Abstract
Background
It is assumed that moderate exercise may improve resistance to infection and reduce inflammation, but there are limited data to support this assumption in an infection model.
Methods
BALB/cJ mice were assigned to the following groups: no exercise (NON-EX), 1 session of acute exercise (A-EX), or chronic exercise for ~3.5 months (C-EX). Mice were infected with influenza (C-EX mice infected at rest; A-EX mice infected 15 min after exercise).
Results
C-EX mice demonstrated the lowest severity of infection, assessed by body weight loss and food intake. There was less virus in the lungs at day 5 after infection in C-EX and A-EX mice compared with NON-EX mice (P = .02) and less virus at day 2 after infection only in C-EX mice (P = .07). Soon after infection (day 2), interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1β, and tumor necrosis factor α in the bronchoalveolar lavage (BAL) fluid were lower in C-EX and A-EX than in NON-EX mice. At day 5 after infection, the BAL fluid from C-EX (but not A-EX) mice had less IL-6, interleukin 12p40, granulocyte colony-stimulating factor, keratinococyte-derived chemokine, and MCP-1 than that from NON-EX mice. A trend toward reduced immunopathologic response was found in C-EX mice.
Conclusions
Chronic exercise resulted in reduced symptoms, virus load, and levels of inflammatory cytokine and chemokines. Acute exercise also showed some benefit, which was limited to the early phase of infection.
Epidemiological evidence suggests that moderate exercise may reduce the risk or severity of infection, whereas exhaustive exercise may increase that risk or severity [1–4]. With respect to animal models of respiratory viral infection, moderate exercise tends to decrease morbidity and mortality, whereas prolonged, strenuous exercise increases mortality [5–8]. The modulation of immune responsiveness that occurs as a result of exercise was well studied and was the subject of a number of review articles [9–11]. However, relatively few studies were conducted in the context of infection to determine which immunological changes may be responsible for improved protection from infection.
The early response to respiratory viral infection involves production of type I interferons by plasmacytoid dendritic cells, alveolar macrophages, and infected epithelial cells [12–14]. Type I interferons (IFNs) have potent antiviral activity [15] and have more recently been shown to modulate adaptive immune responses [16]. Moreover, monocytes, macrophages, and neutrophils accumulate in the lungs and produce inflammatory cytokines that may contribute to immunopathologic responses [17, 18]. Lung natural killer (NK) cells can be detected 48 h after infection; they respond by producing interferom γ (IFN-γ) and lysing virus-infected cells [19]. Respiratory dendritic cells bridge the innate adaptive response by acquiring viral antigen in the lungs, migrating to regional lymph nodes, and activating CD8+ cells that act to lyse virally infected cells [20, 21]. The potential effect of exercise on each of these parameters in the context of infection remains largely unexplored.
Moderate exercise is associated with enhanced activity of several immune parameters that could be important in limiting or clearing viral infection. For example, levels of antigen-specific IFN-γ and interleukin 2 (IL-2) were enhanced by moderate exercise [22], although no effect on immunoglobulin M was found. It was recently found that moderate exercise performed during the early phase of infection is associated with reduced cellular infiltration of the lungs and a shift from a Th1 to a Th2 profile [23]. To our knowledge, that is the only published study to date that has examined the effect of exercise on the immune response at the local site of infection. It remains to be determined whether exercise before infection may have a preventive role in reducing virus load or altering local immune response, given that exercise during infection could potentially have detrimental consequences. It is also unknown whether a single session of exercise, as opposed to regular exercise, may affect the severity of infection.
The purpose of the current study was therefore to evaluate the extent to which acute versus regular moderate exercise could alter the severity of influenza infection and the degree of immune responsiveness at the site of infection. With respect to moderate exercise, the aim was to determine whether repeated, moderate exercise confers a protective effect that persists at rest (24 h after the last exercise session, ie, a “training effect”). To test this aim, exercise-trained mice were infected 24 h after exercise. To examine the possibility that a single session of exercise may have a short-term protective effect for a brief period, other mice were infected 15 min after a single session of exercise. Epidemiological studies support the concept that moderate exercise may improve resistance to infection, but the potential mechanisms involved are unknown. It is also unclear how any benefits from repeated single sessions of acute exercise compare with the potential benefits of the trained but rested state. Therefore, we evaluated the effects of acute as well as chronic exercise in our experiments to address this question.
METHODS
Animals and treatment
Male BALB/cJ mice, 6 weeks of age, were acclimated to the animal housing facility for 2 weeks before experimental intervention. Animal procedures were approved by the Iowa State University Committee on Animal Care. Mice were randomly assigned to 1 of the following groups (8–12 per group at each time point after infection): the non-exercise (NON-EX) group; the chronic exercise (C-EX) group, which included mice that exercised 5 days a week for 14 weeks at moderate intensity; and the acute exercise (A-EX) group, which included mice that ran on a treadmill for 45 min but only on the day of infection. In the C-EX group, exercise duration was gradually increased during weeks 1–4; from weeks 5 to 8, mice ran 40–45 min/day, then 45 min/day for the remainder of the training period. Speed progressed from 8 m/min during week 1 to 18 m/min by week 8. In the A-EX group, mice ran a session equivalent to the last session run by the C-EX mice, 45 min at moderate intensity (18 m/min). Non-infected mice were included as controls.
The C-EX mice were infected with influenza virus 24 h after completing the last exercise session (in a resting condition). The A-EX mice were infected with virus 15 min after completing the single 45-min session of exercise. The exercise session for the A-EX mice was timed such that the time of infection 15 min after exercise coincided with the time at which C-EX mice were infected. NON-EX mice were also infected at the same time of day as the C-EX and A-EX mice. Mice were housed separately so that food and water consumption could be assessed individually for each mouse.
