Abstract
A new metamorphosis-enhancing macrodiolide, luminaolide (1), was isolated from the crustose coralline algae (CCA) Hydrolithon reinboldii. Its structure was determined by spectroscopic analysis. A fraction (1.30 μg/mL) eluted with 80% aqueous MeOH by ODS gel column chromatography of the same CCA extract induced larval metamorphosis (25.9 ± 7.4%) against Leptastrea purpurea, and its metamorphosis-inducing activity was further enhanced to 92.6 ± 2.9% with the addition of 1 (25.6 ng/mL).
Keywords: Luminaolide, Enhancer, Hydrolithon reinboldii, Leptastrea purpurea, Coral larvae, Crustose coralline algae (CCA)
The settlement and metamorphosis of larvae of many marine invertebrates are known to be influenced by crustose coralline algae (CCA). In some species, recruitment is inhibited by CCA.1 However, there are many more reports that coralline algae induce settlement and/or metamorphosis.2 In scleractinian coral, various species of CCA have also been shown to be the primary sources of external inducers of metamorphosis in coral larvae.3 Larvae of the scleractinian coral Agaricia humilis settle and metamorphose when exposed to the extracts of CCA Penyssonnelia sp.4 and to the fragments of Hydrolithon boergesenii.5 These phenomena are thought to produce cell-wall-bound polysaccharides that are recognized by chemoreceptors on the planula larvae.4 While several CCA, such as Lithophyllum insispidum, Hydrolithon onkodes, Neogoniolithon brassica-florida, have been shown to induce metamorphosis of the coral larvae Acropora millepora, the bacteria Pseudoalteromas sp. isolated from the surface of CCA have also been shown to induce the metamorphosis of A. millepora larvae.6 Moreover, the specific substratum preferences of CCA and bacteria have been reported in larvae of two species of scleractinian corals, Goniastrea retiformis and Stylaraea punctata.7 However, the chemical characterization of the external cues that act as natural inducers or enhancers8b of larval metamorphosis has been very limited. In our continuing search for bioactive substances in CCA,8 we found that fragments of coral rubble with the CCA Hydrolithon reinboldii induced larval metamorphosis in the scleractinian coral Leptastrea purpurea (88.9 ± 8.2, n = 6). These biological phenomena were investigated by a simple bioassay9 using larvae of L. purpurea (Fig. 1). We describe here the isolation of a new macrodiolide, luminaolide (1) (Fig. 2), as a natural enhancer of larval metamorphosis, and report its structure and biological activity.
Figure 1.
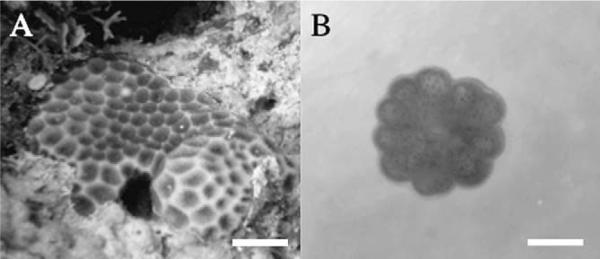
(A) Leptastrea purpurea in Guam. Scale bar = 1.0 cm. (B) Early metamorphosis of L. purpurea larvae, 24 h after exposure to the extract of CCA Hydrolithon reinboldii in a glass dish. The central mouth and septal mesenteries are observed. Scale bar = 0.5 mm.
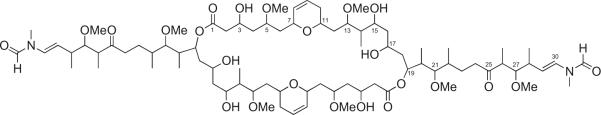
Figure 2.
Structure of luminaolide (1).
