Abstract
Attack of cellular thiols on the antitumor natural product leinamycin is believed to generate a sulfenate intermediate that undergoes subsequent rearrangement to a DNA-alkylating episulfonium ion. Here, 2-(trimethylsilyl)ethyl sulfoxides were employed in a fluoride-triggered generation of sulfenate anions related to the putative leinamycin-sulfenate. The resulting sulfenates enter smoothly into a leinamycin-type rearrangement reaction to afford an episulfonium ion alkylating agent. The results provide evidence that the sulfenate ion is, indeed, a competent intermediate in the leinamycin rearrangement. Further, the molecules examined here may provide a foundation for the design of functional leinamycin analogues that bypass the unstable and synthetically challenging 1,2-dithiolan-3-one 1-oxide moiety found in the natural product.
Introduction
Historically, natural products have represented a rich source of structurally novel organic molecules that generate DNA-damaging reactive intermediates via interesting and unexpected chemical reactions.1,2 The characterization of new chemical reactions by which small molecules can modify cellular DNA is relevant to diverse fields including medicinal chemistry, toxicology, and biotechnology.
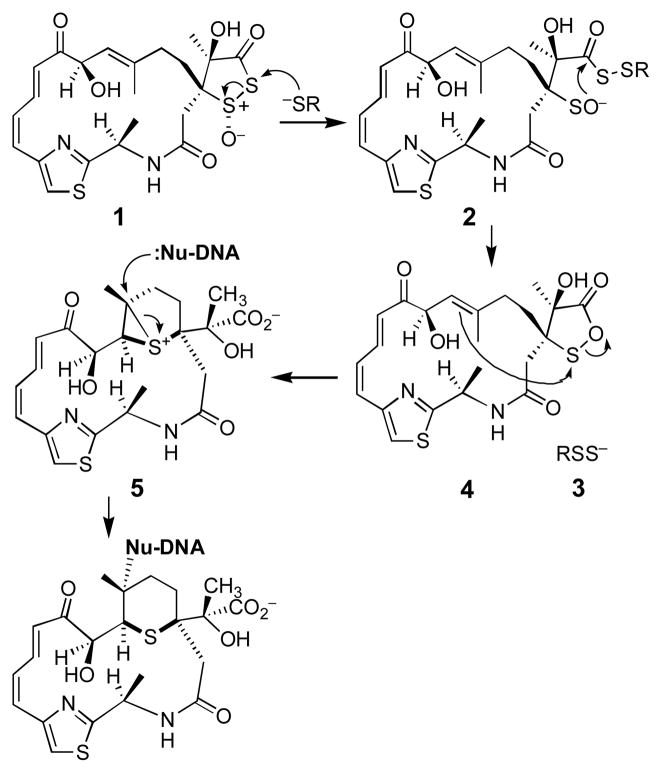
Leinamycin (1) provides an interesting example of a structurally unique natural product that damages DNA via novel chemical mechanisms.3–6 Initial attack of cellular thiols on leinamycin’s 1,2-dithiolan-3-one 1-oxide “triggering unit” is believed to yield a key sulfenate intermediate (2) that undergoes intramolecular cyclization onto the neighboring carbonyl group.7,8 The persulfide (3, RSS−) ejected in this reaction causes oxidative stress,9–13 while the resulting 1,2-oxathiolan-5-one derivative of leinamycin (4) undergoes further rearrangement to yield an episulfonium ion (5) that alkylates guanine residues in duplex DNA (Scheme 1).8–14
Scheme 1.
DNA alkylation by leinamycin (1).
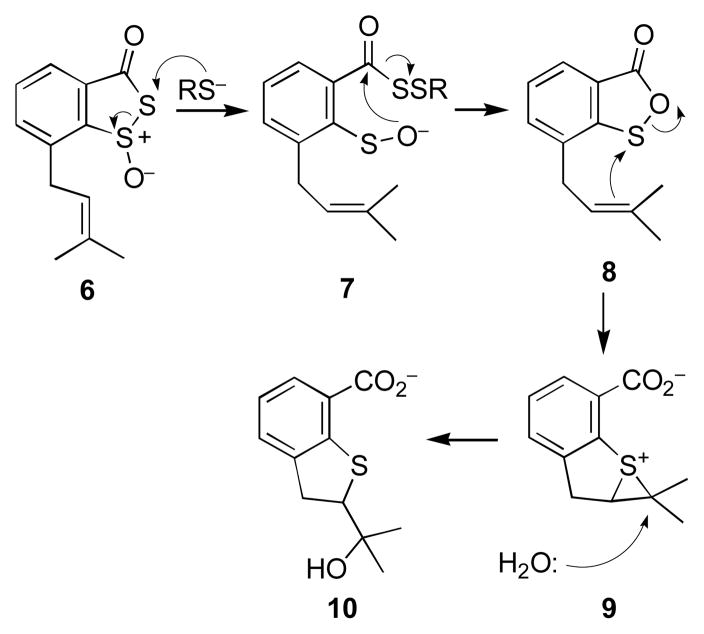
The sulfenate ion (2) is proposed7,8 to be a key intermediate in the thiol-triggered conversion of leinamycin to a DNA-alkylating episulfonium ion; however, to date, there is no experimental support for the existence of this entity. In an effort to fill this gap in our knowledge, we set out to generate discrete sulfenate ions related to 2 and determine whether these intermediates are, in fact, competent to enter the leinamycin rearrangement reaction manifold. For this task, we employed small synthetic molecules containing just the “core” functional groups involved in the leinamycin rearrangement. This approach builds upon our recent finding15 that stripped-down leinamycin analogues such as 6 smoothly undergo a thiol-triggered, leinamycin-type rearrangement to generate the episulfonium alkylating agent 9 (Scheme 2).