Viral infection and illness measures
Before infection, mice were briefly exposed to carbon dioxide and then infected via an intranasal route with 50 µL of influenza A/PR/8/34 virus (10 hemagglutination units; 1010.45 50% egg-infective dose [EID50]/mL) (a dose resulting in ~5% mortality). After infection, all mice were returned to their cages for 2, 5, or 10 days. Mice remained in their cages and did not exercise after infection. Body weight and food intake were monitored daily at 24-h intervals after infection. Initial body weight and food and water intake measures were assessed within 60 min before infection.
Lung bronchoalveolar lavage and tissue collection
Lungs were lavaged 3 times with 1 mL of phosphate-buffered saline. The bronchoalveolar lavage (BAL) fluid was centrifuged, cells were collected for analysis by flow cytometry, and the supernatant was stored at −80°C until subsequent analysis for cytokines and chemokines with a multiplex platform (Bio-Plex, Bio-Rad). Lung lobes were collected and used to determine viral titers (day 2 after infection, all lobes; days 5 and 10, right lobes), or fixed for subsequent analysis by pathology (days 5 and 10, left lobes). In mice euthanized on day 5 or 10, BAL was performed on the left lobes while the right lobes were clamped off to leave the lung lobes undisturbed for analyses of lesions.
Viral titer quantification and lung lesion scoring
Virus titers were measured by quantitative fluorogenic real-time reverse-transcription polymerase chain reaction (RT-PCR) with TaqMan chemistry. Using sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov/Genbank/index.html) and the Influenza Sequence Database (http://www.flu.lanl.gov), we engineered virus-specific oligonucleotide primers and a fluorescent probe to target a highly conserved region of the swine influenza virus nucleoprotein. The forward primer (SIVRTF: 5′-CGGACGAAAAGGCAACGA-3′) and reverse primer (SIVRTR: 5′-CTGCATTGTCTCCGAAGAAATAAG-3′) were synthesized by a commercial vendor (Integrated DNA Technologies). A TaqMan MGB probe with a 5′ reporter 6-carboxyfluorescein (FAM) and a 3′ nonfluorescent quencher (SIVRTP: 5′-6-FAM-CCGATCGTGCCYTC) was synthesized by Applied Biosystems. To conduct the assay, viral RNA was first extracted from 50 µL of lung sample, positive control (H1N1 and H3N2 swine influenza viruses) and negative control (elution buffer) using the Ambion MagMAX Viral RNA Isolation Kit (Applied Biosystems) and KingFisher 96 magnetic particle processor (Thermo Scientific). Real-time RT-PCR was then carried out with the QuantiTect Probe RT-PCR Kit (Qiagen) in a 20-µL reaction volume using 4 µL of extracted template. Primers were added at a final concentration of 0.4 µmol/L each, and the probe was added at a final concentration of 0.2 µmol/L.
Polymerase chain reaction amplification was performed on the ABI 7900HT Sequence Detection System (Applied Biosystems) with the 384-well format. Cycling conditions were as follows: (1) reverse transcription for 30 min at 50°C, (2) a 15-min activation step at 95°C, and (3) 40 cycles of 15 sec at 94°C and 60 sec at 60° C. A set of influenza preparations, each with a known virus titer (EID50/mL), were used to generate a standard curve. Samples with threshold cycle values of ≤35 were considered positive. The amount of influenza in each sample was calculated by converting the threshold cycle value to a virus titer by using the standard curve.
Lung pathology
Lung tissue collected at necropsy was fixed in 10% buffered formalin for histopathological examination. After adequate fixation, the tissues were embedded in paraffin wax, sectioned at 5-µm thickness, stained with hematoxylineosin, and examined with light microscopy. The lungs were examined for bronchiolar epithelial changes, including attenuation, proliferation, degeneration, and necrosis. The amount and severity of peribronchiolar and alveolar inflammation were also evaluated. Sections of lung were given a score from 0 to 3 to reflect an estimate of the percentage of lung tissue containing lesion and the severity of lesions, according to methods described elsewhere [24]. The lung sections were scored according to the following criteria: 0, no significant lesions or minimal epithelial cells change in <25% of the lung tissue; 1, mild to moderate epithelial cell changes and interstitial pneumonia in ~25%–50% of the tissue; 2, moderate epithelial cell changes and moderate interstitial pneumonia in ~50%–75% of the tissue; and 3, significant epithelial cell changes and moderate to severe interstitial to bronchointerstitial pneumonia in ~75%–100% of the tissue. For each mouse, the number of lung lobes examined and a score for each lobe was recorded. A single pathologist scored all slides and was blinded to the treatment groups.
Identification of cell populations and cytokine/chemokine analysis
BAL supernatants were analyzed for cytokines and chemokines with a Luminex Platform (Bio-Plex, Bio-Rad) and a 23-plex kit (Bio-Rad). Cytokines and chemokines included interleukin 1α (IL-1α), interleukin 1β (IL-1β), interleukin 2 (IL-2), interleukin 3 (IL-3), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 9 (IL-9), interleukin 10 (IL-10), interleukin 12p40 (IL-12p40), interleukin 12p70 (IL-12p70), interleukin 13 (IL-13), interleukin 17 (IL-17), eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, keratinococyte-derived chemokine (KC), monocyte chemoattractant protein 1 (MCP 1), macrophage inflammatory protein 1α (MIP 1α), macrophage inflammatory protein 1β (MIP-1β), RANTES (regulated on activation, normal T cell expressed and secreted), and tumor necrosis factor a (TNF α). The following antibodies and appropriate isotype were used to identify cell populations collected in BAL fluid: allophycocyanin–cyanine 7–conjugated anti-mouse CD8 as cytotoxic T cells and fluorescein isothiocyanate–conjugated anti-mouse CD11b and phycoerythrin-conjugated anti-mouse Gr1 as neutrophils. Cells were analyzed with a BD FACSCanto flow cytometer (BD Biosciences).