The CCA H. reinboldii (248 kg, wet weight, including coral rubble) which had overgrown a skeleton of dead Acroporidae (coral rubble) was collected by skin-diving at a depth of 0.5–1.5 m at Luminao Reef, Guam, USA. The coral rubble was extracted with methanol for 12 h. The extract was filtered, concentrated and partitioned between water and EtOAc. The EtOAc layer was further partitioned between 90% aqueous MeOH and hexane. The 90% aqueous MeOH layer was subjected to silica gel column chromatography (CHCl3–MeOH) and then ODS gel column chromatography (40% aqueous MeOH–MeOH). The fractions eluted with 80% aqueous MeOH produced a lower percentage of larval metamorphosis compared to that in untreated live CCA. However, the combination of the fractions eluted with 80% aqueous MeOH and MeOH showed higher metamorphosis-inducing activity than the fraction eluted with 80% aqueous MeOH alone (data not shown). This effect guided isolation of the fraction eluted with MeOH by reversed-phase HPLC (MeOH) and preparative TLC (CHCl3–MeOH, 20:1) to give luminaolide (1) [3.2 mg] as a white amorphous solid −49 (c 0.2, MeOH); IR (KBr) 3440 and 1650 cm−1. The molecular weight of 1 was determined by ESIMS [1562.0 (M+Na)+, 792.5 (M+2Na)2+]. The molecular formula of 1 was found to be C82H142N2O24 [(M+Na)+, m/z 1561.9845, Δ−0.5 mmu, calcd for C82H142N2O24Na 1561.9850] by HR-FABMS.
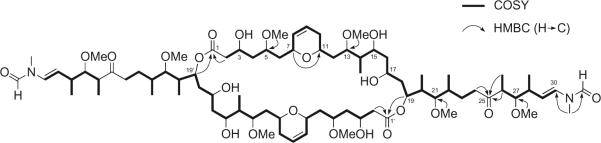
1H and 13C NMR (Table 1) and HSQC spectra of 1 in CD3OD revealed the presence of four O-methyl, one N-methyl, and five secondary methyl groups, and nine methylenes, 10 oxygen-bearing methines, five C-methines, two disubstituted double bonds, one formamide, and two ketones. A terminal N-methyl-N-vinylformamide structure in 1 was deduced based on a comparison of the 1H NMR data with those for aplyronines,10 scytophycins,11 sphinxolide,12 and macrocyclic trisoxazoles.13 Due to the limited rotation about the N-methyl-N-vinylformamide terminus, doubled NMR signals for some protons and carbons were observed in a ratio of approximately 2:1, as shown in Table 1. The Δ29,30 double bond was assigned an E geometry based on a coupling constant of 14.2 Hz between the H29 and H30 signals. A detailed analysis of the 1H–1H COSY spectra of 1 allowed the construction of two partial structures: C2–C24 and C26–C30 (Fig. 3). The connection between C24 and C26 through a ketone carbonyl carbon (C25) was suggested from HMBC for H24/C25, H26/C25, and Me-26/C25. Similarly, an HMBC was observed for H2/C1 (δC 173.79), indicating that C2 was attached to an ester carbonyl carbon (C1). The existence of a dihydropyran ring at C7–C11 was implied by the HMBC for H7/C11. Furthermore, HMBC for OMe-5/C5, OMe-13/C13, OMe-21/C21, and OMe-27/C27 suggested the attachment of four methoxy groups at C5, C13, C21, and C27, respectively. The ester linkage between C1 and C19 was established based on a relatively lowerfield chemical shift for H19 (δH 5.62) and the HMBC for H19/C1. The remaining three oxymethine carbon signals showed a deuterium shift (C3; δC 66.04, C15; δC 72.68, and C17; δC 68.23, respectively) in the 13C NMR spectra in CD3OH, which suggested that three hydroxyl groups were located at C3, C15, and C17, respectively. Overall, analysis of the NMR data indicated that 1 had a molecular formula of C41H71NO12, which was exactly half of the molecular formula C82H142N2O24 determined by HR-FABMS. Thus, it was clear that 1 was a symmetrical macrodiolide dimer composed of two identical C30 units containing two N-methyl-N-vinylformamide groups.
Table 1.