Scheme 2.
A small model compound that mimics leinamycin (Ref. 15).
Sulfenate ions (RSO−) and sulfenic acids (RSOH) typically are not stable, isolable species;16–18 however, methods exist for their in situ generation.16,19–28 In the present study, we employed the 2-(trimethylsilyl)ethyl sulfoxide group as a sulfenate precursor. 2-(Trimethylsilyl)ethyl sulfoxides can undergo both fluoride-triggered and spontaneous elimination of sulfenate species.29 For ease of synthesis, we targeted sulfenates containing a neighboring phenyl thioester group in place of the acyl persulfide moiety found in the putative intermediates 2 and 7 (Schemes 1 and 2). Importantly, the leaving group ability of the PhS− group is similar to that expected for RSS−, as judged by the pKa values of the conjugate acids.30,31
Results and discussion
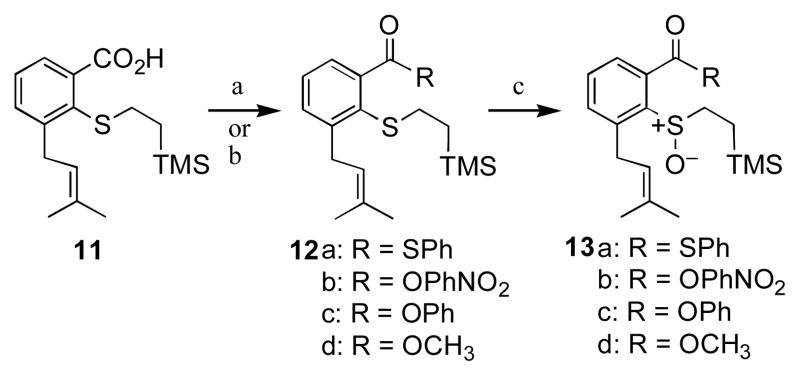
The sulfenate precursors were prepared as shown in Scheme 3. The known15 carboxylic acid 11 was activated with DCC/DMAP and converted to the thioester 12a by reaction with thiophenol. In addition, we prepared ester derivatives 12b and c by analogous reactions. The methyl ester 12d was synthesized by treatment of 11 with diazomethane. The desired sulfenate precursors 13 were then obtained via oxidation of the sulfide group in 12 with dimethyl dioxirane (DMD).32
Scheme 3.
Preparation of sulfenate precursors 13. Reagents and conditions: a. DCC, DMAP, PhSH or p-NO2PhOH, or PhOH (for 12a–c), b. CH2N2 (for 12d), c. DMD, acetone.
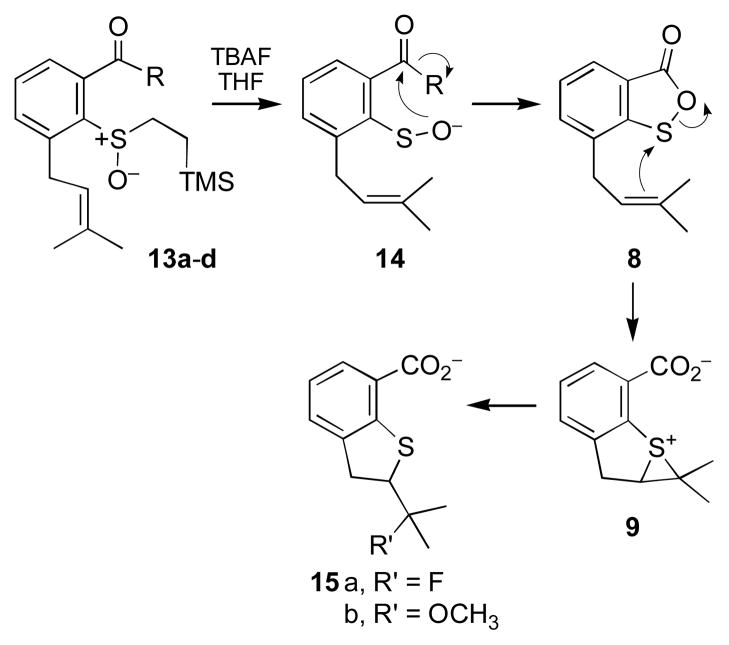
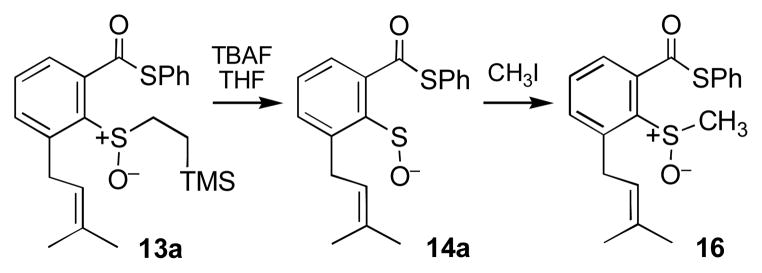
Treatment of the thioester 13a with tetrabutylammonium fluoride (TBAF) in THF rapidly (3 h) affords the rearrangement product 15a (65%, Scheme 4). This product is envisioned to arise from reaction of the episulfonium ion 9 with excess fluoride ion. When the TBAF-triggered reaction is carried out in a 4:1 mixture of THF and methanol, the product (15b) resulting from trapping of the episulfonium ion 9 with methanol is obtained in 22% yield alongside 15a (45%). The acids were characterized as the methyl ester derivatives obtained following treatment of the products with diazomethane.