Statistical analysis
A 1-way analysis of variance was used to compare viral titer, lobe score, cytokine and chemokine levels, and cell populations at each day after infection. SPSS software (version 14.0; SPSS) was used in all analyses, and Bonferroni post hoc analyses were performed as needed. To assess changes in body weight and food and water intake, a mixed analysis of variance (treatment group by repeated measures) was used to evaluate change over time.
RESULTS
Illness measures
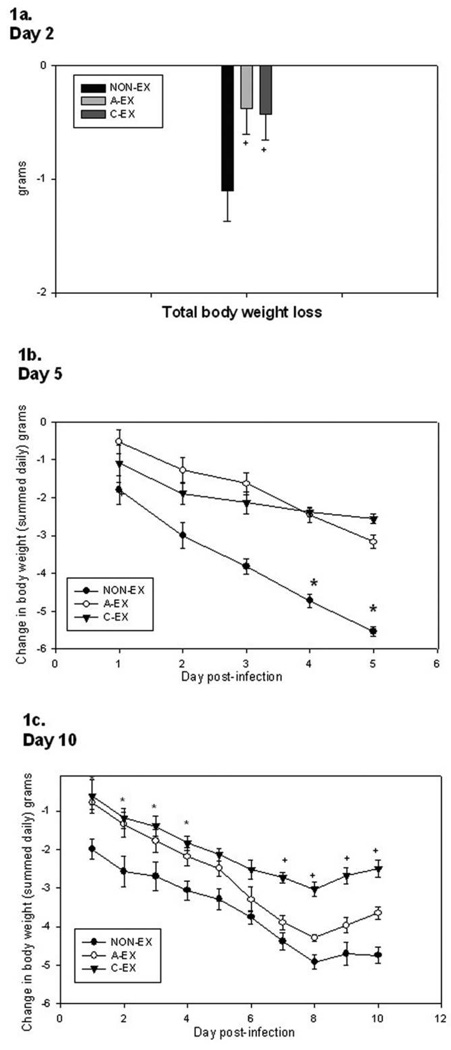
Body weight and food intake were summed over time in the mice that were euthanized on day 2 after infection. A-EX and C-EX mice tended to lose less weight than control mice (P = .10) (Figure 1A). In the experiment in which mice were euthanized on day 5 after infection, NON-EX mice had greater weight loss over time (Figure 1B) (main effect of time, P = .002; treatment-by-time interaction, P = .049). Follow-up analysis showed that A-EX mice had less weight loss at day 1 (P = .04) than NON-EX mice, but at days 4 and 5 after infection C-EX mice had less weight loss than NON-EX or AEX mice (P < .05). In the experiment in which mice were euthanized on day 10 after infection, a benefit was demonstrated for acute and chronic exercise during the early phase of infection (days 1–5), such that NON-EX mice lost more weight than either of the exercise groups (treatment-by-time interaction, P = .04). However, over the entire course of infection (days 1–10), only the C-EX mice showed a trend toward reduced weight loss (Figure 1C).
Figure 1.
Change in body weight over the course of infection is seen in mice euthanized on day 2 (A), 5 (B), or 10 (C) after infection. For mice euthanized on day 2 after infection, the total body weight loss is summed, whereas the change in body weight for each day of infection is shown for those euthanized on day 5 or 10. (A) On day 2 after infection, there is a trend toward reduced total weight loss in the chronic exercise (C-EX) and acute exercise (A-EX) groups compared with the nonexercise (NON-EX) group; + P = .10. (B) In mice euthanized on day 5 after infection, the treatment-by-time interaction was significant (P < .05), such that the rates of weight loss over time differed in the 3 groups. In follow-up analyses, C-EX mice had less weight loss than NON-EX or A-EX mice; *P < .05.(C) Findings in mice euthanized on day 10 after infection show an early-phase (days 1–5) treatment-by-time interaction, such that the rates of weight loss differed over time for the 3 groups. In follow-up analyses, A-EX and C-EX mice had less weight loss than NON-EX mice; *P < .05. During the entire 10-day course of infection, there was a trend toward a treatment-by-time interaction such that the rate of weight loss in C-EX mice differed from that in NON-EX mice; +P = .08.
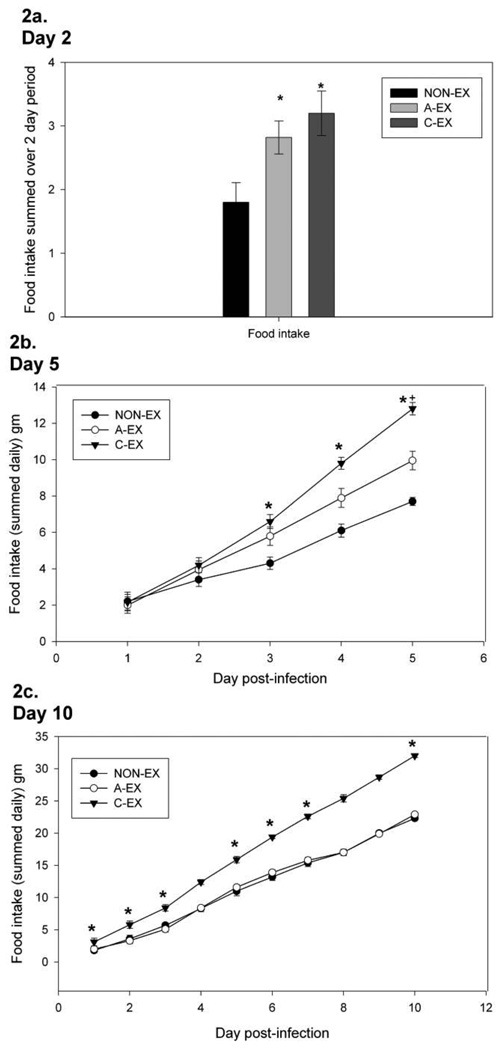
The total food intake of A-EX and C-EX mice was greater than that of NON-EX mice in mice euthanized on day 2 after infection (P = .02) (Figure 2A). In mice euthanized on day 5 after infection, C-EX mice consumed more food than NON-EX mice on days 3, 4, and 5 (main effect of time, P = .004; treatment-by-time interaction, P = .025) (Figure 2B). In mice euthanized on day 10, C-EX mice consumed more food than either A-EX or NON-EX mice over time (on days 1–3, 5, and 6, C-EX mice consumed more food than A-EX and NON-EX mice; on day 10, C-EX consumed more food than NON-EX mice) (main effect of time, P = .001; treatment-by-time interaction, P = .008) (Figure 2C).