NMR data for luminaolide (1) in CD3OD
| No. | 1Ha | 13Cb | HMBC |
|---|---|---|---|
| 1 | 173.79 | ||
| 2 | 2.50 dd (9.8, 16.2)c | 44.00 | C1, 3, 4 |
| 2.59m | C1, 3 | ||
| 3 | 4.07m | 65.95 | |
| 4 | 1.46m | 42.71 | C5 |
| 1.85 ddd (3.7, 10,3, 14.1) | |||
| 5 | 3.73m | 76.16 | C5-OMe |
| 5-OMe | 3.40s | 57.22 | C5 |
| 6 | 1.35m | 39.90 | C5 |
| 1.90 ddd (2.3, 11.5, 13.7) | C7 | ||
| 7 | 4.45 br d (10.6) | 70.82 | C6, 8, 9, 11 |
| 8 | 5.67 br d (10.1) | 131.07 | C7, 10 |
| 9 | 5.83 ddd (2.5, 5.5, 7.8) | 125.11 | C7 |
| 10 | 1.97m 2H | 32.76 | C9 |
| 11 | 3.55m | 65.50 | |
| 12 | 1.64 ddd (1.3, 10.6, 14.2) | 37.67 | |
| 1.82m | C11 | ||
| 13 | 3.92 br d (9.7) | 77.60 | C11, 13-OMe |
| 13-OMe | 3.37 s | 57.22 | C13 |
| 14 | 1.51m | 43.82 | C14-Me, 15 |
| 14-Me | 0.82 d (7.4) | 9.64 | C13, 14, 15 |
| 15 | 3.62m | 72.62 | C14, 17 |
| 16 | 1.74m | 43.34 | C17 |
| 1.71m | |||
| 17 | 3.81m | 68.14 | |
| 18 | 1.44m | 42.29 | C17 |
| 1.95m | |||
| 19 | 5.62 br dd (1.4, 10.6) | 71.94 | C1 |
| 20 | 1.80m | 41.69 | C21 |
| 20-Me | 0.98 d (7.8) | 17.73 | C19, 20 |
| 21 | 2.85 dd (3.2, 7.8) | 89.40 | C19, 20, 21-OMe, 23 |
| 21-OMe | 3.44 s | 61.89 | C21 |
| 22 | 1.74m | 35.89 | |
| 22-Me | 0.97 d (7.3) | 11.62 | C21,22, 23 |
| 23 | 1.35m | 25.31 | |
| 1.74m | |||
| 24 | 2.54m | 42.83 | C22, 23, 25 |
| 2.59m | |||
| 25 | 216.74 | ||
| 25e | 216.64 | ||
| 26 | 2.76 dq (6.9, 9.2) | 50.28 | C25, 26-Me, 27 |
| 26e | 2.74 | 50.31 | |
| 26-Me | 0.95 d (6.9) | 13.90 | C25, 26, 27 |
| 26-Mee | 0.94 | 14.44 | |
| 27 | 3.30 dd [2.6, 9.2]d | 89.05 | C27-OMe |
| 27e | 88.97 | ||
| 27-OMe | 3.341 s | 61.68 | C27 |
| 27-OMee | 3.338 s | 61.68 | |
| 28 | 2.46m | 38.72 | C28-Me, 29, 30 |
| 28e | 2.50 | 38.94 | |
| 28-Me | 1.17 d (6.9) | 19.71 | C27, 28, 29 |
| 28-Mee | 19.61 | ||
| 29 | 5.19 dd (9.2, 14.2) | 113.16 | C30 |
| 29e | 5.26 | 115.32 | |
| 30 | 6.71 d (14.2) | 130.50 | C28, 29, 30-NMe, CHo |
| 30e | 7.10 | 125.71 | |
| 30-NMe | 3.03 s | 27.68 | C30, CHO |
| 30-NMee | 3.12 | 33.58 | |
| CHO | 8.32 s | 164.73 | C30, 30-NMe |
| CHOe | 8.09 | 163.36 |
800 MHz.
150 MHz.
Coupling constants (Hz) are in parentheses.
Coupling constants (Hz) based on homo-J-resolved 1H NMR spectral data are in parentheses.
Signal for minor conformer.
Figure 3.
Partial structure of luminaolide (1), based on 2D NMR correlations.
A fraction (1.30 μg/mL) eluted with 80% aqueous MeOH by ODS gel column chromatography induced larval metamorphosis (25.9 ± 7.4%) of L. purpurea, as shown in Table 2. A higher concentration (6.50 μg/mL) of this fraction showed malformed or dead individuals without an increase in the percentage of those undergoing metamorphosis (data not shown). These results suggest that this fraction may contain at least one compound that acts as a natural inducer of larval metamorphosis, although no such inducer has yet been isolated from this fraction. On the other hand, when luminaolide (1) was offered to L. purpurea larvae at a concentration of 25.6 ng/mL together with this fraction by ODS gel column chromatography (1.30 μg/mL), metamorphosis significantly increased to 92.6 ± 2.9% compared to that with the fraction obtained by ODS gel column chromatography alone (Table 2). Furthermore, at different concentrations, 1 alone (12.8, 25.6, and 128 ng/mL, respectively) did not show any induction of larval metamorphosis in L. purpurea (n = 4). These results indicate that 1 serves to enhance larval metamorphosis in L. purpurea and does not act as an inducer. This kind of enhancing effect in larval metamorphosis has been generally reported for carotenoids in the scleractinian coral Pseudosiderastrea tayamai.8b
Table 2.