Scheme 4.
Fluoride-triggered rearrangement of sulfenate precursors 13.
Consistent with the expectation that this process proceeds via the desired sulfenate ion 14a, when the reaction is conducted in the presence of excess methyl iodide, the characteristic16,33 sulfenate trapping product 16 is obtained in 35% yield along with 15a (25%, Scheme 5). In the context of this reaction, it is useful to note that sulfenate ions are ambident nucleophiles that can react at either sulfur or oxygen.33 In the leinamycin rearrangement, the oxygen atom of the sulfenate is the nucleophile, whereas the sulfur atom serves as a nucleophile in typical reactions of this functional group with methyl iodide and other alkyl halides.16,33,34
Scheme 5.
Trapping the sulfenate intermediate.
The ester derivatives (13b–d) also undergo fluoride-triggered rearrangement in THF to provide 15a in yields comparable to those obtained from 13a. Evidently, a good leaving group (e.g. PhS− or p-NO2PhO−) on the carbonyl is not required for the rearrangement to proceed. The cyclization of 14 to 8 may be favored by the potent nucleophilicity of the sulfenate anion.35
Extended incubation of 13a for 20 h in THF:MeOH in the absence of TBAF does not afford any rearranged product 15b, yielding instead only the product 13d resulting from methanolysis of the thioester group in the starting material. However, in a different solvent mixture consisting of 1:1 CH3CN and sodium phosphate buffer (50 mM, pH 7), compounds 13a and 13b undergo a slow (48 h), fluoride-independent conversion to the episulfonium-derived product 10, albeit in somewhat lower yields (30%) than those obtained in the fluoride-triggered process.36
Initially, we suspected that this fluoride-independent reaction might proceed via the same sulfenate intermediate (14, Scheme 4) generated in the fluoride-triggered reactions, because it is known that 2-(trimethylsilyl)ethyl sulfoxides can undergo fluoride-independent release of sulfenate species.29 However, the intermediacy of a free sulfenate anion or sulfenic acid in these reactions was called into question by our inability to trap this intermediate with methyl iodide under our standard trapping conditions used previously.34 Further evidence arguing against a straightforward elimination of sulfenate from 13a and 13b in this fluoride-independent process was provided by the observation that the reaction occurs only with the activated esters 13a and 13b. The less reactive esters 13c and 13d return starting material under these reaction conditions. Thus, the 2-(trimethylsilyl)ethyl sulfoxide group is inherently stable in the context of 13c and 13d; however, interaction of this functional group with the adjacent activated ester groups in 13a and 13b stimulates rearrangement to 10. This transformation may proceed via initial attack of the sulfoxide oxygen on the adjacent activated carbonyl to yield 17, followed by loss of the 2-(trimethylsilyl)ethyl group to generate the oxathiolanone intermediate 8 that, in turn, yields the episulfonium ion 9 (Scheme 6).37
Scheme 6.
Proposed mechanism for fluoride-independent conversion of 13a and 13b to 10.
Conclusions
In summary, we utilized 2-(trimethylsilyl)ethyl sulfoxides as precursors in the fluoride-triggered generation of sulfenate ions related to a key intermediate (2) proposed previously in the thiol-triggered alkylation of DNA by leinamycin (Scheme 1). Our results provide evidence that the sulfenate ion is, indeed, a competent intermediate in the leinamycin rearrangement reaction. In addition, for two of the 2-(trimethylsilyl)ethyl sulfoxides (13a and b), we observed an unexpected fluoride-independent reaction in which attack of the sulfoxide group on a neighboring activated ester, followed by loss of the 2-(trimethylsilyl)ethyl group, affords entry into the leinamycin rearrangement via the oxathiolanone intermediate 8. Overall, these studies provide a better grasp of the intermediates involved in the thiol-triggered conversion of leinamycin to a DNA-alkylating agent. In addition, the molecules examined here may provide a foundation for the design of functional leinamycin analogues that bypass the unstable38 and synthetically challenging39,40 1,2-dithiolan-3-one 1-oxide moiety found in the natural product.