Figure 2.
Changes in food intake over the course of infection seen in mice euthanized on day 2 (A), 5 (B), or 10 (C) after infection. For mice euthanized on day 2 after infection, the total food intake is summed, whereas the amount of food consumed daily is shown for mice euthanized on day 5 or 10. (A) In mice euthanized on day 2, total food intake was greater in the acute exercise (A-EX) and chronic exercise (C-EX) groups than in the nonexercise (NON-EX) group; *P < .05. (B) Rates of food intake over time in mice euthanized on day 5 after infection revealed a treatment-by-time interaction, such that food intake rates differed among the treatment groups (P = .025). C-EX mice consumed more food than NON-EX mice; *P < .05. (C) A treatment-by-time interaction (P = .008) was observed with respect to food intake in mice euthanized on day 10 after infection. In follow-up analyses, C-EX mice consumed more food than either A-EX or NON-EX mice; *P < .05.
Viral titers
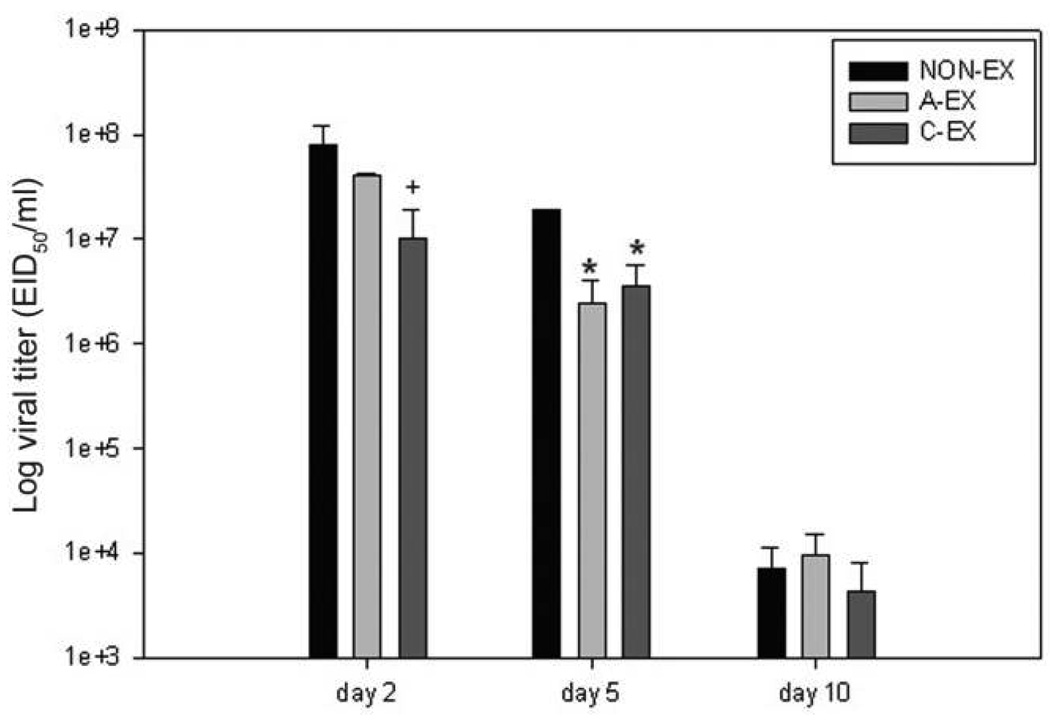
At day 2 after infection, there was a trend (P = .07) toward lower lung viral titer in C-EX mice than in NON-EX mice (Figure 3). At day 5 after infection, both A-EX and C-EX mice had significantly reduced viral titers compared with NON-EX mice (P = .02). At day 10 after infection, the majority of mice no longer had detectable virus in the lung lobes, and there were no differences between treatment groups.
Figure 3.
Lung viral titers assessed with polymerase chain reaction are shown for days 2, 5, and 10 after infection. On day 2 after infection, mice in the chronic exercise (C-EX) group show a trend toward less virus; +P = .07. At day 5 after infection, viral titer is lower in the acute exercise (A-EX) and C-EX groups than in the nonexercise (NON-EX) group; *P < .05. At day 10, virus is cleared or at low levels, and there were no significant differences between treatment groups. EID50, 50% egg infective dose.
