resulting effects of these conditions in PCOS patients. Alternatively, some studies support the use of thiazolidinediones, such as rosiglitazone, to help improve insulin resistance, ovarian androgen production attenuation, and spontaneous ovulation.38
Weight Loss and Nutrition
Although there is a high prevalence of obesity in PCOS patients, the actual statistics vary, even in large case studies.39 According to 1 study of 1741 English PCOS patients, 38.4% of those enrolled in the series had a BMI of 26 or greater.40 Obesity is an independent risk factor for cardiovascular disease itself and should not be neglected in the treatment of the PCOS patient. In a study by Pasquali and colleagues,41 20 PCOS patients with an average BMI of 32 were treated with a hypocaloric diet of 1000 to 1500 kcal/d, consisting of 50% carbohydrate, 30% lipid, and 20% protein for 8 months. Researchers found that after this treatment period, insulin values in oral glucose tolerance tests were similar to those of normal-weight women.41 In a study comparing weight loss to use of OCPs, Wahrenberg and colleagues42 found that insulin sensitivity improved in the weight-loss group but not in the OCP group. Therefore, weight loss can improve insulin resistance in obese patients with PCOS, although there are no long-term studies to correlate the overall risk of diabetes.36
It is well known that obesity, as an independent risk factor, can predispose patients to an increase in cardiovascular risk. In patients with a BMI exceeding 28, the incidence of stroke, ischemic heart disease, and diabetes is as high as 3 to 4 times that of the general population.43 Truncal obesity, as measured by waist to hip ratio, is a known marker for increased cardiovascular risk.44 Pasquali and colleagues45 demonstrated that a hypocaloric diet in conjunction with metformin, as compared with placebo, resulted in a greater reduction of total visceral adipose tissue in obese PCOS patients, which ultimately improved hirsutism and menses abnormalities.
Fertility Options
In light of their anovulation, patients with PCOS may require ovulationinduction agents. The options at the present time include clomiphene citrate (50–150 mg), recombinant FSH, or urinary human menopausal gonadotropins with intrauterine insemination of washed sperm or with natural intercourse. Depending on the patient’s age, basal antral follicle count, day 3 FSH serum level, and tubal status, in vitro fertilization (IVF) may be an option using controlled ovarian hyperstimulation with oocyte retrieval and, ultimately, embryo transfer. One must be extremely cautious with these patients due to their high risk of ovarian hyperstimulation syndrome (OHSS), an exaggerated response of the ovaries to stimulation. OHSS is a serious complication associated with exogenous gonadotropin administration, especially in women with PCOS. It is characterized by ovarian enlargement and increased capillary permeability. Classification of OHSS is based on a 5-grade scale, with grade 5 (severe) including ascites, hydrothorax, hemoconcentration, coagulation abnormalities, and decreased renal perfusion and function. Inpatient management is required for these patients, with supportive care, paracentesis for symptomatic improvement, and thrombosis prophylaxis. Use of the minimum effective dosage to prevent this complication is essential for both gonadotropins with insemination and IVF. In addition, patients with PCOS are at high risk for higher order multiple pregnancy. Ultimately, the goal of ovulation induction is a healthy, full-term singleton gestation.
Cardiovascular Risk Factors
Hypertension
Blood pressure should be treated to a goal level below 140/90 mm Hg in patients with simple hypertension and below 130/80 mm Hg in complex hypertensive patients. Complex hypertensive patients are those who have end-organ manifestations, including left-ventricular hypertrophy, diastolic dysfunction, atherosclerosis, diabetes, stroke, or chronic kidney disease. With the high prevalence of obesity and metabolic syndrome, it would be prudent to avoid use of antihypertensive agents that impair insulin sensitivity (atenolol, metoprolol) or may lead to weight gain.
Dyslipidemia
As mentioned above, treatment goals should be dictated by the presence or absence of either overt or subclinical cardiovascular disease. In patients with any evidence of cardiovascular disease, achievement of an LDL-C of less than 100 mg/dL is the primary goal, and a non-HDL-C (total cholesterol without HDL-C) of less than 130 mg/dL is a secondary goal. Primary treatment should be statin-based if tolerated, with the addition of either fibric acid derivatives or niacin to treat persistently elevated levels of triglycerides, non-HDL-C, and/or low HDL-C. Normalization of glucose levels in patients with diabetes should lead to improvement in hypertriglyceridemia.
Conclusions
The diagnosis of PCOS implies increased cardiac risk. Weight loss and proper nutrition are paramount to decreasing this risk. Approximately half of these patients will be insulinresistant, and treatment may have far-reaching potential by decreasing their lifetime risk of type 2 diabetes. If the patient is planning to conceive, treatment of dyslipidemia with statins is not recommended until childbearing is completed. Ultimately, longstanding oligomenorrhea may lead to endometrial hyperplasia and, possibly, to endometrial adenocarcinoma, and thus OCPs are protective and a key recommendation. Future studies are warranted to establish cardiovascular screening guidelines for women with PCOS. Clinicians may consider early cardiovascular screening and treatment of all modifiable cardiovascular risk factors to secondary prevention levels.
Main Points.
Polycystic ovary syndrome (PCOS) is a symptom complex that includes amenorrhea, hirsutism, and enlarged polycystic ovaries.
The incidence of impaired glucose tolerance, type 2 diabetes mellitus, obesity, hypertension, and dyslipidemia, as well as of coronary and vascular disease, may be higher in women with PCOS during their reproductive years than in patients with the cardiometabolic syndrome.
In a recent study of women with PCOS, 46% also had the metabolic syndrome.
Studies using noninvasive vascular assessment of atherosclerotic plaque, such as coronary calcium and carotid intimal medial thickness assessments, have confirmed a greater prevalence of cardiovascular disease in PCOS patients relative to the general population.