Experimental
Materials were purchased from the following suppliers: HPLC grade solvents, Fisher; silica gel 60 (0.04–0.063 mm pore size) for column chromatography, Merck; TLC plates coated with general purpose silica containing UV254 fluorophore, Aldrich Chemical Company; all chemicals were purchased from Aldrich Chemical Company and were of the highest purity available unless otherwise noted. Water was distilled, deionized and glass redistilled. All reactions were carried out under an atmosphere of nitrogen, unless otherwise noted.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethylsulfanyl]-benzoic acid S-phenyl ester 12a
To a solution of 1115 (200 mg, 0.62 mmol) in dry THF (2 mL) maintained under an atmosphere of nitrogen, dicyclohexyl carbodiimide (153 mg, 0.74 mmol) and a catalytic amount of 4-dimethylaminopyridine (7.6 mg, 0.06 mmol) were added. The solution was allowed to stir for 30 min and thiophenol (76μL, 0.74 mmol) was added. The resulting mixture was stirred at 24 °C for 48 h. The dicyclohexylurea precipitate was filtered off and the solvent evaporated under reduced pressure to yield a yellow oil. The crude product was purified by flash column chromatography on silica gel eluted with 19:1 hexane:ethylacetate to yield 12a as a colorless oil (211 mg, 82%, Rf = 0.5 in 10:1 hexane:ethylacetate). 1H-NMR (250 MHz, CDCl3) δ 7.60-7.28 (m, 8H, aromatic), 5.28 (m, 1H), 3.72 (d, 2H), 2.85 (m, 2H), 1.77 (s, 6H), 0.87 (m, 2H), 0.0 (s, 9H) ppm. 13C-NMR (62.9 MHz, CDCl3) δ 193.05, 147.58, 145.64, 134.47, 133.12, 131.49, 130.80, 129.43, 129.24, 128.39, 125.02, 122.78, 33.9, 32.78, 25.77, 18.03, 17.56, −1.80 ppm. HRMS (ESI) calcd for C23H30OS2SiNa [M + Na]+ 437.1399, found 437.1419.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethylsulfanyl]-benzoic acid p-nitro-phenyl ester 12b
To a stirred solution of 1115 (200 mg, 0.62 mmol) in dry, distilled THF (2 mL) under nitrogen, dicyclohexyl carbodiimide (153 mg, 0.74 mmol) and a catalytic amount of 4-dimethylaminopyridine (7.6 mg, 0.06 mmol) were added. After about 30 min of stirring, p-nitrophenol (103 mg, 0.74 mmol) in THF (1 mL) was added and stirring was continued for 48 h. The dicyclohexyl precipitate was removed by filtration and the filtrate was evaporated under reduced pressure to give a dark yellow oil. Flash column chromatography on silica gel eluted with 19:1 hexane:ethylacetate gave 12b as a pale yellow oil (247 mg, 90% yield, Rf = 0.55 in 10:1 hexane:ethylacetate). 1H-NMR (250 MHz, CDCl3) δ 8.38 (d, 2H), 7.57-7.44 (m, 5H), 5.35 (m, 1H), 3.78 (d, 2H), 2.89 (m, 2H), 1.82 (s, 6H), 0.87 (m, 2H), 0.0 (s, 9H) ppm. 13C-NMR (62.9 MHz, CDCl3) δ 166.39, 155.75, 147.78, 145.43, 138.38, 133.36, 132.27, 128.68, 126.04, 125.28, 122.61, 122.47, 33.51, 32.78, 25.76, 18.03, 17.52, −1.91 ppm. HRMS (ESI) calcd for C23H29NO4SSiNa [M + Na]+ 466.1478, found 466.1490.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethylsulfanyl]-benzoic acid phenyl ester 12c
To a stirred solution of 1115 (200 mg, 0.62 mmol) in dry, distilled THF (2 mL) under nitrogen, dicyclohexyl carbodiimide (153 mg, 0.74 mmol) and a catalytic amount of 4-dimethylaminopyridine (7.6 mg, 0.06 mmol) were added. After about 30 min of stirring, phenol (69.64 mg, 0.74 mmol) in dry THF (1 mL) was added and stirring was continued for 48 h. At the end of the reaction, the dicyclohexylurea precipitate was removed by filtration and the filtrate evaporated under reduced pressure to give a pale yellow oil. Flash column chromatography on silica gel eluted with 19:1 hexane:ethylacetate gave 12c as a colorless oil (187 mg, 76% yield, Rf = 0.51 in 10:1 hexane:ethylacetate). 1H-NMR (250 MHz, CDCl3) δ 7.50-7.31 (m, 8H, aromatic), 5.34 (m, 1H), 3.80 (d, 2H), 2.91 (m, 2H), 1.82 (s, 6H), 0.90 (m, 2H), 0.0 (s, 9H) ppm. 13C-NMR (62.9 MHz, CDCl3) δ 167.47, 151.02, 147.49, 139.44, 133.09, 132.09, 131.65, 129.47, 128.51, 125.96, 122.85, 121.58, 33.42, 32.86, 25.76, 18.03, 17.49, −1.90 ppm. HRMS (ESI) calcd for C23H30O2SSiNa [M + Na]+ 421.1627, found 421.1637.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethylsulfanyl]-benzoic acid methyl ester 12d
To a solution of 1115 (50 mg, 0.15 mmol) in ether (1 mL) freshly prepared diazomethane (1 mL of a 0.66 M solution in ether, warning; EXLOSION HAZARD) was added.41 When the reaction was complete as judged by thin layer chromatography the solvent was evaporated under reduced pressure to give 12d as a colorless oil (44 mg, 85%, Rf = 0.68 in 9:1 hexane:ethylacetate) as a pure compound. 1H-NMR (250 MHz, CDCl3) δ 7.32 (m, 3H), 5.27 (m, 1H), 3.93 (s, 3H), 3.70 (d, 2H), 3.81 (m, 2H), 1.76 (s, 1H), 0.83 (m, 2H), 0 (s, 9H) ppm. 13C-NMR (62.9 MHz, CDCl3) δ 169.58, 147.14, 139.97, 132.95, 131.85, 131.20, 128.28, 125.71, 122.91, 52.29, 33.23, 32.94, 25.75, 18.01, 17.48, −1.86 ppm. HRMS (ESI) calcd for C18H28O2SSiNa [M + Na]+ 359.1471, found 359.1460.