BAL fluid cytokines and chemokines
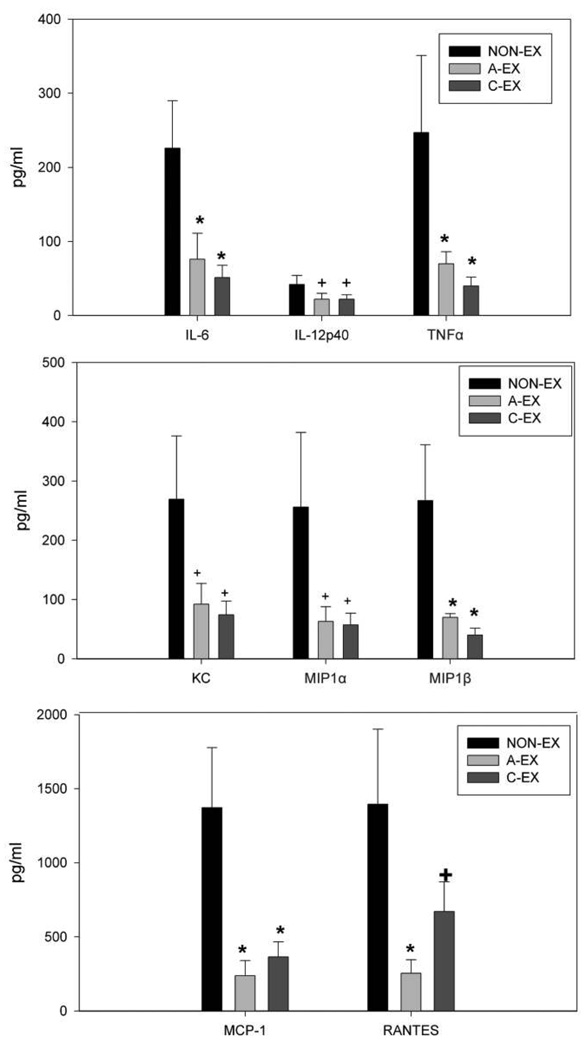
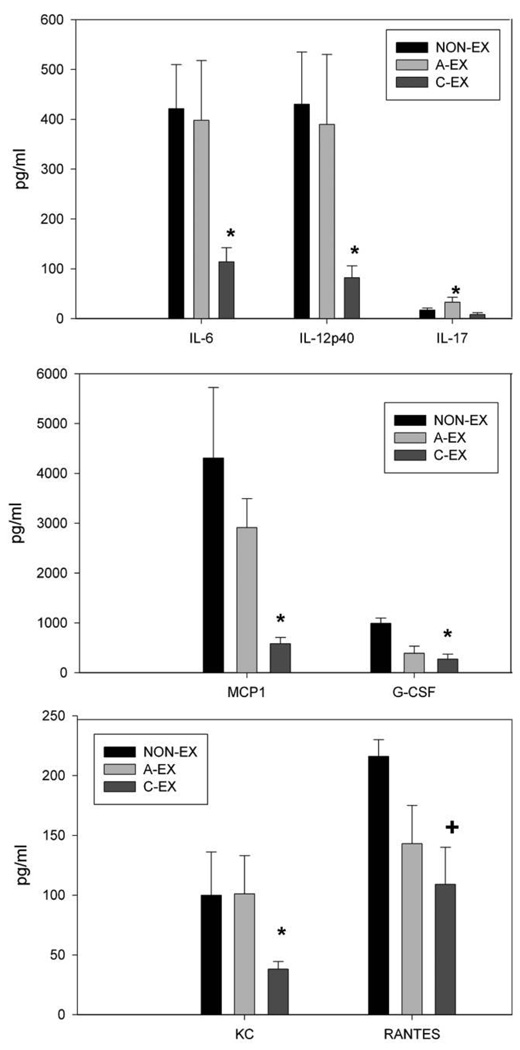
At day 2 after infection, levels of IL-6 and TNF-α were significantly lower in C-EX and A-EX mice than in NON-EX mice (P < .05) (Figure 4). The chemokines MCP-1 and MIP-1β were also found at lower levels in C-EX and A-EX mice than in NON-EX mice (P < .05). For RANTES, a trend was observed toward reduced levels in C-EX compared with NON-EX mice, and levels in A-EX mice were significantly lower than those in NON-EX mice (P < .05). At day 5 after infection, levels of cytokines IL-6 and IL-12p40 were significantly lower in C-EX mice than in NON-EX and A-EX mice (P < .05) (Figure 5). Also at day 5 after infection, levels of MCP-1, KC, and G-CSF in C-EX mice were lower than those in NON-EX mice (P < .05) but not different from those in A-EX mice, with the exception of KC levels, which were significantly lower in C-EX than in A-EX mice (Figure 5). RANTES levels tended to be lower in C-EX than in NON-EX mice (P = .08). At day 10 after infection, there was a trend toward higher levels of IL-6, IL-9, and IL-10 in C-EX mice than in NON-EX mice (P between .05 and .1). By day 10, many of the cytokine and chemokines had returned to the same level observed in noninfected mice, with the exception of IL-6, IL-9, IL-10, IL-12p40, eotaxin, G-CSF, MCP-1, and RANTES. Finally, there were multiple chemokines and cytokines induced by infection but not significantly affected by either exercise treatment at any of the time points measured (IL-1α, IL-1β, IL-5, IL-12p70, IL-13, IFN-γ, GM-CSF; data not shown).
Figure 4.
Chemokines or cytokines measured in bronchoalveolar lavage (BAL) fluid by multiplex assay at day 2 after infection. Levels of interleukin 6 (IL-6), tumor necrosis factor α (TNFα), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1β (MIP-1β) in BAL fluid were lower in the chronic exercise (C-EX) and acute exercise (A-EX) groups than in the nonexercise (NON-EX) group; *P < .05. There was a trend toward lower levels of keratinocyte-derived chemokine (KC), interleukin 12p40 (IL-12p40), RANTES (regulated on activation, normal T cell expressed and secreted), and MIP-1α in C-EX and A-EX mice than in NON-EX mice; +P ≤ .10.
Figure 5.
