Abstract
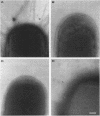
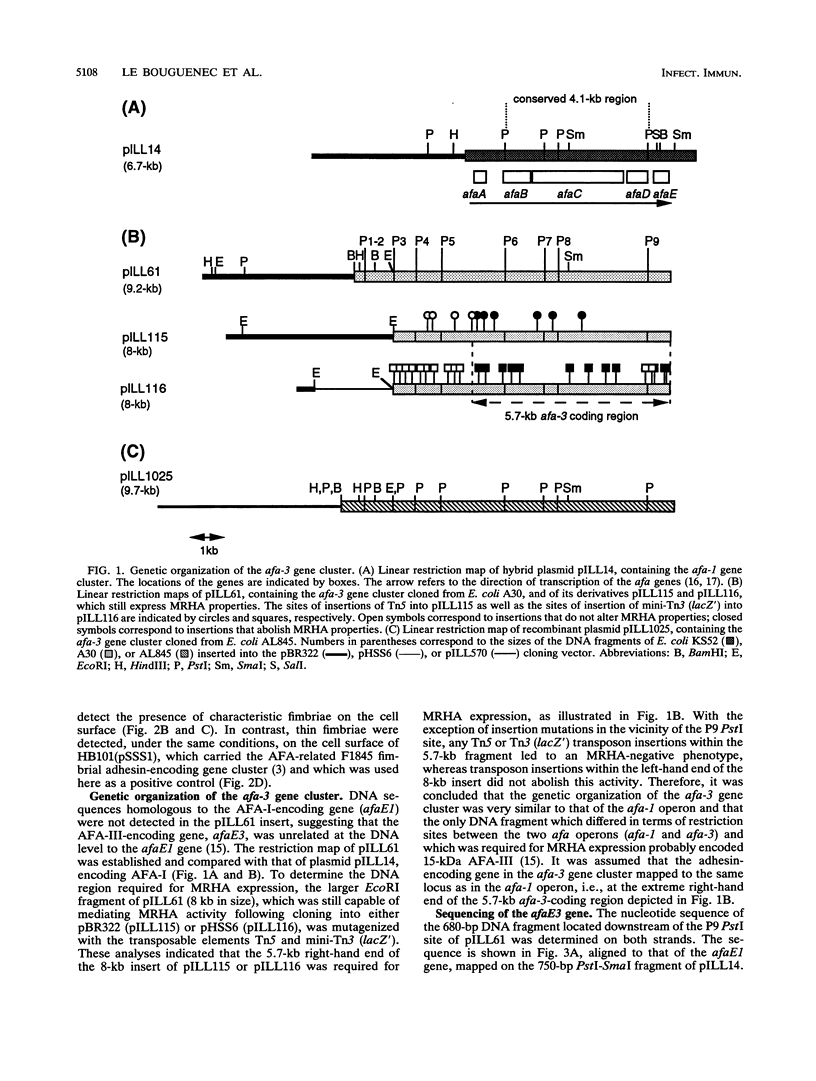
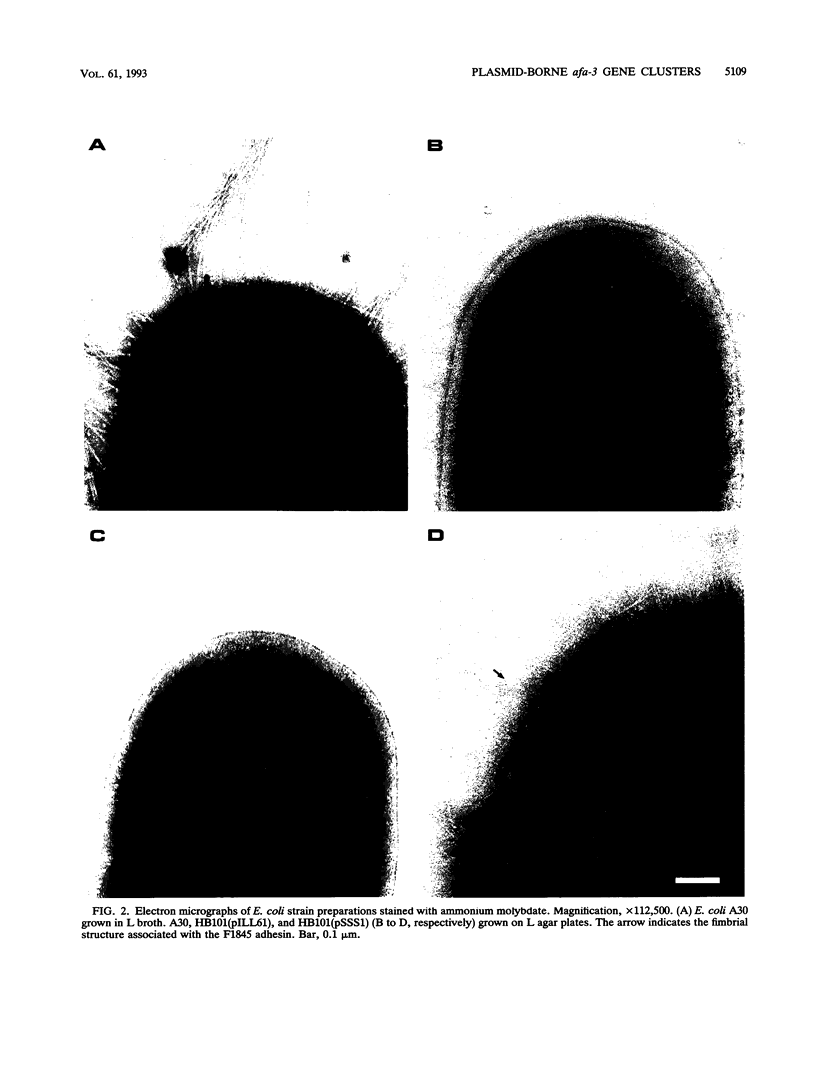
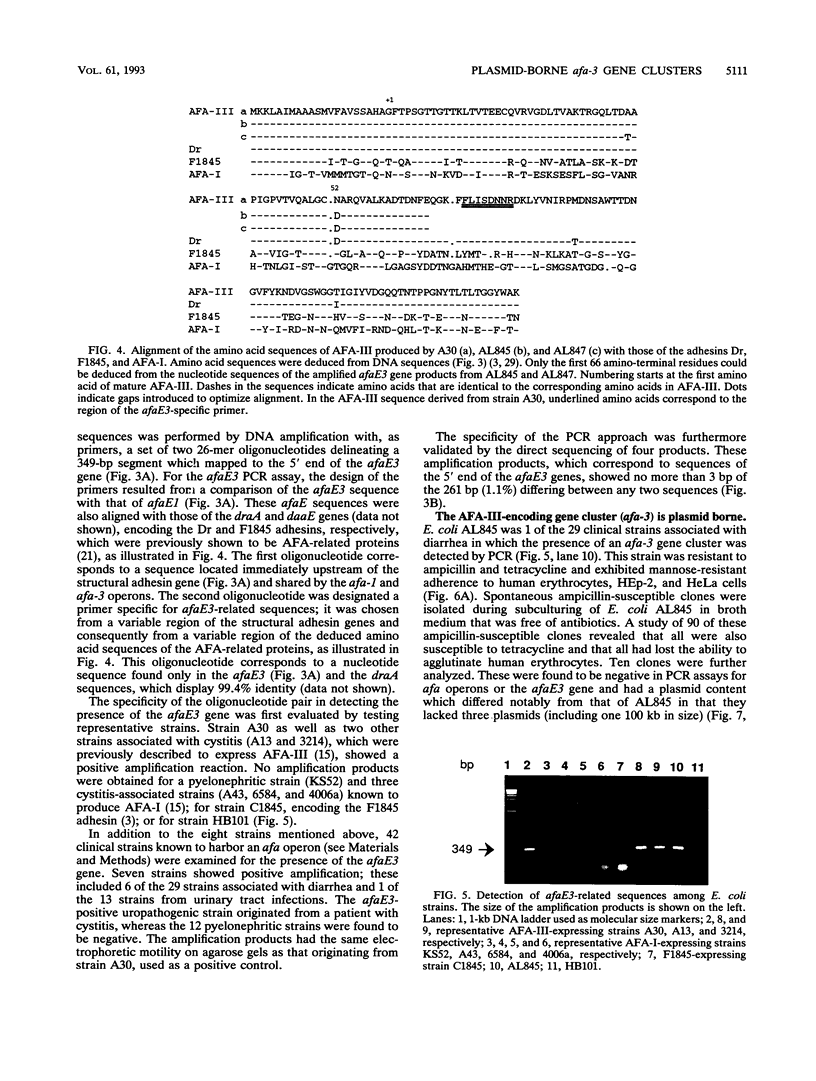
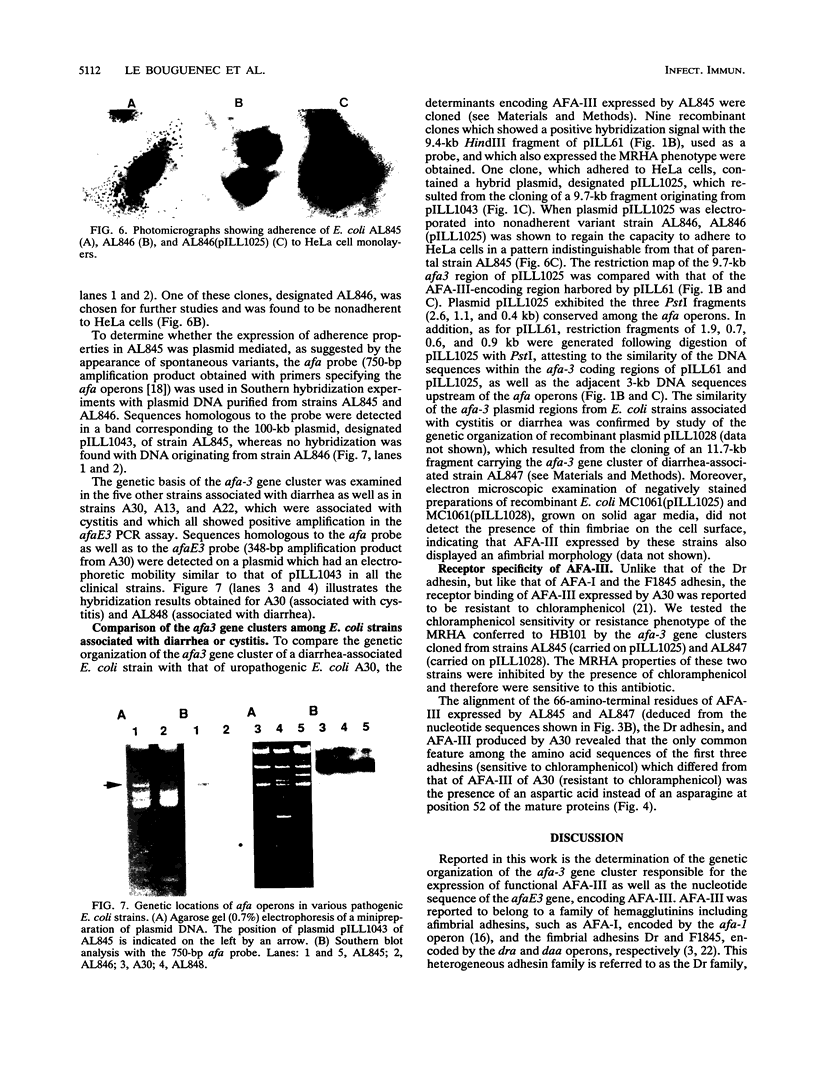
The afa gene clusters encode afimbrial adhesins (AFA) that are expressed by uropathogenic and diarrhea-associated Escherichia coli strains and belong to a family of hemagglutinins recognizing the Dr blood group antigen as a receptor. This family so far includes AFA-I and AFA-III as well as the Dr and F1845 adhesins (B. Nowicki, A. Labigne, S. Moseley, R. Hull, S. Hull, and J. Moulds, Infect. Immun. 58:279-281, 1990). Reported in this work is the genetic organization of the afa-3 gene cluster cloned from a uropathogenic E. coli strain (A30) which expressed a subtype of AFA designated AFA-III. The amino acid sequence of AFA-III was deduced from the nucleotide sequence of the afaE3 gene and was found to be highly homologous to that of the Dr adhesin (98.1% identity). A polymerase chain reaction assay was developed to detect the presence of afa-3 gene clusters in E. coli strains. Study of the genetic support of the afa-3 gene clusters in the strains which showed positive amplification revealed that they were always located on large, 100-kb plasmids whether the strains originated from patients with cystitis or with diarrhea. Moreover, the cloned afa-3 gene clusters