General procedure for the conversion of sulfides 12a–d to sulfoxides 13a–d
To a rapidly stirred dilute solution of the sulfide 12a–d (50 mg, 0.12 mmol) in HPLC grade acetone (10 mL) freshly prepared dimethyl dioxirane32 (1.5 mL of a ~ 0.09 M solution in acetone) was added slowly. The reaction was fast and careful monitoring of TLC was essential to limit overoxidation. The solvent mixture was evaporated under reduced pressure to give the sulfoxide 13a–d (37.2 mg, 72%, Rf = 0.56 in 4:1 hexane:ethylacetate) as a colorless oil and as a pure compound. These compounds are unstable and were used without further purification.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethanesulfinyl]-benzoic acid S-phenyl ester 13a
1H-NMR (250 MHz, CDCl3) δ 7.59-7.55 (m, 3H), 7.48-7.41 (m, 5H), 5.22 (m, 1H), 3.79 (m, 2H), 3.34 (m, 1H), 2.98 (m, 1H), 1.73 (s, 6H), 1.22 (m, 1H), 0.78 (m, 1H), 0.03 (s, 9H) ppm. 13C-NMR (62.9 MHz, CDCl3) δ 192.09, 143.16, 140.14, 138.78, 134.61, 133.79, 130.29, 129.59, 129.27, 127.79, 126.38, 122.25, 50.86, 30.37, 25.69, 18.13, 10.89, −1.90 ppm. LRMS (ESI) calcd for C23H31O2S2Si [M + H]+ 431.15, found 431.12.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethanesulfinyl]-benzoic acid p-nitrophenyl ester 13b
1H-NMR (300 MHz, CDCl3) δ 8.34 (d, 2H), 7.29–7.63 (m, 3H), 5.22 (m, 1H), 3.55 (d, 2H), 3.48 (m, 1H), 2.95 (m, 1H), 1.78 (s, 6H), 1.24 (m, 1H), 0.87 (m, 1H), 0.0 (s, 9H) ppm. LRMS (ESI) calcd for C23H30NO5SSi [M + H]+ 460.16, found 460.19.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethanesulfinyl]-benzoic acid phenyl ester 13c
Obtained in 96% yield as a colorless oil (Rf = 0.32 in 5:1 hexane:ethylacetate). 1H-NMR (250 MHz, CDCl3) δ7.64-7.29 (m, 8H, aromatic), 5.23 (m, 1H), 3.66 (d, 2H), 3.49 (m, 1H), 2.99 (m, 1H), 1.76 (s, 6H), 1.27 (m, 1H), 0.84 (m, 1H), 0.02 (s, 9H) ppm. 13C-NMR (62.9 MHz, CDCl3) δ 166.61, 150.74, 141.52, 133.95, 133.04, 131.78, 130.48, 129.46, 127.99, 126.01, 122.05, 121.71, 50.11, 33.87, 32.13, 31.55, 18.14, 11.14, −1.99 ppm. HRMS (ESI) calcd for C23H30O3SSiNa [M + Na]+ 437.1577, found 437.1564.
3-(3-Methyl-but-2-enyl)-2-[2-(trimethylsilanyl)-ethanesulfinyl]-phenyl]-benzoic acid methyl ester 13d
Obtained in 53% as a pure compound (Rf = 0.38 in 5:1 hexane:ethylacetate). 1H-NMR (250 MHz, CDCl3) δ 7.40-7.28 (m, 3H), 5.14 (m, 1H), 3.84 (s, 3H), 3.60 (d, 2H), 3.39 (m, 1H), 2.90 (m, 1H), 1.67 (s, 6H), 1.22 (m, 1H), 0.79 (m, 1H), 0.0 (s, 9H) ppm. 13C-NMR (62.9 MHz, CDCl3) δ 168.45, 141.83, 141.16, 133.73, 132.82, 132.25, 130.33, 127.58, 122.25, 52.63, 50.20, 30.67, 25.70, 18.12, 11.22, −1.91 ppm. HRMS (ESI) calcd for C18H28O3SSiNa [M + Na]+ 375.1420, found 375.1428.
