Abstract
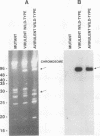
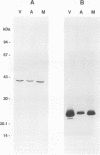
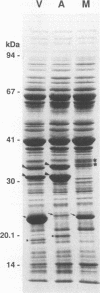
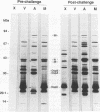
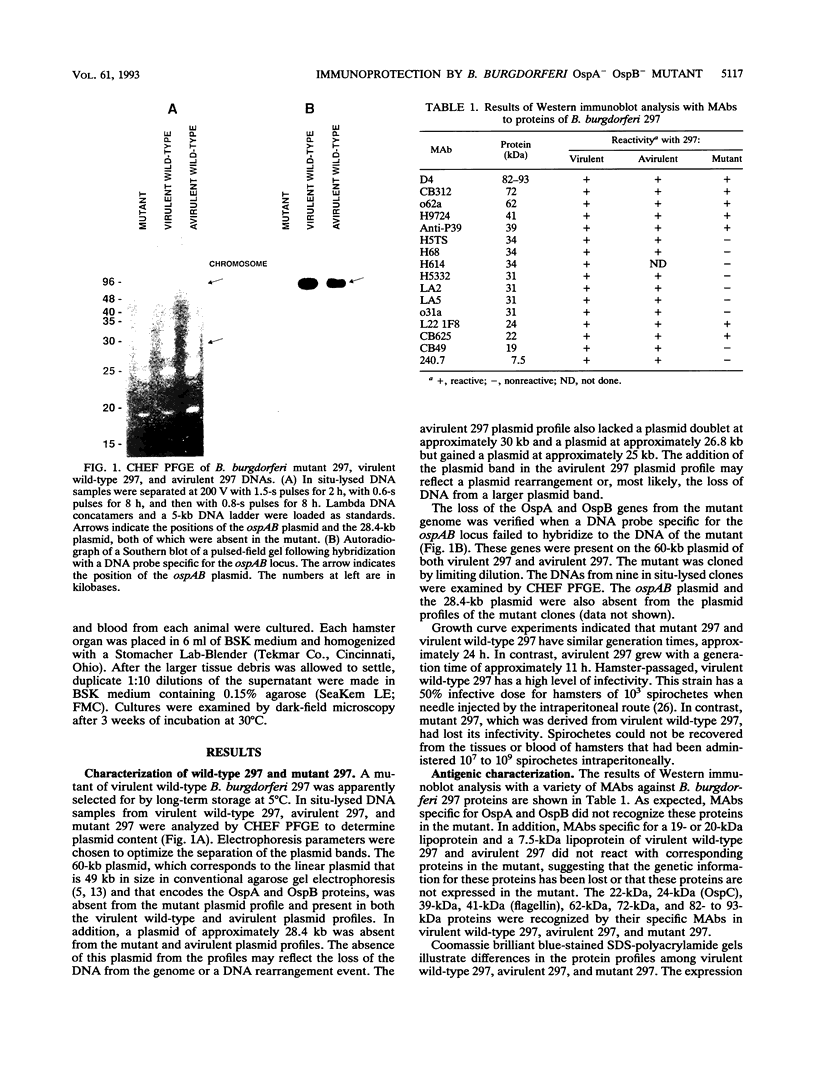
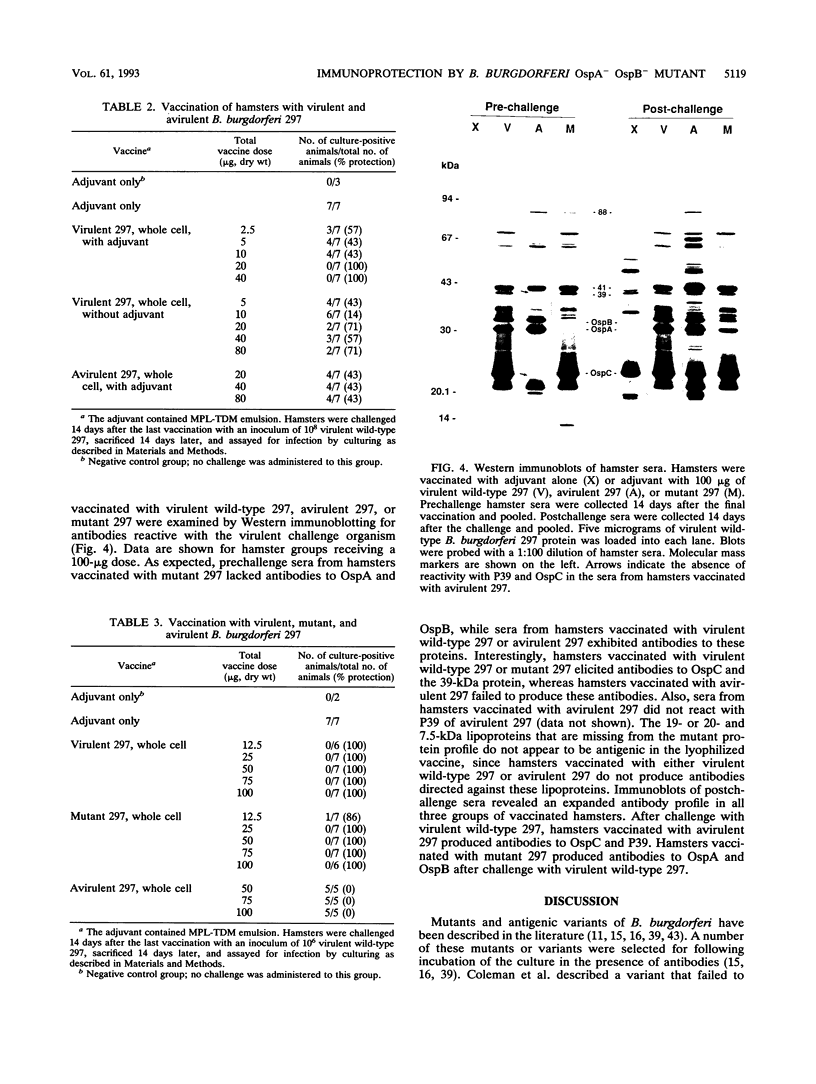
A mutant of virulent Borrelia burgdorferi 297 was apparently selected for by long-term storage at 5 degrees C. This mutant was found to lack the plasmid which encodes outer surface protein A (OspA) and OspB. In addition to the loss of the OspA and OspB proteins, the mutant lacked two lipoproteins, of 20 and 7.5 kDa, that were observed in the wild type. Since the mutant was not recovered from the tissues or blood of hamsters injected with the mutant, the mutant was determined to be noninfectious. Hamsters vaccinated with noninfectious mutant 297 were protected completely from challenge with virulent wild-type 297 spirochetes. Prechallenge sera from hamsters vaccinated with mutant 297 lacked antibodies to OspA and OspB, while those from hamsters vaccinated with virulent wild-type 297 or avirulent 297 exhibited antibodies to these proteins. Hamsters vaccinated with virulent wild-type 297 or mutant 297 elicited antibodies to OspC and a 39-kDa protein (P39), whereas hamsters vaccinated with avirulent 297 lacked these antibodies. These results suggest that OspC and/or P39 are important for the development of a protective immune response. Study of this mutant may elucidate factors important to the development of a Lyme disease vaccine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel M. J., Allan S., Jacobson R. H., Lauderdale T. L., Chang Y. F., Shin S. J., Thomford J. W., Todhunter R. J., Summers B. A. Experimental Lyme disease in dogs produces arthritis and persistent infection. J Infect Dis. 1993 Mar;167(3):651–664. doi: 10.1093/infdis/167.3.651. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Hansen G. M., Terwilliger G. A., Moody K. D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990 Jul;162(1):133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Berger B. W., Johnson R. C., Kodner C., Coleman L. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J Clin Microbiol. 1992 Feb;30(2):359–361. doi: 10.1128/jcm.30.2.359-361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Casjens S., Huang W. M. Linear chromosomal physical and genetic map of Borrelia burgdorferi, the Lyme disease agent. Mol Microbiol. 1993 May;8(5):967–980. doi: 10.1111/j.1365-2958.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Chu H. J., Chavez L. G., Jr, Blumer B. M., Sebring R. W., Wasmoen T. L., Acree W. M. Immunogenicity and efficacy study of a commercial Borrelia burgdorferi bacterin. J Am Vet Med Assoc. 1992 Aug 1;201(3):403–411. [PubMed] [Google Scholar]