Enhancing effects of luminaolide (1) on the induction of larval metamorphosis by a fraction eluted with 80% aqueous MeOH by ODS gel column chromatography
| Additive (ng/mL) | % Metamorphosis ± SE |
|---|---|
| Control | 0 ± 0.0 a |
| None | 25.9 ± 7.4 b |
| Lunimaolide (12.8) | 27.8 ± 6.8 b |
| Lunimaolide (25.6) | 92.6 ± 2.9 c |
Metamorphosis was scored 24 h after the addition of L. purpurea larvae (1–3 days after larval release). Each value represents the mean (±SE) of nine replicates with six larvae in each replicate. Values with different letters were significantly different from each other (P <0.01; Tukey's test).
Compounds with structures similar to that of 1, such as tolytoxin,14 scytophycines,14 swinhoide A,15 and their analogs, have been isolated from cyanobacteria, and some related compounds are also thought to originate from cyanobacteria.16 Fenical and co-workers reported that lobophorolide was isolated from the seaweed Lobophora variegata. However, due to the similarity of its structure to related macrolides, they concluded that the true producer could be a microbial symbiont of L. variegata.17 Based on these previous reports, we can speculate that 1 is produced by epiphytic bacteria on the surface of the CCA H. reinboldii and not directly by the CCA itself, although no such symbiont has yet been identified.
In conclusion, a new metamorphosis-enhancing dimeric macrolide, luminaolide (1), was isolated from the CCA H reinboldii. Compounds related to 1 which possess an N-methyl-N-vinylformamide terminus10–13,18 and macrodiolides19 with a wide range of activities, such as cytotoxic, antibiotic, antifungal, sea urchin egg cleavage-inhibitory, actin depolymerizing, and proteasome inhibitory activities, have been reported. However, this is the first report of a metamorphosis-enhancing macrolide for scleractinian coral larvae.
Acknowledgments
We thank Ciemon Caballes (University of Guam) for assisting in CCA collection and Chie Takase and Ingeborg Ipping Petterson (University of Guam) for the collection of larvae. This work was supported in part by Grants-in-Aid for Creative Scientific Research (16GS0206) and Scientific Research (C) (20611003) from the Japan Society for the Promotion of Science (JSPS) and the 21st century COE program (Establishment of COE on Materials Science) from the Ministry of Education, Science, Sports, and Culture (MEXT), Japan. We are indebted to Ono Pharmaceutical Co., Ltd for their financial support.
References and notes
- 1.Breitburg DL. Ecology. 1984;65:1136. [Google Scholar]
- 2.Hadfield MG, Paul VJ. In: Marine Chemical Ecology. McClintock JB, Baker BJ, editors. CRC Press; Boca Raton: 2001. p. 443. [Google Scholar]; (a) Hayakawa J, Kawamura T, Horii T, Watanabe Y. Fish. Sci. 2007;73:371. [Google Scholar]; (b) Williams EA, Craigie A, Yeates A, Degnan SM. Biol. Bull. 2008;215:98. doi: 10.2307/25470687. [DOI] [PubMed] [Google Scholar]
- 3.Morse ED, Hooker N, Morse ANC, Jensen RA. J. Exp. Mar. Biol. Ecol. 1988;116:193. [Google Scholar]
- 4.Morse DE, Morse ANC. Biol. Bull. 1991;181:104. doi: 10.2307/1542493. [DOI] [PubMed] [Google Scholar]
- 5.Morse ANC, Iwao K, Baba M, Shimoike K, Hayashibara T, Omori M. Biol. Bull. 1996;191:149. doi: 10.2307/1542917. [DOI] [PubMed] [Google Scholar]
- 6.(a) Heyward AJ, Negri AP. Coral Reefs. 1999;18:273. [Google Scholar]; (b) Negri AP, Webster NS, Hill RT, Heyward AJ. Mar. Ecol. Prog. Ser. 2001;223:121. [Google Scholar]
- 7.Golbuu Y, Richmond RH. Mar. Biol. 2007;152:639. [Google Scholar]
- 8.(a) Kitamura M, Koyama T, Nakano Y, Uemura D. Chem. Lett. 2005;34:1272. [Google Scholar]; (b) Kitamura M, Koyama T, Nakano Y, Uemura D. J. Exp. Mar. Biol. Ecol. 2007;340:96. [Google Scholar]
- 9.Bioassay: Assays for larval metamorphosis were performed in a glass dish at the same environmental conditions as stock larvae cultures. Samples of the test chemicals were dissolved in methanol and added into glass dishes. After the solvent was dried, 10 mL of filtered seawater and mobile planula larvae (six individuals) were added into each dish, and the dishes were incubated in the dark for 24 h at 25–28 °C. The number of young polyps was then counted under an optical microscope. Our criteria were the major change from planula larva to developing primary polyp. For these experiments, we define metamorphosis to have occurred when the larvae changed into disk-shaped structures with septal mesenteries radiating from the central mouth region (Fig. 1B).