Fluoride-triggered production of 2-(1-fluoro-1-methyl-ethyl)-2,3-dihydro-benzo[b]thiophene-7-carboxylic acid methyl ester (15a) by treatment of (13a–d) with tetrabutylammonium fluoride in THF, followed by diazomethane workup
A solution of 13a (20 mg, 0.046 mmol) in THF (1 mL) was placed in a flame-dried flask flushed with nitrogen. To this solution, tetrabutylammonium fluoride (0.37 mL of a 1 M solution in THF, 0.37 mmol, 0.27 M) from a freshly opened bottle was added. The reaction mixture turned deep yellow and was stirred for 3 h. Dilute HCl (1 mL of a 1M solution, ~ pH 3) was added, followed by addition of diazomethane (2 mL of a 0.66 M solution in ether, warning: EXLOSION HAZARD) with vigorous stirring. Stirring was continued for 30 min and the mixture was extracted with diethyl ether (3 × 5 mL). The ether extracts were combined and washed with water (1 × 5 mL) followed by brine (1 × 5 mL). The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to yield a pale yellow oil. Flash column chromatography on silica gel eluted with 9:1 hexane:ethylacetate gave 15a as a colorless oil (7.7 mg, 65%, Rf = 0.42 in 6:1 hexane:ethylacetate). 1H-NMR (500 MHz, d6-acetone) δ 7.78 (dd, J = 8, 1 Hz, 1H), 7.41 (qd, J = 8, 1 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 4.06 (ddd, J = 12, 9.5, 6.5 Hz, 1H), 3.86 (s, 3H), 3.46 (dd, J = 16.5, 9.5 Hz, 1H), 3.36 (dd, J = 16.5, 6.5 Hz, 1H), 1.42 (d, J = 21.5, 3H) 1.37 (d, J = 21.5 Hz, 3H) ppm. 19F-NMR (235.35 MHz, CDCl3) −139.35 (J = 21.5, 12 Hz) ppm. (19F NMR chemical shift was determined relative to internal CFCl3 at δ 0.0) 13C-NMR (125.75 MHz, CDCl3) δ 166.67, 145.14, 141.32, 128.90, 127.79, 124.19,123.63, 96.95 (J = 170.27 Hz), 55.88 (J = 25.15), 52.16, 36.29 (J = 4.28 Hz), 25.48 (J = 23.89 Hz), 22.74 (J = 24.15 Hz) ppm. HRMS (EI) calcd for C13H15FO2S [M+] 254.0776, found 254.0781. Similarly, compounds 13b–d generate 15a in comparable yields (13b, 50%; 13c, 56%; 13d, 54%).
Generation of 2-(1-methoxy-1-methyl-ethyl)-2,3-dihydro-benzo[b]thiophene-7-carboxylic acid methyl ester 15b by treatment of 13a with tetrabutylammonium fluoride in THF-MeOH, followed by diazomethane workup
To a solution of 13a (20 mg, 0.046 mmol) in THF (0.8 mL) under nitrogen, dry distilled methanol (200 μL) was added followed by tetrabutylammonium fluoride (0.37 mL of a 1 M solution in THF, 0.37 mmol, 0.27 M). The reaction mixture turned dark yellow and was stirred for 3 h. Dilute HCl (1 mL of a 1M solution, ~ pH 3) was added and the resulting biphasic mixture was treated with diazomethane (2 mL of a ~ 0.66 M solution in ether, warning: EXLOSION HAZARD) with vigorous stirring. After 30 min and the mixture was extracted with diethyl ether (3 × 5 mL). The ether extracts were combined, washed with water and brine (1 × 5 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to yield a pale yellow oil. Flash column chromatography on silica gel eluted with 6:1 hexane:ethylacetate gave 15a (5.3 mg, 45%) as a colorless oil and 15b (2.7 mg, 22%, Rf = 0.33 in 6:1 hexane:ethylacetate) as a colorless oil. All spectral data for this compound matched that reported previously.15
Trapping by methyl iodide of the sulfenate intermediate 14a generated from 13a
To a stirred solution of 13a (20 mg, 0.046 mmol) in THF (1 mL) under nitrogen tetrabutylammonium fluoride (0.37 mL of a 1 M solution in THF, 0.37 mmol, 0.27 M), and excess methyl iodide (0.14 mL, 2.3 mmol, for a final concentration of 1.7 M) were added. The reaction was stirred for 3 h and quenched by dilute HCl (1 mL of a 1 M solution, ~ pH 3). To this biphasic reaction mixture, diazomethane (2 mL of a ~ 0.66M solution in ether, warning: EXLOSION HAZARD) was added with vigorous stirring. After 30 min, the mixture was extracted with diethyl ether (3 × 5 mL). The combined ether extracts were washed with water (1 × 5 mL) followed by brine (1 × 5 mL), dried over anhydrous sodium sulfate and evaporated under reduced pressure. Flash column chromatography on silica gel eluted with 4:1 hexane:ethylacetate yielded 15a (3 mg, 25%) and 16 (4.3 mg, 35%, Rf = 0.09 in 4:1 hexane:ethylacetate). 1H-NMR (500 MHz, CDCl3) δ 7.46 (d, J = 3.75 Hz, 1H), 7.42 (t, J = 3.75 Hz, 1H), 7.37 (d, J = 3.75 Hz), 5.20 (m, 1H), 3.93 (s, 3H), 3.68 (d, J = 3.5 Hz, 2H), 3.05 (s, 3H), 1.74 (d, 6H) ppm. 13C-NMR (125.75 MHz, CDCl3) δ 168.34, 141.87. 141.40, 133.93, 132.92, 131.87, 130.45, 127.58, 121.93, 52.65, 40.17, 30.37, 25.62, 18.05 ppm. HRMS (EI) calcd for C14H18O3S [M+] 266.0976, found 266.0973.