from A30 and from the diarrhea-associated strain AL845 appeared to be carried by 9-kb plasmid regions which displayed a similar genetic organization. Chloramphenicol was reported to be a potent inhibitor of receptor binding by the Dr adhesin (Nowicki et al., Infect. Immun. 58:279-281, 1990). AFA-III expressed by strains AL845 and AL847 appeared to mediate, like the Dr adhesin, chloramphenicol-sensitive hemagglutination, whereas AFA-III produced by A30 conferred chloramphenicol-resistant adherence. A comparison of the sequences of these four proteins indicated that the amino acid at position 52 of the processed AFA could be part of the receptor-binding domain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archambaud M., Courcoux P., Labigne-Roussel A. Detection by molecular hybridization of pap, afa, and sfa adherence systems in Escherichia coli strains associated with urinary and enteral infections. Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):575–588. doi: 10.1016/0769-2609(88)90156-1. [DOI] [PubMed] [Google Scholar]
- Archambaud M., Courcoux P., Ouin V., Chabanon G., Labigne-Roussel A. Phenotypic and genotypic assays for the detection and identification of adhesins from pyelonephritic Escherichia coli. Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):557–573. doi: 10.1016/0769-2609(88)90155-x. [DOI] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goldhar J., Perry R., Golecki J. R., Hoschutzky H., Jann B., Jann K. Nonfimbrial, mannose-resistant adhesins from uropathogenic Escherichia coli O83:K1:H4 and O14:K?:H11. Infect Immun. 1987 Aug;55(8):1837–1842. doi: 10.1128/iai.55.8.1837-1842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. A., Beverley-Clarke H., High N. J., Jann K., Perry R., Goldhar J., Boulnois G. J. Molecular cloning and characterisation of the genes for a non-fimbrial adhesin from Escherichia coli. Microb Pathog. 1988 Jul;5(1):9–17. doi: 10.1016/0882-4010(88)90076-9. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Johnson J. R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991 Jan;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A. Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis. 1991 Jul-Aug;13(4):721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Falkow S. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect Immun. 1988 Mar;56(3):640–648. doi: 10.1128/iai.56.3.640-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguenec C., Archambaud M., Labigne A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J Clin Microbiol. 1992 May;30(5):1189–1193. doi: 10.1128/jcm.30.5.1189-1193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Labigne A., Moseley S., Hull R., Hull S., Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990 Jan;58(1):279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Svanborg-Edén C., Hull R., Hull S. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):446–451. doi: 10.1128/iai.57.2.446-451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Libaek L. B., Schoolnik G. K. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun. 1987 Sep;55(9):2052–2056. doi: 10.1128/iai.55.9.2052-2056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson T. N., Bilge S. S., Nowicki B., Moseley S. L. Molecular structure of the Dr adhesin: nucleotide sequence and mapping of receptor-binding domain by use of fusion constructs. Infect Immun. 1991 Jan;59(1):261–268. doi: 10.1128/iai.59.1.261-268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W., Schmidt M. A., Labigne-Roussel A. F., Falkow S., Schoolnik G. AFA-I, a cloned afimbrial X-type adhesin from a human pyelonephritic Escherichia coli strain. Purification and chemical, functional and serologic characterization. Eur J Biochem. 1985 Oct 15;152(2):315–321. doi: 10.1111/j.1432-1033.1985.tb09200.x. [DOI] [PubMed] [Google Scholar]
- Willshaw G. A., Smith H. R., McConnell M. M., Gaastra W., Thomas A., Hibberd M., Rowe B. Plasmid-encoded production of coli surface-associated antigen 1 (CS1) in a strain of Escherichia coli serotype O139.H28. Microb Pathog. 1990 Jul;9(1):1–11. doi: 10.1016/0882-4010(90)90035-o. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]