Chemokines or cytokines measured in bronchoalveolar lavage (BAL) fluid at day 5 after infection by multiplex assay. Levels of interleukin 6 (IL-6), interleukin 12p40 (IL-12p40), and keratinocyte-derived chemokine (KC) were reduced in the BAL fluid of mice in the chronic exercise (CEX) group compared with those in the acute exercise (A-EX) or nonexercise (NON-EX) group; *P < .05. Levels of monocyte chemoattractant protein 1 (MCP-1) and granulocyte colony-stimulating factor (G-CSF) were significantly lower in C-EX mice than in NON-EX mice (*P < .05), but the reduction in RANTES (regulated on activation, normal T cell expressed and secreted) in C-EX mice compared with NON-EX mice only approached statistical significance; +P = .08.
Cell populations
The numbers of neutrophils and CD8+ T cells tended to be lowest in C-EX mice at day 2 after infection and lower in both exercise groups (C-EX and A-EX) at day 5 after infection (Table 1). At day 10 after infection, no significant treatment differences were found.
Table 1.
Neutrophil and CD8+ Cell Counts by Exercise Group on Days 2, 5, and 10 After Infection
| Cell type, mouse group |
Cell count, mean ± standard error | ||
|---|---|---|---|
| Day 2 | Day 5 | Day 10 | |
| Neutrophils | |||
| NON-EX mice | 12,706 ± 1304 | 7202 ± 2064 | 4084 ± 584 |
| A-EX mice | 19,692 ± 4021 | 3786 ± 790 | 4593 ± 648 |
| C-EX mice | 9394 ± 815 | 2634 ± 892 | 2099 ± 747 |
| CD8+ cells | |||
| NON-EX mice | 980 ± 115 | 6379 ± 4715 | 8846 ± 4802 |
| A-EX mice | 772 ± 278 | 1019 ± 308 | 29,481 ± 5589 |
| C-EX mice | 464 + 115 | 1371 + 1040 | 11,569 + 1127 |
Note. Values represent relative counts at flow cytometry, with cells adjusted to 1 × 105 cells/mL and 500 µL analyzed (50,000 cells total). The numbers indicate the percentage of cells staining positive multiplied by the total number of cells analyzed. P values for the comparisons were as follows: neutrophils on day 2,P = .09; neutrophils on day 5, P = .15; CD8+ cells on day 2, P = .31; and CD8+ cells on day 5 (P = .13). A-EX, 1 session of acute exercise; C-EX, chronic exercise for ~3.5 months; NON-EX, no exercise.
Lesion scores
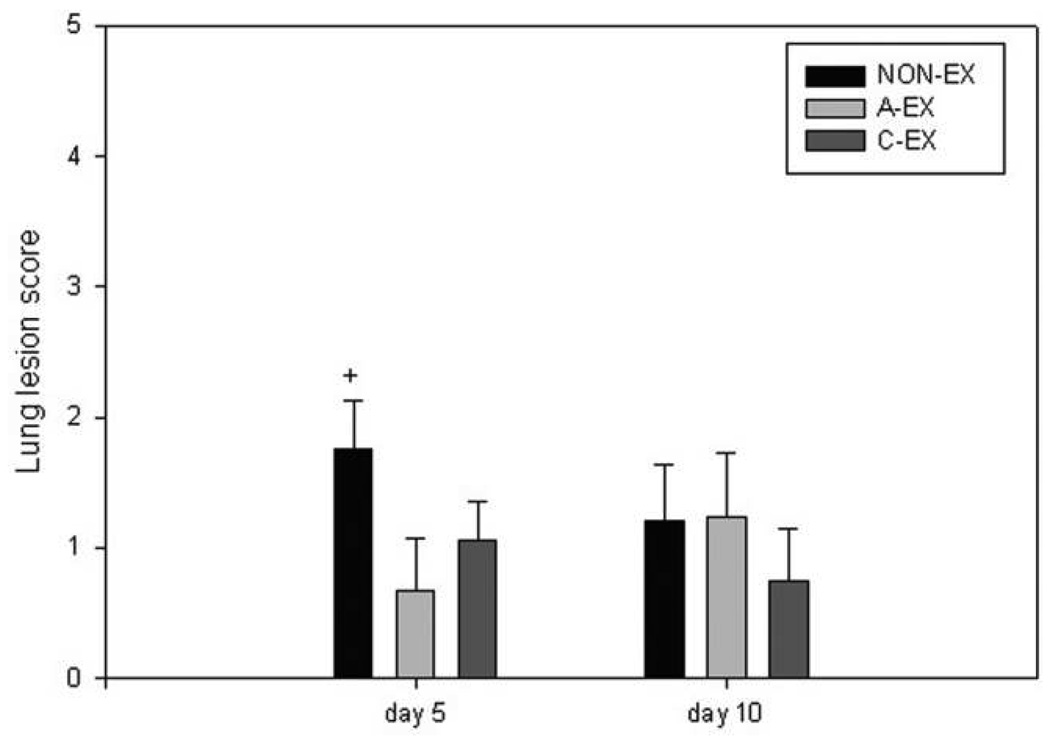
Lung lesion scores suggest that lesion severity tends to be reduced in A-EX and C-EX mice compared with NON-EX mice at day 5 after infection (P = .07) (Figure 6). At day 10 after infection, the apparent reduction in lesion score in C-EX mice compared with NON-EX and A-EX mice did not reach statistical significance.
Figure 6.
Lung lesion scores. The lesion scores from 0 to 3 were averaged across mice in each treatment group. There was a trend toward greater lesion scores in the nonexercise (NON-EX) group; +P = .07. A-EX, acute exercise; C-EX, chronic exercise.
DISCUSSION
Chronic exercise appeared to have the greatest benefit in terms of the reduction of illness symptoms (eg, decreased appetite, weight loss). Acute and chronic exercise resulted in reduced infection-associated weight loss and improved food intake. This effect appeared to persist throughout the course of infection in C-EX mice, whereas the benefit appeared to be limited to the early phase of infection in the A-EX group. The immunopathologic findings correlate with the symptoms of illness and lend further support to the finding that illness is reduced by moderate exercise training.