PCOS patients have been found to have a higher prevalence of aortic calcification, a marker of atherosclerotic disease.
Weight loss and proper nutrition are paramount to decreasing the increased cardiac risk associated with PCOS.
References
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- 2.The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, authors. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 4.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 36–37. [Google Scholar]
- 5.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 471–472. [Google Scholar]
- 6.Guzick DS. Polycystic ovary syndrome. Obstet Gynecol. 2004;103:181–193. doi: 10.1097/01.AOG.0000104485.44999.C6. [DOI] [PubMed] [Google Scholar]
- 7.Glueck CJ, Papanna R, Wang P, et al. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–915. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. 1998;81(4A):18B–25B. doi: 10.1016/s0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 10.Yildirim B, Sabir N, Kaleli B. Relation of intraabdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2003;79:1358–1364. doi: 10.1016/s0015-0282(03)00265-6. [DOI] [PubMed] [Google Scholar]
- 11.Bergman RN, Van Citters GW, Mittelman SD, et al. Central role of adipocyte in metabolic syndrome. J Investig Med. 2001;49:119–126. doi: 10.2310/6650.2001.34108. [DOI] [PubMed] [Google Scholar]
- 12.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192:585–594. doi: 10.1677/joe.1.07070. [DOI] [PubMed] [Google Scholar]
- 13.Third Report of the National Cholesterol Education Program (NCEP), authors Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 14.Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos P. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18:280–285. doi: 10.1016/j.tem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 16.Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136–150. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 17.Lo JC, Feigenbaum SL, Yang J, et al. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1357–1363. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease: detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 19.Boulman N, Levy Y, Leiba R, et al. Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160–2165. doi: 10.1210/jc.2003-031096. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Yildiz BO, Haznedaroğglu IC, Kirazli S, Bayraktar M. Global fibrinolytic capacity is decreased in polycystic ovary syndrome, suggesting a prothrombotic state. J Clin Endocrinol Metab. 2002;87:3871–3875. doi: 10.1210/jcem.87.8.8716. [DOI] [PubMed] [Google Scholar]
- 24.Christian RC, Dumesic DA, Behrenbeck T, et al. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562–2568. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- 25.Talbott EO, Zborowski JV, Rager JR, et al. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5454–5461. doi: 10.1210/jc.2003-032237. [DOI] [PubMed] [Google Scholar]
- 26.Guzick DS. Cardiovascular risk in women with polycystic ovarian syndrome. Semin Reprod Endocrinol. 1996;14:45–49. doi: 10.1055/s-2007-1016308. [DOI] [PubMed] [Google Scholar]
- 27.Talbott EO, Guzick DS, Sutton-Tyrrell K, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414–2421. doi: 10.1161/01.atv.20.11.2414. [DOI] [PubMed] [Google Scholar]
- 28.Talbott EO, Zborowski JV, Boudreaux MY, et al. The relationship between C-reactive protein and carotid intima-media wall thickness in middleaged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:6061–6067. doi: 10.1210/jc.2003-032110. [DOI] [PubMed] [Google Scholar]
- 29.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005;90:5711–5716. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 30.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 31.Adams J, Franks S, Polson DW, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2:1375–1379. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 32.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, authors. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Burkman RT., Jr The role of oral contraceptives in the treatment of hyperandrogenic disorders. Am J Med. 1995;98:130S–136S. doi: 10.1016/s0002-9343(99)80071-0. [DOI] [PubMed] [Google Scholar]
- 34.Vrbíková J, Cibula D. Combined oral contraceptives in the treatment of polycystic ovary syndrome. Hum Reprod Update. 2005;2:277–291. doi: 10.1093/humupd/dmi005. [DOI] [PubMed] [Google Scholar]
- 35.Barbieri RL. Clomiphene versus metformin for ovulation induction in polycystic ovary syndrome: the winner is…. J Clin Endocrinol Metab. 2007;92:3399–3401. doi: 10.1210/jc.2007-1393. [DOI] [PubMed] [Google Scholar]
- 36.Mathur R, Alexander CJ, Yano J, et al. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Moghetti P, Castello R, Negri C, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. Obstet Gynecol Surv. 2000;55:365–366. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 38.Sepilian V, Nagamani M. Effects of rosiglitazone in obese women with polycystic ovary syndrome and severe insulin resistance. J Clin Endocrinol Metab. 2005;90:60–65. doi: 10.1210/jc.2004-1376. [DOI] [PubMed] [Google Scholar]
- 39.Hoeger K. Obesity and weight loss in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:85–97. doi: 10.1016/s0889-8545(05)70187-x. [DOI] [PubMed] [Google Scholar]
- 40.Balen AH, Conway GS, Kaltsas K, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 41.Pasquali R, Antenucci D, Casimirri F, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab. 1989;68:173–179. doi: 10.1210/jcem-68-1-173. [DOI] [PubMed] [Google Scholar]
- 42.Wahrenberg H, Ek I, Reynisdottir S, et al. Divergent effects of weight reduction and oral anticonception treatment on adrenergic lipolysis regulation in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:2182–2187. doi: 10.1210/jcem.84.6.5794. [DOI] [PubMed] [Google Scholar]
- 43.Van Itallie TB. Health implications of overweight and obesity in the United States. Ann Intern Med. 1985;103:983–988. doi: 10.7326/0003-4819-103-6-983. [DOI] [PubMed] [Google Scholar]
- 44.Lapidus L, Bengtsson C, Larsson B, et al. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12-year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:2767–2774. doi: 10.1210/jcem.85.8.6738. [DOI] [PubMed] [Google Scholar]
- 46.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. p. 193. [Google Scholar]
- 47.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. p. 491. [Google Scholar]