Fluoride-independent conversion of 13a and 13b in aqueous buffer followed by diazomethane workup to yield 2-(1-hydroxy-1-methylethyl)-2,3-dihydro-benzo[b]thiophene-7-carboxylic acid methyl ester (10) in acetonitrile:aqueous buffer
Compound 13a (20 mg, 0.046 mmol) was stirred in a solution of acetonitrile (2.5 mL), sodium phosphate buffer (0.5 mL of a 500 mM, pH 7), and water (2 mL). Final concentrations in the reaction mixture were: 13a, 9.2 mM; sodium phosphate, 50 mM, pH 7, acetonitrile 50% by volume. Dilute HCl (1 mL of a 1 M solution, ~ pH 3) was added to the reaction, followed by diazomethane (2 mL of a ~ 0.66 M solution in ether, warning: EXLOSION HAZARD). The mixture was stirred for 30 min and then extracted with diethyl ether (3 × 5mL). The combined ether extracts were washed with water (1 × 5 mL) and brine (1 × 5 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to yield a pale yellow oil. Flash column chromatography on silica gel eluted with 6:1 hexane:ethylacetate yielded 10 as a colorless oil (3.9 mg, 33%, Rf = 0.15 in 6:1 hexane:ethylacetate). All spectral data for this compound matched that reported previously. Similarly, 13b affords 10 (32% yield) under these reaction conditions. It is noteworthy that addition of KF (50 mM) does not alter the rate or yield of this reaction. Finally, compounds 13c, 13d remained unchanged when subjected to the conditions described above (either with or without KF).
Acknowledgments
We thank the National Institutes of Health (CA 83925 and CA 119131) for support of this research.
Notes and references
- 1.Gates KS. In: Comprehensive Natural Products Chemistry. Kool ET, editor. Vol. 7. Pergamon; New York: 1999. pp. 491–552. [Google Scholar]
- 2.Tse WC, Boger DL. Chem Biol. 2004;11:1607–1617. doi: 10.1016/j.chembiol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Gates KS. Chem Res Toxicol. 2000;13:953–956. doi: 10.1021/tx000089m. [DOI] [PubMed] [Google Scholar]
- 4.Hara M, Asano K, Kawamoto I, Takiguchi T, Katsumata S, Takahashi K, Nakano H. J Antibiotics. 1989;42:1768–1774. doi: 10.7164/antibiotics.42.1768. [DOI] [PubMed] [Google Scholar]
- 5.Hara M, Saitoh Y, Nakano H. Biochemistry. 1990;29:5676–5681. doi: 10.1021/bi00476a005. [DOI] [PubMed] [Google Scholar]
- 6.Hara M, Takahashi I, Yoshida M, Kawamoto I, Morimoto M, Nakano H. J Antibiotics. 1989;42:333–335. doi: 10.7164/antibiotics.42.333. [DOI] [PubMed] [Google Scholar]
- 7.Behroozi SB, Kim W, Gates KS. J Org Chem. 1995;60:3964–3966. [Google Scholar]
- 8.Asai A, Hara M, Kakita S, Kanda Y, Yoshida M, Saito H, Saitoh Y. J Am Chem Soc. 1996;118:6802–6803. [Google Scholar]
- 9.Behroozi SJ, Kim W, Dannaldson J, Gates KS. Biochemistry. 1996;35:1768–1774. doi: 10.1021/bi952257t. [DOI] [PubMed] [Google Scholar]
- 10.Mitra K, Kim W, Daniels JS, Gates KS. J Am Chem Soc. 1997;119:11691–11692. [Google Scholar]
- 11.Chatterji T, Gates KS. Bioorg Med Chem Lett. 1998;8:535–538. doi: 10.1016/s0960-894x(98)00066-3. [DOI] [PubMed] [Google Scholar]
- 12.Chatterji T, Gates KS. Bioorganic Med Chem Lett. 2003;13:1349–1352. doi: 10.1016/s0960-894x(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 13.Chatterji T, Keerthi K, Gates KS. Bioorg Med Chem Lett. 2005;15:3921–3924. doi: 10.1016/j.bmcl.2005.05.110. [DOI] [PubMed] [Google Scholar]
- 14.Zang H, Gates KS. Chem Res Toxicol. 2003;16:1539–1546. doi: 10.1021/tx0341658. [DOI] [PubMed] [Google Scholar]