At 2 days after infection, there was a trend toward less virus in the lungs of exercised mice; this difference approached statistical significance only in the C-EX mice. By 5 days after infection, exercised mice had lower lung virus titers, and the magnitude of the reduction in virus was comparable for mice in the 2 types of exercise groups. These findings suggest that the early innate antiviral defenses appear to be improved by identified. Type I interferons have long been known to exhibit antiviral action and are produced by multiple cell types in the respiratory tract [12–14]. However, we did not observe enhanced production of IFN-α in the BAL fluid of exercised mice measured 48 h after infection, although exercise may have had an effect at earlier time points (data not shown). Others have shown that peak IFN-β levels are found 48 h after infection [13]. A trend toward an exercise-associated increase in IFN-β 24 h after infection was observed when mice were exercised on days 0–3 after infection [23]. IFN-β was not measured in our study owing to limited BAL fluid. NK cells also contribute to the innate defense against influenza virus infection, and depletion of this cell population results in greater mortality [25]. We did not assess lung NK function owing to limited cell numbers in the BAL, and we are not aware of other exercise studies that have evaluated lung NK response during infection.
Other immune-mediated defenses that could contribute to an early reduction in viral titer include the β-defensins, collectins, and lung surfactant proteins A and D. These proteins have been shown to play a role in protection from influenza infection through actions such as neutralization of influenza virus and opsonization [26–29]. It has been shown that an acute session of intense exercise enhances surfactant-mediated phagocytosis by alveolar macrophages [30]. To our knowledge, the effect of regular exercise training on the function of any of these innate antiviral defenses (β-defensins, collectins, or surfactant proteins) during infection has not been evaluated. Studies of airway function and inflammation in elite athletes show a higher prevalence of respiratory symptoms and airway inflammation [31, 32]. Although higher numbers of neutrophils, eosinophils, and lymphocytes have been observed in elite athletes, greater cell activation may not be present [32–34]. Elite athletes may have prolonged periods of hyperpnea with intense exercise, but moderate exercise, like that used in our study, would not be expected to cause hyperpnea. Therefore, further research on lung adaptations to moderate exercise is necessary to provide a better understanding of protective mechanisms.
Exercise also reduced inflammatory factors in the lungs. Levels of IL-6, TNF-α, MCP-1, MIP-1β, KC, and RANTES were reduced at day 2 after infection in both groups of exercised mice. This effect appeared to be transient in the A-EX mice but persisted in the C-EX mice. The decrease in TNF-α may be relevant to the reduced morbidity found in both exercise groups. Elevated TNF-α levels have been linked to increased mortality, supported by the finding that TNF-α knockout mice have improved survival after influenza infection [35].
The early changes in chemokines (MCP-1, MIP-1β, RANTES) observed in both exercise treatments may affect inflammatory cell recruitment. Mice deficient in CCR2+ (MCP-1 receptor) show reduced infiltration of inflammatory cells leading to reduced mortality and immunopathologic response [18]. Perhaps the reduction of MCP-1 that we observed at days 2 and 5 after infection in C-EX mice contributed to the reduced morbidity. We did not assess CCR2 in our study; although the data on lung cell populations did not reach statistical significance, they followed a pattern that would be predicted by the change in chemokines (reduced KC levels would predict the trend toward lower numbers of neutrophils in A-EX and C-EX mice). Neutrophil accumulation in the lungs may play a role in virus elimination, but massive accumulation of neutrophils associated with a secondary infection has been correlated with increased mortality [36, 37]. On the basis of symptoms and lung viral load, one can conclude that the reduction in the chemokine KC along with the tendency toward reduced neutrophil accumulation found in our study did not impair the response to infection. Explanations include the possibility that neutrophils are not critical to recovery from infection or that very early innate defenses reduced viral titer enough that a large neutrophil influx was not necessary.
Our findings can be compared with those of another study in which exercised mice were challenged with influenza virus [23]. The results from both studies showed decreased KC and a tendency to reduced leukocyte infiltration. However, the other researchers concluded that exercise may shift toward a Th2 profile, whereas our results showed a decrease in IL-12. It is not clear whether an enhancement of Th2 responses would be of benefit, because some have suggested that IL-4 may lead to impaired clearance of primary infection or delayed clearance of secondary influenza infection [38, 39]. The studies used different techniques to measure cytokines and chemokines, as well as different exercise models, which may account for the discrepancies in findings.
In summary, the findings presented here first demonstrate that repeated moderate exercise before infection can positively affect infection outcome. A single session of exercise also confers some benefit, although this appears to be present only in the first days after infection. Coupled with the improvement in morbidity and reduction of viral load is a pattern of changes in chemokines and cytokines that provides some insight into potential mechanisms of action, but further research is necessary to identify these mechanisms precisely. Our findings are also the first to show that regular moderate exercise before infection has anti-inflammatory effects at a local site of infection, expanding the current literature on the anti-inflammatory benefits of exercise.
Acknowledgments
Financial support: National Institute of Allergy and Infectious Diseases (grant R01 AI059455-01).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: A portion of this research was presented at the annual American College of Sports Medicine meeting in Indianapolis, IN, 28–31 May 2008.