- 10.Yamada K, Ojika M, Ishigaki T, Yoshida Y. J. Am. Chem. Soc. 1993;115:11020. [Google Scholar]
- 11.(a) Moor RE, Patterson GML, Mynderse JS, Barchi J, Jr., Barchi J, Jr., Norton TR, Furusawa E, Furusawa S. Pure & Appl. Chem. 1986;58:263. [Google Scholar]; (b) Ishibashi M, Moor RE, Patterson GML, Xu C, Clardy J. J. Org. Chem. 1986;51:5300. [Google Scholar]
- 12.Guella G, Mancini I, Chiasera G, Pietra F. Helv. Chim. Acta. 1989;72:237. [Google Scholar]
- 13.(a) Roesener J, Scheuer PJ. J. Am. Chem. Soc. 1986;108:846. [Google Scholar]; (b) Matsumoto S, Fusetani N, Hashimoto K. J. Am. Chem. Soc. 1986;108:847. [Google Scholar]
- 14.Carmeli S, Moore RE, Patterson GML. J. Nat. Prod. 1990;53:1533. doi: 10.1021/np50072a021. [DOI] [PubMed] [Google Scholar]
- 15.Andrianasolo EH, Gross H, Goeger D, Musafija-Girt M, McPhail K, Leal RM, Mooberry SL, Gerwick WH. Org. Lett. 2005;7:1375. doi: 10.1021/ol050188x. [DOI] [PubMed] [Google Scholar]
- 16.(a) Tsukamoto S, Ishibashi M, Sasaki T, Kobayashi J. J. Chem. Soc., Perkin Trans. 1991;1:3185. [Google Scholar]; (b) Todd JS, Alvi KA, Crews P. Tetrahedron Lett. 1992;33:441. [Google Scholar]
- 17.Kubanek J, Jensen PR, Keifer PA, Sullards MC, Collins DO, Fenical W. PNAS. 2003;100:6916. doi: 10.1073/pnas.1131855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Matsumoto S, Fusetani N, Hashimoto K, Noguchi H, Sankawa U. J. Org. Chem. 1989;54:1360. [Google Scholar]; (b) Saito S, Watabe S, Ozaki H, Fusetani N, Karaki H. J. Biol. Chem. 1994;269:29710. [PubMed] [Google Scholar]; (c) Saito S, Watabe S, Ozaki H, Kigoshi H, Yamada K, Fusetani N, Karaki H. J. Biochem. 1996;120:552. doi: 10.1093/oxfordjournals.jbchem.a021449. [DOI] [PubMed] [Google Scholar]; (d) Tsukamoto S, Koimaru K, Ohta T. Mar. Drugs. 2005;3:29. [Google Scholar]
- 19.(a) Nakamura H, Iitaka Y, Kitahara T, Okazaki T, Okami Y. J. Antibiot. 1977;30:714. doi: 10.7164/antibiotics.30.714. [DOI] [PubMed] [Google Scholar]; (b) Kaiser H, Keller-Schierlein W. Helv. Chim. Acta. 1981;64:407. [Google Scholar]; (c) Kobayashi M, Tanaka J, Katori T, Matsuura M, Kitagawa I. Tetrahedron Lett. 1989;30:2963. [Google Scholar]; (d) Tsuda M, Izui N, Shimbo K, Sato M, Fukushi E, Kawabata J, Katsumata K, Horiguchi T, Kobayashi J. J. Org. Chem. 2003;68:5339. doi: 10.1021/jo0343634. [DOI] [PubMed] [Google Scholar]; (e) Kwon HC, Kauffman CA, Jensen PR, Fenical W. J. Am. Chem. Soc. 2006;128:1622. doi: 10.1021/ja0558948. [DOI] [PubMed] [Google Scholar]