- 15.Chatterji T, Kizil M, Keerthi K, Chowdhury G, Posposil T, Gates KS. J Am Chem Soc. 2003;125:4996–4997. doi: 10.1021/ja029169y. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell JS, Schwan AL. J Sulfur Chem. 2004;25:183–211. [Google Scholar]
- 17.Goto K, Holler M, Okazaki R. J Am Chem Soc. 1997;119:1460–1461. [Google Scholar]
- 18.Okuyama T, Miyake K, Fueno T, Yoshimura T, Soga S, Tsukurimichi E. Heteroatom Chem. 1992;3:577–583. [Google Scholar]
- 19.Adams H, Anderson JC, Bell R, Neville Jones D, Peel MR, Tomkinson NCO. J Chem Soc Perkin 1. 1998:3967–3973. [Google Scholar]
- 20.Hashmi M, Vamvakas S, Anders MW. Chem Res Toxicol. 1992;5:360–365. doi: 10.1021/tx00027a007. [DOI] [PubMed] [Google Scholar]
- 21.Block E. Angew Chem Int Ed Eng. 1992;31:1135–1178. [Google Scholar]
- 22.Cubbage JW, Guo Y, McCulla RD, Jenks WS. J Org Chem. 2001;66:8722–8736. doi: 10.1021/jo0160625. [DOI] [PubMed] [Google Scholar]
- 23.Nagatsugi F, Kawasaki T, Usui D, Maeda M, Sasaki S. J Am Chem Soc. 1999;121:6753–6754. [Google Scholar]
- 24.Katritzky AR, Takahashi I, Marson CM. J Org Chem. 1986;51:4914–4920. [Google Scholar]
- 25.Caupene C, Boudou C, Perrio S, Metzner P. J Org Chem. 2005;70:2812–2815. doi: 10.1021/jo0478003. [DOI] [PubMed] [Google Scholar]
- 26.Aversa MC, Barattucci A, Bonaccorsi P, Giannetto P. J Org Chem. 2005;70:1986–1992. doi: 10.1021/jo048662k. [DOI] [PubMed] [Google Scholar]
- 27.Rose P, Whiteman M, Moore PK, Zhu YZ. Nat Prod Rep. 2005;22:351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]; Kubec R, Kim S, McKeon DM, Musah RA. J Nat Prod. 2002;65:960–964. doi: 10.1021/np020064i. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell JS, Schwan AL. Tet Lett. 2003;44:6293–6296. [Google Scholar]
- 29.Chambert S, Desire J, Decout JL. Synthesis. 2002:2319–2334. [Google Scholar]
- 30.Everett SA, Folkes LK, Wardman P. Free Rad Res. 1994;20:387–400. doi: 10.3109/10715769409145638. [DOI] [PubMed] [Google Scholar]
- 31.Otto AH, Steudel R. Eur J Inorg Chem. 1999:2057–2061. [Google Scholar]
- 32.Adam W, Bialas J, Hadjiarapoglou L. Chem Ber. 1991;124:2377. [Google Scholar]
- 33.Hogg DR, Robertson A. Tet Lett. 1974;43:3783–3784. [Google Scholar]
- 34.Sulfenic acids (RSOH) can also act as electrophiles that are susceptible to nucleophilic attack at the sulfur atom. See reference 17 and Sivaramakrishnan S, Keerthi K, Gates KS. J Am Chem Soc. 2005;127:10830–10831. doi: 10.1021/ja052599e.
- 35.For a related example involving intramolecular attack of an α-effect nucleophile, hydroxylamine (RNHOH), on a methyl ester, see: Cox BG, Grieve DMA, Porter AEA. JCS Perkin. 1975;2:1512–1515.
- 36.Added KF has no effect on either the rates or yields of this reaction, presumably because hydration of the fluoride ion in aqueous solution diminishes the rate of its reaction with the 2-(trimethylsilyl)ethyl group. For example, see: Hogrefe RI, McCaffrey AP, Borozdina LU, McCampbell ES, Vaghefi MM. Nucleic Acids Res. 1993;21(20):4739–4741. doi: 10.1093/nar/21.20.4739.
- 37.In a related reaction, removal of a 2-(trimethylsilyl)ethyl protecting group from sulfur can be stimulated by acylating agents, see: Wang Y, Koreeda M, Chatterji T, Gates KS. J Org Chem. 1998;63:8644–8645.
- 38.Kanda Y, Ashizawa T, Kakita S, Takahashi Y, Kono M, Yoshida M, Saitoh Y, Okabe M. J Med Chem. 1999;42:1330–1332. doi: 10.1021/jm9900366. [DOI] [PubMed] [Google Scholar]
- 39.Kanda Y, Fukuyama T. J Am Chem Soc. 1993;115:8451–8452. [Google Scholar]
- 40.Pattenden G, Shuker AJ. J Chem Soc Perkin 1. 1992:1215–1221. [Google Scholar]
- 41.Aldrich Technical Bulletin AL-180