References
- 1.Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, Hebert JA. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34:1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Wong CM, Lai HK, Ou CQ, et al. Is exercise protective against influenza-associated mortality? PLoS ONE. 2008;3:e2108. doi: 10.1371/journal.pone.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieman DC, Johanssen LM, Lee JW, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30:316–328. [PubMed] [Google Scholar]
- 4.Peters EM, Bateman ED. Ultramarathon running and upper respiratory tract infections: an epidemiological survey. S Afr Med J. 1983;64:582–584. [PubMed] [Google Scholar]
- 5.Davis JM, Kohut ML, Colbert LH, Jackson DA, Ghaffar A, Mayer EP. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J Appl Physiol. 1997;83:1461–1466. doi: 10.1152/jappl.1997.83.5.1461. [DOI] [PubMed] [Google Scholar]
- 6.Kiel RJ, Smith FE, Chason J, Khatib R, Reyes MP. Coxsackievirus B3 myocarditis in C3H/HeJ mice: description of an inbred model and the effect of exercise on virulence. Eur J Epidemiol. 1989;5:348–350. doi: 10.1007/BF00144836. [DOI] [PubMed] [Google Scholar]
- 7.Lowder T, Padgett DA, Woods JA. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun. 2005;19:377–380. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Murphy EA, Davis JM, Carmichael MD, Gangemi JD, Ghaffar A, Mayer EP. Exercise stress increases susceptibility to influenza infection. Brain Behav Immun. 2008;22:1152–1155. doi: 10.1016/j.bbi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Gleeson M, Bishop NC. The T cell and NK cell immune response to exercise. Ann Transplant. 2005;10:43–48. [PubMed] [Google Scholar]
- 10.Nieman DC. Immune response to heavy exertion. J Appl Physiol. 1997;82:1385–1394. doi: 10.1152/jappl.1997.82.5.1385. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai Y, Takeuchi O, Kato H, et al. Alveolar macrophages are the primary interferon-α producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Jewell NA, Vaghefi N, Mertz SE, et al. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J Virol. 2007;81:9790–9800. doi: 10.1128/JVI.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronni T, Matikainen S, Sareneva T, et al. Regulation of IFN-α/β, MxA, 2′5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J Immunol. 1997;158:2363–2374. [PubMed] [Google Scholar]
- 15.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 16.Koyama S, Ishii KJ, Coban C, Akira S. Innate immune response to viral infection. Cytokine. 2008;43:336–341. doi: 10.1016/j.cyto.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 18.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 19.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 20.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 21.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 22.Kohut ML, Boehm GW, Moynihan JA. Moderate exercise is associated with enhanced antigen-specific cytokine, but not IgM antibody production in aged mice. Mech Ageing Dev. 2001;122:1135–1150. doi: 10.1016/s0047-6374(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 23.Lowder T, Padgett DA, Woods JA. Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev. 2006;12:97–111. [PubMed] [Google Scholar]
- 24.Richt JA, Lager KM, Janke BH, Woods RD, Webster RG, Webby RJ. Pathogenic and antigenic properties of phylogenetically distinct reassortant H3N2 swine influenza viruses cocirculating in the United States. J Clin Microbiol. 2003;41:3198–3205. doi: 10.1128/JCM.41.7.3198-3205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein-Streilein J, Guffee J, Fan W. Locally and systemically derived natural killer cells participate in defense against intranasally inoculated influenza virus. Reg Immunol. 1988;1:100–105. [PubMed] [Google Scholar]
- 26.Benne CA, Benaissa-Trouw B, van Strijp JA, Kraaijeveld CA, van Iwaarden JF. Surfactant protein A, but not surfactant protein D, is an opsonin for influenza A virus phagocytosis by rat alveolar macrophages. Eur J Immunol. 1997;27:886–890. doi: 10.1002/eji.1830270413. [DOI] [PubMed] [Google Scholar]
- 27.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong KT, Thangavel RR, Tang X. Enhanced expression of murine beta-defensins (MBD-1, -2, -3, and -4) in upper and lower airway mucosa of influenza virus infected mice. Virology. 2008;380:136–143. doi: 10.1016/j.virol.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Hartshorn KL, Webby R, White MR, et al. Role of viral hemagglutinin glycosylation in anti-influenza activities of recombinant surfactant protein D. Respir Res. 2008;23:9–65. doi: 10.1186/1465-9921-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su SH, Chen HI, Jen CJ. Exercise enhances surfactant-mediated phagocytosis in bronchoalveolar macrophages. Chin J Physiol. 2005;48:210–216. [PubMed] [Google Scholar]
- 31.Langdeau JB, Boulet LP. Prevalence and mechanisms of development of asthma and airway hyperresponsiveness in athletes. Sports Med. 2001;31:601–616. doi: 10.2165/00007256-200131080-00005. [DOI] [PubMed] [Google Scholar]
- 32.Bonsignore MR, Morici G, Vignola AM, et al. Increased airway inflammatory cells in endurance athletes: what do they mean? Clin Exp Allergy. 2003;33:14–21. doi: 10.1046/j.1365-2222.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 33.Bermon S. Airway inflammation and upper respiratory tract infection in athletes: is there a link? Exerc Immunol Rev. 2007;13:6–14. [PubMed] [Google Scholar]
- 34.Helenius I, Lumme A, Haahtela T. Asthma, airway inflammation and treatment in elite athletes. Sports Med. 2005;35:565–574. doi: 10.2165/00007256-200535070-00002. [DOI] [PubMed] [Google Scholar]
- 35.Peper RL, Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog. 1995;19:175–183. doi: 10.1006/mpat.1995.0056. [DOI] [PubMed] [Google Scholar]
- 36.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol. 2008;82:2772–2783. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MW, Schmidt JE, Rehg JE, Orihuela CJ, McCullers JA. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp Med. 2007;57:82–89. [PMC free article] [PubMed] [Google Scholar]
- 38.Bot A, Holz A, Christen U, et al. Local IL-4 expression in the lung reduces pulmonary influenza-virus-specific secondary cytotoxic T cell responses. Virology. 2000;269:66–77. doi: 10.1006/viro.2000.0187. [DOI] [PubMed] [Google Scholar]
- 39.Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]