- Cinco M. Selection of a Borrelia burgdorferi antigenic variant by cultivation in the presence of increasing amounts of homologous immune serum. FEMS Microbiol Lett. 1992 Apr 1;71(1):15–18. doi: 10.1016/0378-1097(92)90534-u. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Rogers R. C., Benach J. L. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992 Aug;60(8):3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdile L. F., Brandt M. A., Warakomski D. J., Westrack G. J., Sadziene A., Barbour A. G., Mays J. P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993 Jan;61(1):81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect Immun. 1992 Mar;60(3):773–777. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Persing D. H., Sun X., Kantor F. S., Flavell R. A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992 Apr 1;148(7):2256–2260. [PubMed] [Google Scholar]
- Goodman J. L., Jurkovich P., Kramber J. M., Johnson R. C. Molecular detection of persistent Borrelia burgdorferi in the urine of patients with active Lyme disease. Infect Immun. 1991 Jan;59(1):269–278. doi: 10.1128/iai.59.1.269-278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch J., Barbour A. G. Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J Bacteriol. 1991 Nov;173(22):7233–7239. doi: 10.1128/jb.173.22.7233-7239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Kodner C. B., Johnson R. C. DNA analysis of Borrelia burgdorferi NCH-1, the first northcentral U.S. human Lyme disease isolate. J Clin Microbiol. 1992 Mar;30(3):698–703. doi: 10.1128/jcm.30.3.698-703.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M. Active immunization of hamsters against experimental infection with Borrelia burgdorferi. Infect Immun. 1986 Dec;54(3):897–898. doi: 10.1128/iai.54.3.897-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M., Duray P. H. Experimental infection of the hamster with Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:258–263. doi: 10.1111/j.1749-6632.1988.tb31859.x. [DOI] [PubMed] [Google Scholar]
- Jonsson M., Noppa L., Barbour A. G., Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992 May;60(5):1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashige S., Bissett M., Oshiro L. Characterization of a tick isolate of Borrelia burgdorferi that possesses a major low-molecular-weight surface protein. J Clin Microbiol. 1990 Jun;28(6):1362–1366. doi: 10.1128/jcm.28.6.1362-1366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ma B., Christen B., Leung D., Vigo-Pelfrey C. Serodiagnosis of Lyme borreliosis by western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol. 1992 Feb;30(2):370–376. doi: 10.1128/jcm.30.2.370-376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Konkel M. E., Garon C. F. Variability of osp genes and gene products among species of Lyme disease spirochetes. Infect Immun. 1993 Jun;61(6):2611–2617. doi: 10.1128/iai.61.6.2611-2617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis N., Rosa P. A. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M. T., Aydintug M. K., Bohm R. P., Jr, Cogswell F. B., Dennis V. A., Lanners H. N., Lowrie R. C., Jr, Roberts E. D., Conway M. D., Karaçorlu M. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993 Jul;61(7):3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Patsouris E., Jauris S., Will G., Soutschek E., Rainhardt S., Lehnert G., Klockmann U., Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992 Nov-Dec;20(6):342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- Sadziene A., Barbour A. G., Rosa P. A., Thomas D. D. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun. 1993 Sep;61(9):3590–3596. doi: 10.1128/iai.61.9.3590-3596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A., Wilske B., Ferdows M. S., Barbour A. G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993 May;61(5):2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. L., Schell R. F., Hejka A. G., England D. M. Passive immunization prevents induction of Lyme arthritis in LSH hamsters. Infect Immun. 1990 Jan;58(1):144–148. doi: 10.1128/iai.58.1.144-148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. M., Schaible U. E., Wallich R., Kramer M. D. A mouse model for Borrelia burgdorferi infection: approach to a vaccine against Lyme disease. Immunol Today. 1991 Jan;12(1):11–16. doi: 10.1016/0167-5699(91)90106-4. [DOI] [PubMed] [Google Scholar]
- Simpson W. J., Burgdorfer W., Schrumpf M. E., Karstens R. H., Schwan T. G. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J Clin Microbiol. 1991 Feb;29(2):236–243. doi: 10.1128/jcm.29.2.236-243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Sădziene A., Rosa P. A., Thompson P. A., Hogan D. M., Barbour A. G. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992 Sep 1;176(3):799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmoen T. L., Sebring R. W., Blumer B. M., Chavez L. G., Jr, Chu H. J., Acree W. M. Examination of Koch's postulates for Borrelia burgdorferi as the causative agent of limb/joint dysfunction in dogs with borreliosis. J Am Vet Med Assoc. 1992 Aug 1;201(3):412–418. [PubMed] [Google Scholar]
- Wilske B., Luft B., Schubach W. H., Zumstein G., Jauris S., Preac-Mursic V., Kramer M. D. Molecular analysis of the outer surface protein A (OspA) of Borrelia burgdorferi for conserved and variable antibody binding domains. Med Microbiol Immunol. 1992;181(4):191–207. doi: 10.1007/BF00215765. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Jauris S., Hofmann A., Pradel I., Soutschek E., Schwab E., Will G., Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Zumstein G., Fuchs R., Hofmann A., Preac-Mursic V., Soutschek E., Wilske B. Genetic polymorphism of the gene encoding the outer surface protein A (OspA) of Borrelia burgdorferi. Med Microbiol Immunol. 1992;181(2):57–70. doi: 10.1007/BF00189424. [DOI] [PubMed] [Google Scholar]
- Zöller L., Burkard S., Schäfer H. Validity of western immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991 Jan;29(1):174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]